Abstract
Variations among stamens and styles are widespread in angiosperms. In both heteranthery and heterostyly, variation in styles and stamens is considered an outcome of selective forces driven mainly by pollinators. Macairea radula (Melastomataceae) has two stamen sets, poricidal anthers, pollen as the main floral resource and flowers visited by vibrating bees, a set of traits related to heteranthery. However, tristyly, which also predicts stamens differences within the same flower, has been proposed as a possible floral system for this species. We describe the variation in length of stamens and styles in two populations of this species considering the interaction with their pollinators. Both populations showed high variation in stamen and style lengths with length overlap between floral types. These populations also showed low reciprocity between stamens and styles. In one chosen population, where we evaluated fruit formation after controlled pollination and checked the individual floral types, plants were partially self-incompatible and there was no proportional representation of floral types (anisoplethy). These results suggest that M. radula does not follow the theoretically expected patterns of the tristylous systems, because there is a flexibility in all heterostylous traits analyzed within populations. Considering the length of bee bodies as well as their behavior, stamens and styles could be associated with bee feeding or plant reproduction, as occurs in heteranthery. Our study points out that the evaluation of floral systems only based in floral morphological traits without considering the morphology and behavior of pollinators may be misleading in some cases.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The huge diversity of floral traits is widely proposed to be an outcome of selection exerted by pollinators since interactions with them are essential to ensure crossbreeding (Nabhan and Buchmann 1997; Harder and Johnson 2009; van der Niet and Johnson 2012). Such selective force can promote the development and evolution of a correlated set of floral traits that, in its turn, can be called floral system (Cardoso et al. 2018). The association between a set of floral traits to floral systems has helped to understand the functioning and evolution of many flowers. This is specifically true when considering morphological traits because their structure, position, and temporal variation may directly affect reproductive success and, ultimately, plant fitness (Barrett 2002). However, the association between a set of floral morphological traits and floral systems is not always straightforward. Morphological variation among and within populations can hamper the direct assessment of the floral system, and some floral systems may even overlap in their expected set of morphological traits.
Heteranthery is a floral system in which two sets of different stamens with feeding and pollination functions occur within the same flower (Luo et al. 2008). This system is thought to be a solution to the "pollen dilemma" in flowers that offer only pollen as rewards to pollinators, mainly bees (Vogel 1978). In such flowers, pollen grains serve simultaneously as a food source for bee offspring and as a reproductive resource for plants (Luo et al. 2008). Therefore, in heterantherous flowers, short stamens would provide pollen grains for bee feeding, while the long ones would make pollen available for plant reproduction, solving this evolutionary conflict (Luo et al. 2008; Vallejo-Marín et al. 2009, 2010). In this system, stamens and styles that are longer than the bee's body are mainly involved in plant reproduction, while those that are below this threshold are directly in contact with unsafe sites on the bee’s body where pollen can be effectively groomed. Along with the difference in length, it is also common to find a difference in shape, color and scent of these structures (Vallejo-Marín et al. 2009; Velloso et al. 2018; Solís-Montero et al. 2018).
Heterostyly is another type of floral system involving the length of the stamens and styles related to optimization of reproductive success (Darwin 1877; Ornduff 1966; Barrett 1990, 2019). However, unlike heteranthery, this system is not directly involved with pollen partitioning, but rather to the promotion of cross-pollination among individuals of different morphs (i.e., disassortative mating) and to decrease self-interference and gamete loss. Distyly and tristyly are types of heterostyly characterized by the arrangement of the length of the stamens and styles resulting in two (long- and short-styled) and three (long-, mid- and short-styled) floral morphs, respectively, each one distributed in different individuals, within populations (Ganders 1979; Bawa and Beach 1983; Barrett 1993, 2019; Barrett et al. 2000; Barrett and Shore 2008). In tristyly, the stigma of the long-styled flowers is placed above the two whorls of anthers, in the mid-styled flowers the stigma is placed between them, and in the short-styled flowers the stigma is below the two anthers whorls (Barrett 1993, 2019; Barrett et al. 2000; Barrett and Shore 2008). Theoretically, it is expected that the stigmas of a floral morph correspond to the same position (length) of the anthers of the other floral morphs for that level, an arrangement referred to as reciprocal herkogamy in an heterostylous system (Ganders 1979; Lloyd and Webb 1992; Barrett 2002, 2019). In this system, the occurrence of stamens and styles with the same length within the same morph is possible and it is considered maladaptive because it would not prevent intra-morph crossing. In most heterostyly cases, species present a self- and intra-morph incompatibility system, associated with reciprocal herkogamy, which limits self- and intra-morph mating and often favors inter-morph (disassortative) mating (Bawa and Beach 1983; Kohn and Barrett 1992; Barrett 2019). In a theoretical scenario, the disassortative mating may drive heterostylous populations to an isoplethic equilibrium (1:1:1) of morph ratio (Fisher 1941; Heuch 1979). However, the occurrence of biased morph ratios is also possible. Such biased morph ratios can be caused by historical factors, such as genetic drift, or even by inbreeding, differences in selfing rates and male and female mating success (Barrett et al. 1983; Morgan and Barrett 1988; Cunha and Barrett 2019).
The appearance of both heteranthery and tristyly is quite recurrent in the evolutionary history of angiosperms. Heteranthery has evolved in more than 20 families, while tristyly has been reported in seven unrelated families (Barrett 2002; Vallejo-Marín et al. 2010; Naiki 2012). Among them, Fabaceae, Lythraceae and Pontederiaceae have already been reported for exhibiting both heteranthery and tristyly within its genera and species (Vallejo-Marín et al. 2010). Both heteranthery and tristyly allow the deposition of pollen loads on different pollinator body parts, but at the population level, species with heteranthery present only two sets of stamens, while tristylous populations present three sets of stamens with different lengths. However, in the tristylous populations, no matter which floral morph is considered, each flower presents only two stamen sets, just as heterantherous flowers. This may confuse the interpretation of the floral system in plants that possess two kinds of stamens within the same flower. Such confusion may be hard to untangle when studying only the intrafloral morphological data without considering the distribution of the stamen and style lengths at the population level, the pollinator behavior and the mechanical fit between flowers and pollinators during floral visits (Baker et al. 2000; Ferrero et al. 2009).
Most Melastomataceae species have flowers with poricidal anthers, providing only pollen as floral reward and the bee buzzing behavior determines its reproductive success (Fracasso 2008; Luo et al. 2009; Renner 1989). Melastomataceae is also commonly known for their numerous heterantherous species (Vallejo-Marín et al. 2010; Melo et al. 2021), which seems to be the case for Macairea radula (Bonpl.) DC, a species with a visible variation in the length of stamens within flowers and of stamens and styles among individuals. Such variation, especially the positioning of the style in relation to the two sets of stamens, made M. radula be formally described as the first heterostylous pollen flower species (Fracasso 2008), but accurate morphological data about the distribution of the length of stamens and styles are still lacking. Fracasso (2008) described three floral morphs in similar proportions in different populations of M. radula. Short- and long-styled flowers showed better reciprocity in the length of one of their stamen and style sets, unlike to what was observed in mid-styled flowers, which showed intermediate characteristics.
In this study, we investigated the floral biology of M. radula, analyzing whether the morphology of stamens and styles linked to the interaction with effective pollinators make this species more likely to be either heterantherous or tristylous. Specifically, we aimed to investigate whether, as it is theoretically expected by tristylous systems, (I) the variation in stamen and style lengths among individuals determines three different floral types, (II) there is a precise reciprocity in stamen and style positions among the possible floral types, (III) the possible floral types possess a strongly expressed self-incompatibility system and (IV) the population of M. radula is isoplethic. Our hypothesis is that heteranthery better describes the floral system of M. radula than tristyly. Thus, we predict to find a large variation in stamen and style lengths among different plants, which makes it difficult to determine the number of floral types within populations and to promote precise reciprocity between stamens and styles as theoretically expected by tristylous systems. In addition, we also predict that M. radula displays variation in its self-incompatibility system and population morph ratio.
Material and methods
Species and area of study
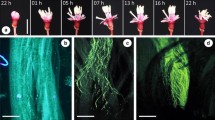
Macairea radula is a shrub ca. 1.5–2 m tall. Its flowers have eight alternately dimorphic stamens in two whorls, the short antepetalous set (AP) and the large antesepalous set (AS) (Fig. 1). Although M. radula flowers produce eadible oils in staminal glands (Oliveira et al. 2022), pollen grains are the only floral resource and are concealed in anthers with poricidal dehiscence. Pollen removal from this type of anther is only possible through the vibration of the wing muscles of bees that are specialized in a process known as buzz pollination (Buchmann 1983; De Luca and Vallejo-Marín 2013). The styles are curved upward with a punctiform stigma, once the flowers are mostly perpendicular to the ground at the anthesis (Bacci et al. 2016; Oliveira et al. 2020). The length of the stamens and styles varies among different individuals, which can be characterized into three floral types according to style position related to both stamen sets: short-styled (hereafter S-styled)—when the style is shorter than the two sets of stamens, mid-styled (hereafter M-styled)—when the length of the style is intermediate between the two sets of stamens, and long-styled (hereafter L-styled)—when the style is longer than the two sets of stamens (Fig. 1). Intra-individual variation in the length of stamens and styles is very low in comparison with the inter-individual variation, with no variation in the floral type within the individual (Online Resource 1).
We collected morphological data of M. radula between August and November of 2017 and 2018 in two distinct populations located at Uberlândia (hereafter UDI; 19º 03’ S 48º 21’ W; 801 m.a.s.) and Delfinópolis (hereafter DEL; 20° 25' S 46° 40' W; 844 m.a.s.) municipalities, both in Minas Gerais State, Brazil. The study areas lie within the Brazilian savanna, in a kind of swamp vegetation known as “vereda”. In DEL, the climate is Cwa according to Köppen classification, with the rainy period from October to April and dry period from May to September, an average annual temperature of 18° C and annual rainfall of approximately 1250 mm (Alvares et al. 2013; MMA 2020). In UDI, the climate is Aw, with the rainy period from October to March and dry period from April to September, an average annual temperature of 22° C and annual rainfall of approximately 1500 mm (Alvares et al. 2013; Rosa et al. 1991).
Stamen and style morphology
To analyze whether variations in the length of stamens and styles are related to tristylous morphological patterns, we collected flowers from 35 and 39 individuals haphazardly selected in UDI and DEL populations, respectively. We measured the length of the two sets of stamens and styles in three newly opened flowers of each individual, taking digital photographs of the stamens and styles on a glass plate with graph paper. For each flower, we arranged one AS stamen, one AP stamen and the style on the same glass plate. Then, using a digital camera (Canon EOS30D) coupled with an interchangeable lens (EF-S 18-135mm f/3.5-5.6 IS STM) on a regular tripod, we took one picture per flower. We positioned the camera lens parallel to the plate and standardized both the camera distance to ± 1 m and the focal length of the lens to 50 mm to prevent any differential size distortion in the pictures (Oliveira et al. 2020). We took length measurements from the base to the apex of the AS and AP stamens and of the style (Solís-Montero and Vallejo-Marín 2017) using ImageJ software (Online Resource 2).
Relationship between pollen deposition sites in pollinators and alternative functions of stamens and styles
Fracasso (2008) observed that M. radula flowers are visited by Centris, Bombus, Oxaea and Augochloropsis species. Most of these bees have large bodies and similar foraging behavior. Generally, such bees land on the flowers, grab the set of stamens and style and vibrate them to release pollen (buzz pollination). Consequently, the stigma contacts the surface of their body, and pollination occurs. Once Augochloropsis bees have a smaller size, they can vibrate just one or a few stamens by time, sometimes contacting the stigma which occasionally leads to pollination (Mesquita-Neto et al. 2018). Bombus sp. and Oxaea sp. bees are infrequently visitors and take longer to visit just a few flowers and plants. On the other hand, Centris sp. bees are quite fast in their visits and explore many flowers in their foraging bout, seeming to be the most effective pollinators of M. radula (Fracasso 2008). In fact, the most frequently identified bee species visiting M. radula flowers, over 90% of visits viewed, was Centris aenea (Lepeletier) in both UDI and DEL populations (LC. Oliveira personal observation).
We measured the length of the abdomen of 20 bees belonging to C. aenea to graphically understand how the length variation of stamens and styles is related to the pollinator body length. We took the bee abdomen as a reference measurement because we observed that stamens and styles contact different areas (i.e., venter and dorsum) of this region during the visits (Fig. 2). During their visits, bees grab and vibrate all the stamens and the style, generating a cloud of dry pollen that partially adheres to their body. After some visits, bees mainly groom the ventral region of the abdomen possibly changing the initial distribution of pollen grains (Koch et al. 2017). Pollen deposited on the ventral region is frequently manipulated and may be easily lost for bee feeding, whereas pollen on the dorsal region usually remains on the body of the bee and may be destined for pollination (Luo et al. 2008). Thus, stamens and styles that are longer than the abdomen of the bees can be considered mainly reproductive. On the other hand, stamens and styles that are shorter or equal to the bees’ abdomen would touch the region of the bee body directly involved with grooming behavior, and pollen would often be involved with feeding function.
Two floral types of Macairea radula and its effective pollinator Centris aenea during a flower visit. a A flower with style shorter than the two sets of stamens, likely performing both feeding and pollination functions; b a flower with the style longer than the two sets of stamens, both performing only the feeding function. Illustrator: Pedro Lorenzo
Self-incompatibility and dependence on pollinators
We selected individuals with each predefined floral type in the UDI population, as previously described. We also tested whether fruit production is dependent on pollinators and whether the plants possess a strong self-incompatibility system, as theoretically expected in tristylous systems. To test for spontaneous self-pollination, we bagged flower buds that were not manipulated in any other way (n = three flowers per individual/20 individuals per floral type). To test for self-incompatibility, we hand self-pollinated flowers previously bagged in bud stage (n = 20–25 flowers per individual/three individuals per floral type). To assess natural levels of pollination (control), we used two different methods: 1) Non-bagged marked flowers on the same individuals of the other treatments were kept accessible to flower visitors (n = three flowers per individual/ca. 10 individuals per floral type) and 2) the number of flower scars during the fruiting phase was counted as an indicative of the total number of flowers produced in the flowering season. The fruit set in all treatments was estimated as the ratio between the number of fruits produced and the number of flowers or scars counted. Although inter- and intra-morph crosses have not been performed, we consider that fruit production from self-pollination could indicate a certain deviation from the disassortative mating pattern theoretically expected in heterostylous systems.
Population floral type ratio
The floral type ratio was assessed in the UDI population. In the field, we classified plants as S-, M- or L-styled, as above mentioned, and calculated the number of individuals of each floral type in a haphazardly sampling, in order to verify their proportion in this population. A total of 178 individuals were sampled.
Data analysis
In both populations, we tested if the style and stamen lengths would be arranged in a way similar to theoretically expected as if a tristylous system would determine floral types in M. radula, i.e., if there is an intra-morph difference in the length of stamens and styles, and if there is no inter-morph difference within equivalent floral organ lengths. To do that, we compared the length of each stamen and style (response variable) among floral organs sets (style, AP stamen or AS stamen) and floral types (S-, M- or L-styled) using a linear mixed model. Plant individuals were considered a random factor. We compared the fitted model against a null model, and we found them different from each other (UDI: Chisq = 708.63, DF = 8, p < 0.001; DEL: Chisq: 839.19, DF:8, p < 0.01). We used the function anova type III from the lmerTest package (Kuznetsova et al. 2017) to obtain the significance of each independent variable and the interaction term. Afterward, we applied a post hoc pairwise t test with pooled standard deviations followed by Bonferroni correction to test for differences among groups. To find out which stamens potentially have feeding and/or pollination function, we also tested the hypothesis that the stamen length of each floral type is greater than the length of the abdomen of pollinating bees using a single-tailed t test. The same reasoning was used to determine whether the styles from the different floral types touched the sites of the bee's body that they do not groom.
We also evaluated the variation in the length of stamens and styles graphically as in Sánchez et al. (2013), which considers the dispersion of the styles and stamens to characterize each of the three possible organ levels of a species with tristylous systems. To investigate reciprocity, we predefined the floral types (S-, M- or L-styled) considering the length of the style in relation to the two sets of stamens. We estimated the levels of reciprocity between floral organs for each population by two indexes: (1) the reciprocity index (R2) of Sánchez et al. (2013) and (2) the method of inaccuracy proposed by Armbruster et al. (2017). R2 provides value of reciprocity at the population level that can be compared to other truly heterostylous species (distylous or tristylous), being defined as 1—(R × 10), where R = r x SDr. In this formula, r represents the overall reciprocity index based on all inter-individual comparisons in the population, and SDr is the arithmetic mean of the standard deviations of r calculated for short, long and mid-organs (for a complete description, see Sánchez et al. 2013). The R2 values range between 0 (minimum reciprocity) and 1 (perfect reciprocity).
The method of adaptive inaccuracy proposed by Armbruster et al. (2017) can also be used to measure reciprocity in heterostylous species in general, but comparisons between distylous and tristylous species are not possible. To date, studies have only been developed with distylous species (Armbruster et al. 2017; Jacquemyn et al. 2018; Matias et al. 2020; Raupp et al. 2020; Furtado et al. 2021), and this work is the first to use this index to calculate reciprocity in a species with three possible floral morphs. The inaccuracy index analyzes how populations depart from the optimum and variation of the mean of these populations (known as imprecision) contributes to inaccuracy in pollen transfer and the final phenotypic scenario (Armbruster et al. 2017). With this method, it is possible to estimate reciprocity at the population level, but also verify the inaccuracy in reciprocity of the short, long and mid-organs separately. The inaccuracy between the short, long and mid-organs of each population was calculated from the equations below in which (A) represents the length of the short styles, (B) is the length of the long styles, (C) is the length of the mid-styles, (a) is the length of the short stamens (AP) of long-styled and mid-styled flowers, (b) is the length of the long stamens (AS) of short-styled and mid-styled flowers and (c) represents the length of the mid-stamens (AP and AS, respectively) of long-styled and short-styled flowers.
Letters with bars correspond to the populational average of each organ length, while V corresponds to organ length variance (i.e., the squared deviation from the mean). The unit of inaccuracy is millimeters squared, and the results of short, long and mid-organs inaccuracies were summed to provide a total inaccuracy value per population. To compare levels of inaccuracy between the two populations and for future comparisons with species with tristylous systems, values were standardized in percentage from the total squared mean length of the floral organs (i.e., the sum of the mean lengths of all stamens and styles). An inaccuracy value equal to zero indicates perfect reciprocity. Thus, lower values of inaccuracy represent better levels of reciprocity (Armbruster et al. 2017).
We compared the ratio of the number of individuals of each floral type using a Chi-square test to find out if the abundances of each floral type were balanced in the population (isoplethy) as theoretically expected in large heterostylous populations with a predominance of disassortative mating (Darwin 1877; Zhou et al. 2015).
All tests and graphics were made in software R-4.0.2 (http://www.r-project.org/) using the packages: ggplot2 (Wickham 2016) for graphic analyzes, lme4 (Bates et al. 2014) and lmerTest (Kuznetsova et al. 2017) for analyzes of linear mixed-effects models.
Results
There is a huge variation in stamen and style length in both populations of M. radula (Fig. 3). Stamens ranged from 2.0 mm to 19.7 mm. Styles ranged from 3.4 mm to 16.0 mm. In both populations, the length among floral organ sets (style, AP stamen and AS stamen) and floral types was significantly different (Table 1) and the differences among the floral organs can be found in Online Resource 3. Stamens and styles of different floral types touch different bee body regions (Table 2; Online Resource 3). In UDI population, L-styled flowers have only isometrically short stamens, while both M- and S-styled flowers have long stamens as well. In DEL population, S-styled flowers have only isometrically long stamens, while the other two flower types bear both short and long stamens. In both populations, L- and M-styled flowers possess styles longer than bee abdomen length, while S-styled flowers have styles not longer than this threshold (Table 2; Online Resource 3).
Stamen and style lengths (a and c) and style vs. stamen plots (b and d) for Macairea radula in Uberlândia (a and b) and Delfinópolis (c and d) populations, Minas Gerais, Brazil. Plots show the real values of floral organs of M. radula (dots) as well as the bee body threshold (dashed line) of Centris aenea, the main pollinator in both populations studied. In figure a and c, the individuals were sorted in order of increasing style length as in Ferrero et al. 2011 and Sánchez et al. 2013. Different color tones represent long and short stamens in each flower type
Despite the apparent three levels of stamens and style in both populations, the distribution of stamen and style lengths is mostly continuous, with a certain overlap between M- and L-styled flowers in both populations (Fig. 3). However, there is a gap in style length distribution in both populations that overlaps with the bee body threshold (Fig. 3a and c). Both UDI and DEL populations showed low reciprocity between stamens and styles. The Sánchez index (R2) indicated that the UDI population had a reciprocity value of 0.58 and that the DEL population had a reciprocity value of 0.59. The inaccuracy value (another method used to measure the reciprocity) for the UDI population was 16.52 mm2 (25.27% in the standardized scale), so that the mid-organs contributed 54.67% to the total inaccuracy, followed by long organs with 36.77% and short organs with 8.55%. For the DEL population, inaccuracy value was 15.5 mm2 (16.09% in the standardized scale). The long organs contributed 55.41% to the total inaccuracy in the DEL population, followed by mid-organs with 34.65% and short organs with 9.93%. In both populations, the short organs were more reciprocal than the other organ levels.
Flowers in the UDI population showed dependence on pollinators for reproduction (Table 3). In addition, we detected evidence for partial self-incompatibility, given that some fruits were formed after manual self-pollination (Table 3). Natural pollination (control) produced about 70% more fruits than manual self-pollination. In the UDI population (n = 178), 45% of the individuals had L-styled flowers, 35% had S-styled flowers and 20% had M-styled flowers, resulting in a proportion that differed from that theoretically expected in tristylous systems (X2 = 18.011; Df = 2; p < 0.05).
Discussion
In our study, we investigated the variation in length of stamens and styles in a species with pollen flowers. Macairea radula has been previously characterized by the occurrence of three floral types in its populations (Fracasso 2008). Tristyly has been reported in many families, such as Amaryllidaceae, Pontederiaceae, Connaraceae, Oxalidaceae, Linaceae, Lythraceae and Thymelaeaceae (Naiki 2012), and M. radula could be the first case in Melastomataceae. However, although our results showed three length levels of floral organs in both studied populations, stamen and style lengths vary almost continuously, with an overlap between M- and L-styled flowers within populations. Although heterostylous systems comprise discrete style morphs, variation in the position of stamens and styles is usually continuous, but not overlapping as recorded in M. radula (Cunha et al. 2014; Barrett 2019). Moreover, both populations showed low reciprocity among stamens and styles. Interestingly, there is a gap in style length variation in both populations that overlaps with the length of the pollinating bees’ abdomen, a possible maladaptive contact region for the stigmas given bee grooming behavior. Stamens and styles of different floral types possibly contact different regions in the bees’ abdomen and stamens from different whorls could be categorized as feeding or pollinating (Fig. 2). Our results also showed that the UDI population is anisoplethic; that is, there is an unbalanced proportion of individuals of each floral type, being the L-styled individuals more represented. In addition, self-incompatibility is not strongly expressed within plants as theoretically expected in a heterostylous system, because some fruits were formed in manual self-pollination treatments. Low reciprocity among stamens and styles, anisoplethy and partial self-incompatibility are features found in many tristylous populations (Barrett 2019), but these features rarely occur together within the same population, hampering the classification of M. radula as a tristylous species. Our results suggest a possible specialization of plant individuals in male and female sexual functions, which may explain the unbalanced proportion of floral types found in the UDI population and also a functional reciprocity and role of styles and stamens in this species.
As theoretically expected by tristylous systems, intra-morph variations among stamen and style lengths were recorded in all floral types of M. radula. In addition, both populations studied showed no inter-morph variations within equivalent floral organs lengths, except for mid-organs. Moreover, when the distribution of lengths at the population level is graphically analyzed, we can clearly find an overlap in the variation in both stamen and style lengths between M- and L-styled flowers. One could argue that the variation in stamen and style lengths of M. radula is driven by micro-environmental variation given that such plants occur in high patchy swamp areas of the Brazilian savannah. However, plants of different floral types could occur in the same patch tangled one to another (LC. Oliveira personal observation), which suggests that the morphology of stamens and styles of M. radula is mostly genetically driven. Breeding experiments involving plants of different floral types as pollen donors and/or receivers will be necessary to finally put an end to this debate (Barrett 1993).
Low reciprocity and maladaptive zones for pollen flow among morphs can occur in heterostylous systems because there is a self- and intra-morph incompatibility system, leading to pollen and ovule discounting in flowers with stamens and styles not well-positioned in relation to the pollinator body (Lloyd and Webb 1992; Brys et al. 2008; Matias et al. 2020). Unfortunately, we were unable to investigate the intra-morph incompatibility of M. radula, but the formation of some fruits after manual self-pollination suggests a flexibility in the self-incompatibility system. Thus, individuals may have some degree of assortative mating, and disassortative pollination is not a necessary condition for reproduction. Heterostylous species that have some degree of assortative mating usually have a precise reciprocity to promote sufficient disassortative mating (Barrett et al. 1987; Kohn and Barrett 1992; Zhou et al. 2015). However, we found a low reciprocity and a large morphological flexibility in the length of stamens and styles among individuals of M. radula suggesting a lack of the heterostylous maladaptive zones for stamen and style positions in both populations. We also found an overlap of style lengths of the M- and L-styled flowers and a gap in style length variation in both populations. Interestingly, this gap overlaps with the length of the pollinating bees’ abdomen and creates a bimodal distribution of style lengths in both populations.
Due to the grooming behavior of vibrating bees, the distribution of pollen grains on the pollinator’s body can be altered in relation to its initial deposition sites (Harder and Wilson 1998). Therefore, pollen grains are spatially structured in the bee body, occupying safe or exposed sites (Koch et al. 2017). Pollen grains on the exposed sites are more frequently manipulated and may be lost, while bee legs usually do not reach the safe sites, and the pollen in these regions is more easily destined for pollination (Harder and Barrett 1996; Harder and Wilson 1998; Koch et al. 2017). The effect of grooming behavior is even more pronounced in flowers that offer pollen grains as the main resource to motivate pollinator visits, as is the case of M. radula. When visiting pollen flowers, both the midline of the dorsal abdomen and the bee waist are two of the safest sites on the bee body for stigma contact (Tong and Huang 2018). Therefore, the bimodal distribution of style lengths in both populations could be related to the best contact sites for stigmas considering the grooming behavior of bee pollinators. In fact, short and long styles in M. radula are selected by vibrating bees in both populations when considering the female fitness component (Oliveira et al. 2020). Thus, the low reciprocity verified for floral organ position does not necessarily mean weak functional reciprocity when we contrast the morphological fit between these organs and the pollinator body.
The degree of reciprocity exhibited by heterostylous species can vary widely (Sánchez et al. 2008). However, the reciprocity values indicated by the Sánchez index for M. radula populations (R2: 0.58 and 0.59 in UDI and DEL, respectively) are considered low, and the degree of overlap in the length of floral organs is not as theoretically expected for tristylous systems. The degrees of reciprocity found in our study seem to be within the threshold value of both heterostylous (R2 values ranging from 0.88–0.58; Matias et al. 2016; Sá et al. 2016; Ferrero et al. 2017) and style dimorphic species (values ranging from 0.58–0.25; Ferrero et al. 2017). However, plants with style dimorphism only show variations in the length of their styles, preserving the length of their stamens (Ferrero et al. 2017; Cardoso et al. 2018), which does not occur for M. radula. The most plausible hypothesis to explain the observed reciprocity values of M. radula between the two major classes of stylar polymorphisms concerns the huge variation of style and stamen lengths. Additionally, we found low variation in reciprocity values by the Sánchez index between populations, indicating that low reciprocity is consistent across populations within M. radula. Similar values of reciprocity between populations were also evident using the method of adaptive inaccuracy (with total inaccuracy varying between 16.52 and 15.5 in UDI and DEL, respectively). Although it is still not clear what ultimately drives the variation in the length of the floral organs of M. radula, it is tempting to suggest that the selection pressures needed to increase reciprocity in heterostylous breeding systems may not occur in this plant.
Reciprocity according to inaccuracy values demonstrated that the short organs were the most reciprocal in both populations studied. Adaptive inaccuracy uses the mean and the variance to interpret the adaptive significance of the position of anthers and stigmas in relation to pollen delivery and deposition on stigmas (Armbruster et al. 2009, 2017). In M. radula, lower inaccuracy values in short organs can be explained, in part, by the lower variance of these organs (UDI = 1.11 and DEL = 0.99 mm) than in mid (UDI = 2.61 and DEL = 1.57 mm)- and long organs (UDI = 2.75 and DEF = 4.68 mm). In species of Primula L. (Primulaceae), Pulmonaria L. (Boraginaceae), Erythroxylum P. Browne (Erythroxylaceae), Palicourea L. and Psychotria Aubl. (Rubiaceae) short organs were also most reciprocal, a pattern attributed to developmental variation, which is often lower in small organs (Armbruster et al. 2017; Jacquemyn et al. 2018; Matias et al. 2020; Raupp et al. 2020; Furtado et al. 2021). However, it is believed that other selective pressures may influence the variation at the level of reciprocity among floral types. In M. radula, greater reciprocity between the short organs may have occurred in response to the behavior of bees when obtaining pollen grains from the feeding stamens, which are reciprocal to the styles of the S-styled flowers. Thus, better reciprocity would optimize the deposition of pollen grains specifically on the waist region of the bee's body, a safe site where pollination can still occur after bee grooming (Tong and Huang 2018). Thus, the bee grooming behavior may be influencing selection for the low inaccuracy and greater reciprocity of short organs in M. radula populations.
The lack of fruit formation in bagged flowers shows that M. radula plants need pollinating bees for sexual reproduction, though self-fertilization and apomixis are quite common in some Melastomataceae (Santos et al. 2012; Brito et al. 2017). In fact, M. radula flowers follow the morphological patterns and the pollination system found in most pollinator-dependent species of this family (Brito et al. 2017). Traits such as pronounced herkogamy, the presence of poricidal anthers and pollen as the main floral resource indicate the need of vibrating bees to accomplish buzz pollination (Buchmann 1983; Renner 1989). This specialized interaction favors heteranthery and possibly promotes the division of labor between stamens of different lengths (Luo et al. 2008; Vallejo-Marín et al 2009, 2010). Considering the stamen length variation and the bee behavior, it is likely that M. radula flowers have different functions within their populations: some flowers produce pollen only for food, some produce pollen only for pollination, and other flowers produce pollen grains that are used in both functions. Therefore, the floral types of M. radula provide different pollen loads for feeding and pollination functions and bees access different amounts of reward during their floral bout. Despite this, we still do not know whether such asymmetrical partition of pollen load among floral types influences bee preference or if there is any mechanism to prevent discrimination of floral types by pollinators.
In plant–pollinator interactions, pollen deposition in parts of the pollinator body that are different from the place of stigma contact of the same flower is very common (Harder and Wilson 1998; Koch et al. 2017). However, stamen specialization and the floral polymorphism described here for M. radula may lead not just to floral herkogamy. It may also optimize the mechanical fit of male and female functions on the pollinator’s body. In fact, a kind of sexual specialization in M. radula populations is possibly favored by vibrating bee-mediated phenotypic selection (Oliveira et al. 2020). The proportion of plants with different floral types in the UDI population is closer to a population with sexual specialization (1:1) than a proportion expected in tristylous systems (1:1:1) (Bawa 1980; Oliveira 1996; Ganders 1979; Barrett et al. 2000; Cardoso et al. 2018). However, the hypothesis of sexual specialization still needs more data to be further tested and we should consider that anisoplethy is also common in tristylous populations. Studies have shown that variation in floral type ratio in tristylous populations can be caused by a variety of deterministic and stochastic events, such as some degree of clonal propagation and reduction of population size, which are often accompanied by changes in mating patterns (Barrett et al. 1983; Eckert and Barrett 1992; Cunha et al. 2014; Weller et al. 2016). However, M. radula individuals do not appear to propagate clonally (LC. Oliveira personal observation; Fracasso 2008) and the floral type ratio was studied in a relatively large population (178 individuals sampled). Future studies are needed to investigate mechanisms causing floral type ratio bias in M. radula.
Conclusions
Macairea radula presents three floral types, easily distinguishable by the relative positions of stamens and styles within flowers. However, our results revealed that its populations do not exhibit a strong self-incompatibility system and do not correspond to high reciprocity classic models, both features proposed for species with heterostylous systems. Normally, species with high reciprocity typically does not show overlap in the length of floral organs between the floral morphs (see Brys et al. 2008; Puentes et al. 2013; Sánchez et al. 2013; Cunha et al. 2014; Barrett 2019). In M. radula, there is a huge variation in both stamen and style length, with overlap between M- and L-styled flowers. The absence of distinct floral organ levels has also been reported in other species predicted to be heterostylous only by visual inspection (Richards and Koptur 1993; Eckert and Barrett 1994; Ferrero et al. 2011, 2017). This reinforces the need of detailed measurements and analyses to define complex floral systems. Presenting poricidal anthers and pollen as the main resource, the flowers of M. radula are visited by vibrating bees, which possibly exert a selective pressure that favors heteranthery (Oliveira et al. 2020). In addition, the occurrence of two functional stamen levels with long stamens for pollination and short stamens for feeding may favor sexual specialization and explain why the previously proposed floral types are not in isoplethic equilibrium in these populations. Although the distribution of stamen and style lengths within M. radula populations does not conform to tristylous systems, more studies are needed to better classify the floral polymorphism in this species. Future studies could investigate more populations and other heterostylous traits, such as intra-morph incompatibility system and ancillary polymorphism in pollen grains and stigmatic papillae.
References
Alvares CA, Stape JL, Sentelhas PC, De Moraes Gonçalves JL, Sparovek G (2013) Koppen’s climate classification map for Brazil. Meteorol Z 22:711e728. https://doi.org/10.1127/09412948/2013/0507
Armbruster WS, Hansen TF, Pélabon C, Pérez-Barrales R, Maad J (2009) The adaptive accuracy of flowers: measurement and microevolutionary patterns. Ann Bot (Oxford) 103:1529–1545. https://doi.org/10.1093/aob/mcp095
Armbruster WS, Bolstad GH, Hansen TF, Keller B, Conti E, Pélabon C (2017) The measure and mismeasure of reciprocity in heterostylous flowers. New Phytol 215:906–917. https://doi.org/10.1111/nph.14604
Bacci LF, Versiane AFA, Oliveira ALF, Romero R (2016) Melastomataceae na RPPN do Clube Caça e Pesca Itororó, Uberlândia, MG, Brasil. Hoehnea 43:541–556. https://doi.org/10.1590/2236-8906-27/2016
Baker AM, Thompson JD, Barrett SC (2000) Evolution and maintenance of stigma-length dimorphism in Narcissus. I. Floral variation and style-morph ratios. Heredity 84:502–513. https://doi.org/10.1046/j.1365-2540.2000.00651.x
Barrett SCH (1990) The evolution and adaptive significance of heterostyly. Trends Ecol Evol 5:144–148. https://doi.org/10.1016/0169-5347(90)90220-8
Barrett SCH (1993) The evolutionary biology of tristyly. In: Futuyma D, Antonovics J (eds) Oxford surveys in evolutionary biology, 9. Oxford University Press, Oxford, pp 283–326
Barrett SCH (2002) Evolution of sex: the evolution of plant sexual diversity. Nat Rev Gen 3:274. https://doi.org/10.1038/nrg776
Barrett SCH (2019) ‘A most complex marriage arrangement’: recent advances on heterostyly and unresolved questions. New Phytol 224:1051–1067. https://doi.org/10.1111/nph.16026
Barrett SCH, Shore JS (2008) New insights on heterostyly: comparative biology, ecology and genetics. In: Franklin-Tong VE (ed) Self-incompatibility in flowering plants. Springer, Berlin, Heidelberg, pp 3–32. https://doi.org/10.1007/978-3-540-68486-2_1
Barrett SCH, Price SD, Shore JS (1983) Male fertility and anisoplethic population structure in tristylous Pontederia cordata (Pontederiaceae). Evolution 37:745–759. https://doi.org/10.1111/j.1558-5646.1983.tb05597.x
Barrett SCH, Brown AHD, Shore JS (1987) Disassortative mating in tristylous Eichhornia paniculata (Pontederiaceae). Heredity 58:49–55. https://doi.org/10.1038/hdy.1987.7
Barrett SCH, Lloyd DG, Arroyo J (1996) Stylar polymorphisms and the evolution of heterostyly in Narcissus (Amaryllidaceae). In: Lloyd DG, Barrett SCH (eds) Floral biology. Springer, Boston, pp 339–376. https://doi.org/10.1007/978-1-4613-1165-2_13
Barrett SCH, Cole WW, Arroyo J, Cruzan MB, Lloyd DG (1997) Sexual polymorphisms in Narcissus triandrus (Amaryllidaceae): is this species tristylous? Heredity 78:135–145
Barrett SCH, Jesson LK, Baker AM (2000) The evolution and function of stylar polymorphisms in flowering plants. Ann Bot (Oxford) 85:253–265. https://doi.org/10.1006/anbo.1999.1067
Bates D, Machler M, Bolker B, Walker SC (2014) Fitting Linear Mixed-Effects Models Using lme4. J Stat Softw 67:1–48. https://doi.org/10.18637/jss.v067.i01
Bawa KS (1980) Evolution of dioecy in flowering plants. Annual Rev Ecol Syst 11:15–39. https://doi.org/10.1146/annurev.es.11.110180.000311
Bawa KS, Beach JH (1983) Self-incompatibility systems in the Rubiaceae of a tropical lowland wet forest. Amer J Bot 70:1281–1288. https://doi.org/10.1002/j.1537-2197.1983.tb07917.x
Brito VL, Weynans K, Sazima M, Lunau K (2015) Trees as huge flowers and flowers as oversized floral guides: the role of floral color change and retention of old flowers in Tibouchina pulchra. Frontiers Pl Sci 6:362. https://doi.org/10.3389/fpls.2015.00362
Brito VLG, Maia FR, Silveira FA, Fracasso CM, LemosFilho JP, Fernandes GW, Goldenberg R, Morellato LPC, Sazima M, Staggemeier VG (2017) Reproductive phenology of Melastomataceae species with contrasting reproductive systems: contemporary and historical drivers. Pl Biol 19:806–817. https://doi.org/10.1111/plb.12591
Brys R, Jacquemyn H, Beeckman T (2008) Morphratio variation, population size and female reproductive success in distylous Pulmonaria officinalis (Boraginaceae). J Evol Biol 21:1281–1289. https://doi.org/10.1111/j.1420-9101.2008.01569.x
Buchmann SL (1983) Buzz pollination in angiosperms. In: Jones CE, Little RJ (eds) Handbook of experimental pollination biology. Van Nostrand & Reinhold, New York, pp 73–113
Cardoso JCF, Viana ML, Matias R, Furtado MT, Caetano APS, Consolaro H, Brito VLG (2018) Towards a unified terminology for angiosperm reproductive systems. Acta Bot Brasil 32:329–348. https://doi.org/10.1590/0102-33062018abb0124
Cunha NL, Barrett SCH (2019) Architectural constraints, male fertility variation and biased floral morph ratios in tristylous populations. Heredity 123:694–706
Cunha NL, Fischer E, Lorenz-Lemke AP, Barrett SCH (2014) Floral variation and environmental heterogeneity in a tristylous clonal aquatic of the Pantanal wetlands of Brazil. Ann Bot (Oxford) 114:1637–1649. https://doi.org/10.1093/aob/mcu181
Darwin C (1877) The different forms of flowers on plants of the same species. John Murray, London
De Luca PA, Vallejo-Marín M (2013) What‘s the -buzz about? The ecology and evolutionary significance of buzz-pollination. Curr Opin Pl Biol 16:429–435. https://doi.org/10.1016/j.pbi.2013.05.002
Eckert CG, Barrett SCH (1992) Stochastic loss of style morphs from populations of tristylous Lythrum salicaria and Decodon verticillatus (Lythraceae). Evolution 46:1014–1029. https://doi.org/10.1111/j.1558-5646.1992.tb00616.x
Eckert CG, Barrett SCH (1994) Tristyly, self-compatibility and floral variation in Decodon verticillatus (Lythraceae). Biol J Linn Soc 53:1–30. https://doi.org/10.1111/j.1095-8312.1994.tb01000.x
Ferrero V, Arroyo J, Vargas P, Thompson JD, Navarro L (2009) Evolutionary transitions of style polymorphisms in Lithodora (Boraginaceae). Perspect Pl Ecol Evol Syst 11:111–125. https://doi.org/10.1016/j.Ppees.2009.01.004
Ferrero V, Chapela I, Arroyo J, Navarro L (2011) Reciprocal style polymorphisms are not easily categorised: the case of heterostyly in Lithodora and Glandora (Boraginaceae). Pl Biol 13:7–18. https://doi.org/10.1111/j.1438-8677.2009.00307.x
Ferrero V, Arroyo J, Castro S, Navarro L (2012) Unusual heterostyly: style dimorphism and self incompatibility are not tightly associated in Lithodora and Glandora (Boraginaceae). Ann Bot (Oxford) 109:655–665. https://doi.org/10.1093/aob/mcr222
Ferrero V, Barrett SCH, Rojas D, Arroyo J, Navarro L (2017) Associations between sex organ deployment and morph bias in related heterostylous taxa with different stylar polymorphisms. Amer J Bot 104:50–61. https://doi.org/10.3732/ajb.1600345
Fisher RA (1941) The theoretical consequences of polyploid inheritance for the mid style form of Lythrum salicaria. Ann Eugenic 11:31–38. https://doi.org/10.1111/j.1469-1809.1941.tb02268.x
Fracasso CM (2008) Biologia da polinização e reprodução de espécies de Melastomataceae do Parque da Nacional da Serra da Canastra. PhD Thesis, University of Campinas, Campinas
Furtado MT, Matias R, Pérez-Barrales R, Consolaro H (2021) Do reciprocal herkogamy and pollinators affect legitimate pollen flow in distylous species of Rubiaceae? Bot J Linn Soc 196:524–539. https://doi.org/10.1093/botlinnean/boab004
Ganders FR (1979) The biology of heterostyly. New Zealand J Bot 17:607–635. https://doi.org/10.1080/0028825X.1979.10432574
Harder LD, Barrett SCH (1996) Pollen dispersal and mating patters in animal- pollinated plants. In: Lloyd DG, Barrett SCH (eds) Floral biology: studies on floral evolution in animal-pollinated plants. Chapman & Hall, New York, pp 140–190
Harder LD, Johnson SD (2009) Darwin’s beautiful contrivances: evolutionary and functional evidence for floral adaptation. New Phytol 183:530–545. https://doi.org/10.1111/j.1469-8137.2009.02914.x
Harder LD, Wilson WG (1998) Theoretical consequences of heterogeneous transport conditions for pollen dispersal by animals. Ecology 79:2789–2807. https://doi.org/10.1890/00129658(1998)079[2789:TCOHTC]2.0.CO;2
Heuch I (1979) Equilibrium populations of heterostylous plants. Theor Popul Biol 15:43–57
Jacquemyn H, Gielen M, Brys R (2018) Is sexual organ reciprocity related to legitimate pollen deposition in distylous Pulmonaria (Boraginaceae)? Oikos 127:1216–1224. https://doi.org/10.1111/oik.05122
Koch L, Lunau K, Wester P (2017) To be on the safe site–Ungroomed spots on the bee‘s body and their importance for pollination. PLoS ONE 12:e0182522. https://doi.org/10.1371/journal.pone.0182522
Kohn JR, Barrett SCH (1992) Experimental studies on the functional significance of heterostyly. Evolution 46:43–55. https://doi.org/10.1111/j.1558-5646.1992.tb01983.x
Kuznetsova A, Brockhoff PB, Christensen RHB (2017) lmerTest package: tests in linear mixed effects models. J Stat Softw 82:1–26. https://doi.org/10.18637/jss.v082.i13
Li P, Johnston MO (2001) Comparative floral morphometrics of distyly and homostyly in three evolutionary lineages of Amsinckia (Boraginaceae). Canad J Bot 79:1332–1348. https://doi.org/10.1139/b01-107
Lloyd DG, Webb CJ (1992) The evolution of heterostyly. In: Barrett SCH (ed) Evolution and function of heterostyly. Springer-Verlag, Berlin, pp 151–178
Lunau K, Piorek V, Krohn O, Pacini E (2015) Just spines—mechanical defense of malvaceous pollen against collection by corbiculate bees. Apidologie 46:144–149. https://doi.org/10.1007/s13592014-0310-5
Luo Z, Zhang D, Renner SS (2008) Why two kinds of stamens in buzzpollinated flowers? Experimental support for Darwin’s division-of-labour hypothesis. Funct Ecol 22:794–800. https://doi.org/10.1111/j.1365-2435.2008.01444.x
Luo Z, Gu L, Zhang DX (2009) Intrafloral differentiation of stamens in heterantherous flowers. J Syst Evol 47:43–56. https://doi.org/10.1111/j.1759-6831.2009.00002.x
Matias R, Oliveira ASD, Furtado MT, Sá T, Rodrigues EB, Oliveira PED, Consolaro H (2016) Atypical mating system in two Rubiaceae species: distyly with partial self-incompatibility in the thrum morph? Rodriguésia 67:357–368. https://doi.org/10.1590/2175-7860201667207
Matias R, Pérez-Barrales R, Consolaro H (2020) Patterns of variation in distylous traits and reproductive consequences in Erythroxylum species and populations. Amer J Bot 107:1–13. https://doi.org/10.1002/ajb2.1478
Melo LR, Vasconcelos T, Reginato M, Caetano APS, Brito VLG (2021) Evolution of stamen dimetrism in Melastomataceae, a large radiation of pollen flowers. Perspect Pl Ecol 48:125589. https://doi.org/10.1016/j.ppees.2021.125589
Mesquita-Neto JN, Blüthgen N, Schlindwein C (2018) Flowers with poricidal anthers and their complex interaction networks—disentangling legitimate pollinators and illegitimate visitors. Funct Ecol 32:2321–2332. https://doi.org/10.1111/1365-2435.13204
Morgan MT, Barrett SCH (1988) Historical factors and anisoplethic population structure in tristylous Pontederia cordata L., a re-assessment. Evolution 42:496–504
Nabhan GP, Buchmann SL (1997) Services provided by pollinators. In: Daily GC (ed) Nature’s services: societal dependence on natural ecosystems. Island Press, Washington, DC, pp 133–150
Naiki A (2012) Heterostyly and the possibility of its breakdown by polyploidization. Pl Spec Biol 27:3–29. https://doi.org/10.1111/j.1442-1984.2011.00363.x
Oliveira PE (1996) Dioecy in the Cerrado vegetation of Central Brazil. Flora 191:235–243. https://doi.org/10.1016/S0367-2530(17)30718-1
Oliveira LC, Teixido AL, Trevizan R, Brito VLG (2020) Bee-mediated selection favors floral sex specialization in a heterantherous species: strategies to solve the pollen dilemma. Plants 9:1685. https://doi.org/10.3390/plants9121685
Oliveira LC, Nunes CEP, Brito VLG, Caetano APS (2022) Floral oil production in a family dominated by pollen flowers: The case of Macairea radula (Melastomataceae). Flora 288:152008. https://doi.org/10.1016/j.flora.2022.152008
Ornduff R (1966) The origin of dioecism from heterostyly in Nymphoides (Menyanthaceae). Evolution. https://doi.org/10.2307/2406632
Pannell JR, Dorken ME, Eppley SM (2005) ‘Haldane’s Sieve’ in a metapopulation: sifting through plant reproductive polymorphisms. Trends Ecol Evol 20:374–379. https://doi.org/10.1016/j.tree.2005.05.004
Puentes A, Cole WW, Barrett SCH (2013) Trimorphic incompatibility in Pontederia subovata (Pontederiaceae): an aquatic macrophyte from lowland South America. Int J Pl Sci 174:47–56. https://doi.org/10.1086/668229
Raupp PP, Matias R, Furtado MT, Consolaro H (2020) The role of distyly in pollen flow of the hummingbird-pollinated Palicourea rigida (Rubiaceae). Flora 271:151681. https://doi.org/10.1016/j.flora.2020.151681
Renner SS (1989) Systematic studies in the Melastomataceae: Bellucia, Loreya and Macairea. Mem New York Bot Gard 50:1–112
Richards JH, Koptur S (1993) Floral variation and distyly in Guettarda scabra (Rubiaceae). Amer J Bot 80:31–40. https://doi.org/10.1002/j.1537-2197.1993.tb13764.x
Rosa R, Lima SC, Assunção WL (1991) Abordagem preliminar das condições climáticas de Uberlândia (MG). Soc Nat 3:91–108. https://doi.org/10.14393/SN-v3-1991-60693
Sá T, Furtado MT, Ferrero V, Pérez-Barrales R, Rodrigues EB, Dos Santos IG, Consolaro H (2016) Floral biology, reciprocal herkogamy and breeding system in four Psychotria species (Rubiaceae) in Brazil. Bot J Linn Soc 182:689–707. https://doi.org/10.1111/boj.12476
Sánchez JM, Ferrero V, Navarro L (2008) A new approach to the quantification of degree of reciprocity in distylous (sensu lato) plant populations. Ann Bot (Oxford) 102:463–472. https://doi.org/10.1093/aob/mcn111
Sánchez JM, Ferrero V, Navarro L (2013) Quantifying reciprocity in distylous and tristylous plant populations. Pl Biol 15:616–620. https://doi.org/10.1111/j.1438-8677.2012.00720.x
Santos APM, Fracasso CM, Santos ML, Romero R, Sazima M, Oliveira PE (2012) Reproductive biology and species geographical distribution in the Melastomataceae: a survey based on New World taxa. Ann Bot (Oxford) 110:667–679. https://doi.org/10.1093/aob/mcs125
Solís-Montero L, Vallejo-Marín M (2017) Does the morphological fit between flowers and pollinators affect pollen deposition? an experimental test in a buzz-pollinated species with anther dimorphism. Ecol Evol 7:2706–2715. https://doi.org/10.1002/ece3.2897
Solís-Montero L, Cáceres-García S, Alavez-Rosas D, García-Crisóstomo JF, Vega-Polanco M, Grajales-Conesa J, Cruz-López L (2018) Pollinator preferences for floral volatiles emitted by dimorphic anthers of a buzz-pollinated herb. J Chem Ecol 44:1058–1067. https://doi.org/10.1007/s10886-018-1014-5
Tong ZY, Huang SQ (2018) Safe sites of pollen placement: a conflict of interest between plants and bees? Oecologia 186:163–171. https://doi.org/10.1007/s00442-017-3999-9
Vallejo-Marín M, Manson JS, Thomson JD, Barrett SC (2009) Division of labour within flowers: heteranthery, a floral strategy to reconcile contrasting pollen fates. J Evol Biol 22:828–839. https://doi.org/10.1111/j.1420-9101.2009.01693.x
Vallejo-Marín M, Da Silva EM, Sargent RD, Barrett SC (2010) Trait correlates and functional significance of heteranthery in flowering plants. New Phytol 188:418–425. https://doi.org/10.1111/j.1469-8137.2010.03430.x
van der Niet T, Johnson SD (2012) Phylogenetic evidence for pollinator-driven diversification of angiosperms. Trends Ecol Evol 27:353–361. https://doi.org/10.1016/j.tree.2012.02.002
Velloso MSC, Brito VLG, Caetano APS, Romero R (2018) Anther specializations related to the division of labor in Microlicia cordata (Spreng.) Cham. (Melastomataceae). Acta Bot Brasil 32:349–358. https://doi.org/10.1590/0102-33062017abb0358
Vogel S (1978) Evolutionary shifts from reward to deception in pollen flowers. In: Richards AJ (ed) The pollination of flowers by insects. Academic Press, London, pp 89–96
Weller SG, Sakai AK, Gray T, Weber JJ, Tsyusko OV, Domínguez CA, Fornoni J, Molina-Freaner FE (2016) Variation in heterostylous breeding systems in neighbouring populations of Oxalis alpina (Oxalidaceae). Pl Biol 18:104–110. https://doi.org/10.1111/plb.12340
Wickham H (2009) ggplot2: elegant graphics for data analysis. Springer-Verlag, New York
Zhou W, Barrett SC, Wang H, Li DZ (2015) Reciprocal herkogamy promotes disassortative mating in a distylous species with intramorph compatibility. New Phytol 206:1503–1512. https://doi.org/10.1111/nph.13326
Acknowledgements
We are thankful for the invaluable comments from four anonymous reviewers on previous version of the manuscript. We are also thankful for the previous reading and further suggestions on the first draft of this manuscript by Hélder Consolaro and Paulo Eugênio Oliveira. We also thank the owners of Fazenda Preciosa, the Federal University of Uberlândia (UFU) and the Laboratory of Morphology, Microscopy, and Image (LAMOVI) for their infrastructure and support of the study.
Funding
This study was funded by the Fundação de Apoio à Pesquisa do Estado de Minas Gerais (process FAPEMIG APQ—02497–16 and RED-00253–16), by the Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq ref. 431873/2018–6; ref. 308107/2021-7). This study was also financed in part by the Coordenação de Aperfeiçoamento de Pessoal de Nível Superior—Brasil (CAPES)—Finance Code 001.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Handling editor: Louis P. Ronse De Craene.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Information on Electronic Supplementary Material
Information on Electronic Supplementary Material
Online Resource 1. Descriptive analysis of variance considering intra and inter plants floral organ length variations in Macairea radula in Uberlândia and Delfinópolis populations.
Online Resource 2. Method used for the measurements of stamens and styles of Macairea radula flowers.
Online Resource 3. Stamen and style lengths distribution of Macairea radula according to the different floral types in Uberlândia and Delfinópolis populations.
Rights and permissions
About this article
Cite this article
Oliveira, L.C., Matias, R., Furtado, M.T. et al. What explains the variation in length of stamens and styles in a pollen flower? a study exemplified by Macairea radula (Melastomataceae). Plant Syst Evol 308, 15 (2022). https://doi.org/10.1007/s00606-022-01808-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00606-022-01808-0