Abstract
Elaiophores seems to be uncommon in Orchidaceae; however, the number of known species with floral oil glands has increased in recent years, principally in Oncidiinae. Oil rewards are used by bees of the tribes Centridini, Tapinotaspidini and Tetrapediini. Our aims were to identify the presence of elaiophores and to describe their structure in species of Gomesa, Grandiphyllum and Trichocentrum, and to compare our results with other studies of elaiophores in Oncidiinae. We selected a set of characters presumably associated with oil production in flowers of Oncidiinae, which were evaluated using a cluster analysis to identify different floral morphologies of the oil flowers. The correlation between morphological types of oil flowers and species of pollinators was examined. The cluster analysis distinguished two groups of species, one of them principally linked with pollination by bees of genus Centris and the other type associated to species of Paratetrapedia and Tetrapedia. The evaluation of these results into a phylogenetic framework of the Oncidiinae, adding more evidence that species of this subtribe with similar floral morphology associated with floral oil secretion arise in many independent clades, in parallel evolution with the oil-bee pollination.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The diverse and showy floral morphology and reproductive biology of the Orchidaceae have attracted the attention of many researchers (Darwin 1862; van der Pijl and Dodson 1966; Proctor et al. 1996; van der Cingel 2001). About a third of the species of Orchidaceae deceive pollinators (Tremblay et al. 2005), and the remaining species offer diverse floral rewards such as nectar, oils, fragrances, resin-like substances, pseudopollen and waxes (Davies and Stpiczyn´ska 2008). Vogel (1969, 1974) was the first to report the presence of oil-secretor glands (elaiophores) in Orchidaceae and other families of plants. In Neotropics, this floral reward is used by numerous bees of the tribes Centridini, Tapinotaspidini and Tetrapediini (family Apidae) that collect oil to feed larvae or use it for water proofing larval cells (Buchmann 1987; Dressler 1990; Vinson et al. 1996; Singer and Cocucci 1999; van der Cingel 2001; Torretta et al. 2011; Neubig et al. 2012).
In Orchidaceae, most species that offer oil as reward belong to Oncidiinae (Chase et al. 2009), although this reward is not exclusive of this subtribe (Dressler 1993; Pansarin et al. 2009; Stpiczyńska and Davies 2008). Within Oncidiinae, the presence of elaiophores occurs in different clades of the subtribe, and they are thought to have probably arisen at least 7 times (Renner and Schaefer 2010). Nevertheless, the oil flowers (especially in tropical Orchidaceae) are poorly known (Renner and Schaefer 2010). For this reason, morphological and anatomical detailed studies covering a large number of species are required in order to understand the evolution of elaiophores and to characterize this condition in the subtribe Oncidiinae.
Neubig et al. (2012) commented that a large percentage of Oncidiinae possess flowers that either produce an oil reward (“oil flowers”) or mimic the oil-producing flowers of Malpighiaceae in terms of color and morphology; therefore, anatomical studies and field observations are necessary to discriminate between both types of flowers. In recent years, several anatomical investigations have demonstrated the presence of elaiophores in many species of Oncidiinae (Singer and Cocucci 1999; Pacek and Stpiczyńska 2007; Stpiczyńska et al. 2007, 2013; Stpiczyńska and Davies 2008; Aliscioni et al. 2009; Davies and Stpiczyńska 2009; Pansarin and Pansarin 2011; Pacek et al. 2012; Blanco et al. 2013; Gomiz et al. 2013, 2014; Davies et al. 2014). Thus, the number of species of Oncidiinae that are currently known to produce floral oil as a reward is gradually increasing. In contrast to Malpighiaceae, where oil-secreting flower is a primitive condition (Anderson 1979), floral elaiophores have arisen recently and in many independent episodes within the Oncidiinae (Chase et al. 2009; Renner and Schaefer 2010).
Based on the huge diversity in floral morphology exhibited in Oncidiinae, these species are attractive subjects for evolutionary studies (Neubig et al. 2012). Regarding oil secretion as a floral reward, Stpiczyńska et al. (2013) concluded that many traits of the elaiophores are shared by members of different clades of the Oncidiinae; accordingly, elaiophores must be considered homoplasious in this group. In this same way, Davies et al. (2014) reinforced the idea that the presence of elaiophores is of limited value for establishing phylogenetic relationships in this subtribe; but these assumptions were not tested in an evolutionary context. On the other hand, there is enough evidence to show that the structure and position of the elaiophores varies among different genera or species; although these structures can be modified by selection pressures from pollinators, each linage of the Oncidiinae might be evolved independently. However, since knowledge about the presence or morphology of elaiophores in Oncidiinae is still limited, the exact number of gains and losses is difficult to establish.
In the present work, we undertake a comparative analysis to the morphology and anatomy of elaiophores in six species of Oncidiinae belonging to two different lineages in the phylogeny postulated by Neubig et al. (2012). We analyzed one species of Grandiphyllum Docha Neto and two species of Trichocentrum Poepp. & Endl., located in a more ancestral clade (Trichocentrum clade) and three species of Gomesa R.Br. belonging to a more recent monophyletic group (Gomesa clade). Our aim was to identify the presence of elaiophores in these species, to describe their structure and to postulate how oil is secreted. We compared and discussed our results with information known from anatomical studies in elaiophores in other species of Oncidiinae. Moreover, we selected a set of morphological and anatomical characteristics present in oil flowers, and we analyzed these characteristics using a cluster analysis that includes a significant number of species of Oncidiinae with elaiophores. The importance of these characteristics is evaluated to identify groups of species that represent different floral morphologies associated with oil flowers. Then, we explored if there is any correlation between the different types of oil flowers and species of pollinators exists based on published bibliography. Lastly, the types of oil flowers are discussed in an evolutionary context along the phylogeny published by Neubig et al. (2012) for the subtribe Oncidiinae.
Materials and methods
Plant material
Fresh flowers of Gomesa cornigera (Lindl.) M.W.Chase & N.H.Williams, G. herzogii (Schltr.) Lückel, G. longicornu (Mutel) M.W.Chase & N.H.Williams, Grandiphyllum divaricatum (Lindl.) Docha Neto, Trichocentrum cebolleta (Jacq.) M.W.Chase & N.H.Williams, and T. jonesianum (Rchb.f.) M.W.Chase & N.H.Williams were obtained from plants cultivated at the Botanical Garden Lucien Hauman, Faculty of Agronomy, University of Buenos Aires, Argentina. Reference vouchers were deposited in the Herbarium Gaspar Xuárez (BAA). The identification of the analyzed specimens was corroborated by comparing with herbarium material deposited at BAA and SI (Thiers 2012).
Histochemical reactions
Entire fresh flowers of the six species were examined using a Wild M5 stereomicroscope; the flowers were submerged in saturated alcoholic Sudan III solution to detect the presence of lipids. The labellar areas that reacted positively were sectioned and fixed in FAA (ethyl alcohol 70%: glacial acetic acid: formaldehyde 40%; 90:5:5) for 48 h. and stored in 70% ethanol. These samples were used for optical and scanning electronic microscopes preparations.
Anatomical and micromorphological study
The samples were dehydrated in an ethanol series, transferred to xylene, embedded in paraffin (58 °C), and sectioned at a thickness of 6–7 μm on a rotary microtome (Leitz Wetzlar), using conventional methods. Histological sections were stained with Safranin-Fast Green and mounted in Canada balsam (Zarlavsky 2014). Observations were made using a Motic optical light microscope. Photomicrographs and measurements were taken using Motic images plus 2.0.
For scanning electron microscopy (SEM), the portions of the labellum were dehydrated and subjected to critical-point drying using liquid CO2. The material was then sputter-coated with gold and examined using a Philips XL 30 TMP microscope at an accelerating voltage of 80 kV.
Cluster analysis
We selected 11 morphological and anatomical floral characters commonly present in species of Oncidiinae with elaiophores; characters 1 and 2 are associated with the attraction to the pollinators and the level of resemblance with flowers of Malpighiaceae; characters 3, 4 and 5 are related to the oil production and exposition of the secretory tissue; characters 6, 7, 8, 9, and 10 are associated to the pollinators forage (the alighted of the bee at the flowers, if the bee grasps the tabula infrastigmatica, in what manner the bee scrapes the areas with elaiophores), and character 11 is connected with the position that the insect contact with the viscidium (Table 1). These floral characters were scored based on personal observations and published information for this subtribe (Singer and Cocucci 1999; Pacek and Stpiczyńska 2007; Stpiczyńska et al. 2007, 2013; Stpiczyńska and Davies 2008; Aliscioni et al. 2009; Davies and Stpiczyńska 2009; Pansarin and Pansarin 2011; Pacek et al. 2012; Blanco et al. 2013; Gomiz et al. 2013, 2014; Davies et al. 2014). All states assigned to the characters were corroborated with observations based on herbarium specimens (BAA, SI), description of the species and available digital images (http://www.orchidspecies.com; https://orchid.unibas.ch; http://epic.kew.org; http://www.flmnh.ufl.edu/herbarium/oncids/home; http://www.epidendra.org/). Although Rudolfiella picta belongs to the subtribe Maxillariinae (Chase et al. 2015), we considered this species in our analysis because this species presents elaiophores (Davies and Stpiczyńska 2009) and is included in the molecular phylogeny (Neubig et al. 2012).
The informative features were scored for a total of 46 species of Oncidiinae and 1 species of Maxillariinae whose elaiophores were anatomically described (Online Resource 1). A cluster analysis was carried out using NTSYS-pc version 2.0 (Rohlf 1998). The standardized matrix was used and distances among OTUs were evaluated based on the Manhattan distance coefficient, using the unweighted pair-group method on the arithmetic average algorithm (UPGMA).
Species of potential pollinators
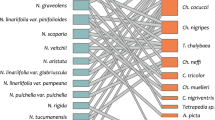
In order to associate the types of the oil flower established in the cluster analysis with their potential pollinators, a bibliographical research was conducted to find records of floral visitors in different species of Oncidiinae, with either confirmed or presumed presence of elaiophores (Dodson 1962; van der Pijl and Dodson 1966; Singer and Cocucci 1999; Parra-Tabla et al. 2000; Reis et al. 2000, 2007; Silvera 2002; Damon and Cruz-López 2006; Singer et al. 2006; Pemberton 2008; Carmona-Díaz and García-Franco 2009; Pansarin and Pansarin 2011; Torretta et al. 2011; Vale et al. 2011; Neubig et al. 2012; Gomiz et al. 2014). This information is listed in Table 2.
Results
General aspects and floral morphology
The six species studied are characterized by having racemose paniculate inflorescences of 20–60 cm long, with approximately 10–20 flowers. We could not detect appreciable fragrance, or it was very subtle. Regarding the size and external aspect, flowers are very variable. Flowers are 1–2 cm diameter in Gomesa spp. and more than 2 cm in G. divaricatum and the two species of Trichocentrum; yellow (in 5 spp.) or yellow cream in Trichocentrum jonesianum, with different spots or transverse markings red-brown in sepals and petals, including the callus (Fig. 1).
The species of Gomesa have the lateral sepals partly fused, contrary to G. divaricatum and the two species of Trichocentrum where the sepals are free. The labellum is three-lobed in all species, with a large mid-lobe and two lateral lobes. The lateral lobes are wing-like in all species, except in G. cornigera where they are narrower and plain; G. herzogii is distinguished by having lateral lobes with fimbriate margins (Fig. 2).
The tabula infrastigmatica is located between the base of the column and the callus, and is very well formed in Gomesa herzogii, T. cebolleta and T. jonesianum, and very reduced in G. cornigera or absent in G. longicornu and G. divaricatum (Fig. 2).
In all species, the calluses are conspicuous and well developed, and situated on the base of the mid-lobe, between the lateral lobes. We defined arbitrarily two areas in the calluses (basal platform and apical region) to homologize the observations among the species. These two areas are either similar or have different aspects depending on the species (see below). The three species of Gomesa present calluses differentiated in the basal platform and the apical region; in G. cornigera the basal platform showed several protuberances and two irregular horns, and the apical region is hoof-shaped with a central furrow (Fig. 2a); in G. herzogii, the basal platform is trichomatose with hairs that are gradually shorter and sparser to the apex, followed by a median keel and several lateral protuberances (Fig. 2b), and G. longicornu differs to have the basal platform surmounted by two pyramidal extensions and a median horn-shaped protrusion in the apical region (Fig. 2c). The callus of G. divaricatum is entirely trichomatous (Fig. 2d), without differentiation between the basal platform and the apical region since both are covered by unicellular hairs. The trichomes are irregularly cylindrical, sometimes geniculate, with dome-shaped tips. Finally, the callus of T. cebolleta has a basal platform scarcely corrugated and an apical region with three keels of which the central one is larger than the lateral (Fig. 2e); whereas in T. jonesianum the basal platform comprises two protuberances followed by a central keel with several lateral protrusions (Fig. 2f).
Histochemical reactions
The reaction with saturated alcoholic solution of Sudan III was positive principally on the calluses in all species, indicating that these areas secrete oils; in addition, in G. cornigera, G. herzogii, T. cebolleta and T. jonesianum, a reduced proximal part of the lateral lobes also reacted positively but with slight intensity, showing that this portion can secrete less oil than callus or only collect oil secreted by the callus (Fig. 1).
Anatomy of the calluses in cross section
In Gomesa spp., the epidermal cells of the calluses contain intensely stained cytoplasm, with basal nuclei, conspicuous nucleoli and frequently with small vacuoles. Gomesa cornigera shows in the basal platform and protuberances, cuboidal to palisade cells, 20–35 × 13–20 μm; in the hoof-like area of the apical region, epidermal cells are radially elongated, 50–60 × 10–15 μm, but in the central furrow the cells are flat and clear, with non-secreting aspect (Fig. 3a–d). Gomesa herzogii differs in that this species presents in the basal platform, unicellular trichomes, cylindrical to flask-like, 150–240 × 20–30 μm, with a central vacuole surrounded by a thin layer of dense cytoplasm and a basal nucleus with evident nucleolus. The remaining epidermal cells are cuboidal with dense cytoplasm and conspicuous nuclei, 20–25 × 15–20 μm, and small papillae in the deeper areas that have translucent cytoplasm (Fig. 3e–i). The epidermal cells of the callus in G. longicornu are palisade-like, 80–70 × 15–20 μm, with dense cytoplasm, contrary to the epidermis of the horn and papillae located in deeper areas which have clear cytoplasm (Fig. 4a–c). In the three species of Gomesa, the external tangential walls are cutinized, 0.5–2.5 μm and lack blisters, and the subepidermal tissue comprises 1–3 layers of isodiametric parenchymatous cells with plastids; idioblasts with raphides in the subjacent parenchyma are observed.
Cross section of the callus view by optical light microscope. a–d Gomesa cornigera. a Basal platform of the callus. b Apical region of the callus. c–d Detail of the secretory epidermis and raphides in the subjacent parenchyma. e–i G. herzogii. e Basal platform of the callus with trichomes. f Apical region of the callus with a median keel. g Detail of the secretory trichomes in the basal platform. h Detail of the secretory cuboidal epidermis in the apical region. i Detail of the non-secretory papillae
The trichomes of the callus in G. divaricatum are 400–470 × 8–12 μm and contain a central vacuole surrounded by a thin layer of dense cytoplasm and nuclei apically positioned. The first subepidermal few layers are composed by more or less isodiametric cells with small vacuoles, central nuclei and dense cytoplasm; also idioblasts with raphides are observed (Fig. 4d–f).
In the two species of Trichocentrum, the epidermal cells of the callus are cubic to palisade, 20–35 × 15–30 μm, with dense cytoplasm, apical vacuole and large nucleus basally positioned, and the cuticle thickness is 1–2 μm, and without blisters. The subepidermal tissue comprises a single layer of small parenchymatous cells with similar characteristics of the other species (Fig. 5).
Cross section of the callus view by optical light microscope. a–c Trichocentrum cebolleta. a Basal platform of the callus. b Detail of the secretory cuboidal epidermis. c Detail of the non-secretory papillae. d–f T. jonesianum. d Basal platform of the callus. e Detail of secretor epidermis. f Oil drops in the subjacent parenchyma
Micromorphology of the calluses
Observations made with SEM revealed that the areas of the calluses have irregular projections covered by plain epidermal cells with smooth to rough cuticles; stomata are absent or scattered, and small papillae (or flask-like cells) can be present in deeper areas of the calluses and proximal parts of the lateral lobes (Figs. 6 and 7). On the other hand, the entire callus of G. divaricatum (Fig. 7a) and only the basal platform of G. herzogii (Fig. 6d) are covered by trichomes with rugose cuticles. Conspicuous blisters or cuticular distensions were not observed in any species.
a–i Scanning electron micrographs of the lateral lobes and callus. a–c Gomesa cornigera. a Entire callus. b Detail of the hoof shape area. c Detail of the papillae in lateral lobes. d–f G. herzogii. d Entire callus. e Detail of protuberance in the apical region. f Detail of trichomes in the basal platform. g–i G. longicornu. g Entire callus. h Detail of the basal platform. i Detail of papillae in a lateral lobe
a–e Scanning electron micrographs of the lateral lobes and callus. a Grandiphyllum divaricatum, detail of trichomes of the callus. b–c Trichocentrum cebolleta. b Entire callus. c Detail of a lateral protuberance of the callus. d–e T. jonesianum. d Entire callus. e Detail of lateral protuberances of the callus
Cluster analysis
The cluster analysis (CA) based on 11 characters and 47 species is shown in Fig. 8. The correlation of the distance and tree matrix is 0.84, indicating a good fit of the phenogram to the distance matrix. The different groups and subgroups are numbered and discussed in the order that appears in Fig. 8. An additional cluster analysis was performance only with species of Oncidiinae (removing Rudolfiella picta), but as the same clusters are recovered, this is not shown.
Cluster A is composed by Cyrtochilum meirax, Gomesa bifolia, G. flexuosa, G. herzogii, G. longicornu, G. longipes, G. varicosa, G. venusta, Oncidium cheirophorum, O. heteranthum, O. ornithorhynchum, Trichocentrum cavendishianum, T. cebolleta, T. jonesianum, and Vitekorchis excavata. These species have medium to large flowers (measuring never less than 1 cm), generally yellow with red spots; the elaiophores are epithelial or intermediate (not entirely trichomatous) and the position is variable: either on the callus, on the lateral lobes or in both areas; the calluses present a central keel and lateral finger-like protuberances; many species have a conspicuous tabula infrastigmatica; callus and column are located at a recto to obtuse angle (≥90°); the rostellum is generally not elongate; and the lateral lobes are usually developed like wings, and their margins would be abaxially reflex.
Cluster B is constituted by three subclusters. The first subcluster to emerge, here indicated as B1, consists of Gomesa echinata, Lockhartia bennettii, L. obtusata and R. picta (Maxillariinae). These species share the position of the elaiophores on the callus; developed central keel absent; tabula infrastigmatica absent; elongate rostellum absent; angle between column and callus acute (<90°) and lateral lobes in the same plane of the labellum. Within this species group, color and size of the flower, type of elaiophore, development of the lateral lobes and relation between positions of lateral lobes and median lobe are variable characters. The subcluster B2 consists of Gomesa radicans, G. recurva, Lockhartia hercodonta, L. micrantha, L. acuta, Oncidium oxyceras, Ornithocephalus ciliatus, O. gladiatus, Phymatidium falcifolium, Trichocentrum pumilum, Zygostates alleniana, Z. grandiflora and Z. lunata. Most of the species of this group have small to medium flowers (smaller than 2 cm), white or yellowish-green without red spots; elaiophores are predominantly trichomatous but epithelial and intermediate are also present; elaiophores are mainly located on the callus (except Ornithocephalus gladiatus); the form of the callus could be diverse but never presents a central keel; usually tabula infrastigmatica is absent; callus and column are located at a recto to obtuse angle (≥90°); the rostellum is frequently elongated on the column and sometime over the callus; the lateral lobes are scarcely developed and in the same plane as the labellum. The subcluster B3 is composed by G. cornigera, G. loefgrenii, G. paranensoides, G. ranifera, G. riograndensis, G. divaricatum, Lockhartia amoena, L. grandibractea, L. lepticaula, L. lunifera, L. niesseniae, L. oerstedii, L. serra, L. verrucosa, and Oncidium amazonicum. This group is morphologically diverse, although some patterns are frequently shared among the species. The flower size is variable, but middle-sized flowers (between 1 and 2 cm) are more common; the predominant color is yellow with red spots; there are epithelial and trachomatous elaiophores but no intermediate type was found in any of these species; elaiophores are generally on the callus; the presence of the callus with a central keel is uncommon, and it has finger-like protuberances; a tabula infrastigmatica is either absent or very reduced; the callus and column are located at a right to obtuse angle (≥90°); the rostellum is most of the times not elongate and the lateral lobes are very diverse in development and degree of curvature.
Species of potential pollinators
Based on personal observations and available works about field observations, we constructed a table that contains information of the species/genera of oil-collecting bees cited for different species of Oncidiinae (Table 2). For those species that were not included in the cluster analyses, but have a register of pollinators, we assign a presumable type of oil flower based on the combination of the characters present in the flowers.
Discussion
Our data confirmed the presence of floral elaiophores in Gomesa cornigera, G. herzogii, G. longicornu, G. divaricatum, Trichocentrum cebolleta and T. jonesianum. The floral morphology, and particularly the position of the callus, are variable among the studied species; however, the anatomical pattern of the secretor area was similar to the elaiophores described for other species of Oncidiinae (Singer and Cocucci 1999; Pacek and Stpiczyńska 2007; Stpiczyńska et al. 2007; Stpiczyńska and Davies 2008; Aliscioni et al. 2009; Davies and Stpiczyńska 2009; Pacek et al. 2012; Blanco et al. 2013; Stpiczyńska et al. 2013; Gomiz et al. 2013, 2014; Davies et al. 2014). In all cases, the secretory tissue is either represented by a single layer of palisade or cuboidal epidermal cells, as in G. cornigera; G. longicornu; T. cebolleta, T. jonesianum; or consists fully of a trichomatous epidermis as in G. divaricatum; or has both trichomatous and cuboidal cells as in G. herzogii. The secretory epidermis is associated with some layers of parenchymatous subepidermal tissue that probably contributes to the secretion because the characteristics of the cells are similar to the epidermis. Both epidermal and subepidermal parenchymatous cells were intensely stained and had basal or central positioned nuclei with conspicuous nucleoli. We assume that these characteristics indicate that these cells are metabolically active in secretion.
The presence of a callus with oil-secretor trichomes in the basal platform and the cuboidal epidermal cells in the apical area in G. herzogii added evidence of the presence of intermediate type of elaiophores in Oncidiinae, as was described in Ornithocephalus gladiatus (Pacek et al. 2012); Gomesa flexuosa (Gomiz et al. 2013); Gomesa longipes, Oncidium heteranthum and Vitekorchis excavata (Davies et al. 2014).
We observed and described in this work all types of elaiophores detected in Oncidiinae: epithelial, trichomatous (Vogel 1974) and also intermediate (Gomiz et al. 2013). Considering the recovered data in the cluster analyses, this included a total of 47 species (46 Oncidiinae and 1 Maxillariinae), 21 had epithelial elaiophores, 19 trichomatous and only 6 intermediate. Thus, epithelial and trichomatous are the most frequent elaiophores in this group of Orchidaceae, with intermediate elaiophores being the most common.
This study represents the first description of elaiophores in the genus Grandiphyllum, where they are situated only on the calluses, as in Gomesa longicornu and most of the Oncidiinae (Davies et al. 2014). Based on a review of the literature, 75% of the studied species present the elaiophore on the callus. The remaining have the elaiophores only in the lateral lobes (five species, 10%) or combined lateral lobes and callus (7 species, 15%), as G. cornigera, G. herzogii, T. cebolleta, and T. jonesianum. These last two positions seem to be uncommon in the subtribe.
Many flowers of Oncidiinae have a tabula infrastigmatica, this structure is a thickened pad on the base of the column. The bees grab this with their mandibles, thereby freeing their legs for gathering oil; this structure is a functional analogue to the flag petal of a flower of a Malpighiaceae (van der Pijl and Dodson 1969; Dressler 1990; van der Cingel 2001). In our analysis, all species with tabula infrastigmatica were positioned in the cluster A, with the exception of T. pumilum, which was considered in the subtype B2.
We observed that none of the considered characters are exclusively associated with a particular type of oil flower; however, each cluster was characterized by a suite of a predominant combination of states of characters. For example, species of Oncidiinae called informally “Oncidium type” by Neubig et al. (2012) for exhibiting bright yellow flowers and a developed tabula infrastigmatica, were principally located in our analysis in cluster A. In this group, the lateral lobes are usually developed like wings and their margins might abaxially curve. This last condition may be associated to oil secretion (in the cases where elaiophores were detected in the lateral lobes) or oil accumulation, for the bees to collect with the legs. Additionally, this group of species does not present an elongate rostellum, a characteristic that seems to be not quite related to the type of oil flower, except in O. cheirophorum (Pacek and Stpiczyńska 2007) and O. ornithorhynchum (Davies and Stpiczyńska 2009) in which rostellum is somewhat elongated up to the base of the column.
In our cluster analysis, two types of oil flower can be distinguished in species of Oncidiinae: A and B (into the cluster B, three subtypes can be differentiated). Many of the analyzed genera were represented in different clusters supporting the idea that this condition is very variable at the generic level. Our observations, together with published works on Oncidiinae, proved that the genus Gomesa is one of which exhibits high variability related to the characteristics associated to the oil flower. In our analysis, species of Gomesa were distributed in all clusters (G. bifolia, G. flexuosa, G. herzogii, G. longicornu, G. longipes, G. varicosa, G. venusta: type A; G. echinata: type B1; G. radicans, G. recurva: type B2; G. cornigera, G. loefgrenii, G. paranensoides, G. ranifera, G. riograndensis: type B3). Likewise Trichocentrum is variable, but three species (T. cavendishianum, T. cebolleta, T. jonesianum) were placed in cluster A, and only T. pumilum was assigned as type B2. The species currently recognized in the genus Oncidium whose elaiophores were described by other authors (Pacek and Stpiczyńska 2007; Davies and Stpiczyńska 2009; Stpiczyńska et al. 2013; Davies et al. 2014) also were associated with different types of oil flower. On the contrary, the species studied of the genus Lockhartia (Blanco et al. 2013) and the members of the clade Ornithocephalinae, as the genera Zygostates, Ornithocephalus and Phymatidium (Pacek and Stpiczyńska 2007; Pacek et al. 2012; Gomiz et al. 2014), were less variable and were located in cluster B, although in different subtypes in the case of Lockhartia.
In the case that more species of Oncidiinae with elaiophores will be described in the future, we will be able to know more precisely how homoplastic is this condition in this subtribe. Unfortunately, many species whose elaiophores were currently studied were not tested in the recent phylogeny of the Oncidiinae (Neubig et al. 2012); however, some preliminary observations can be made (Fig. 9).
Reduced phylogeny of the subtribe Oncidiinae modified from Neubig et al. (2012). The genera in bold were considered in the cluster analysis and species of the underlined genera were anatomically studied in the present work. The cluster/clusters are indicated within brackets; and the number of species with elaiophores on the total number of species of the genus
Davies et al. (2014) asserts that elaiophores are of limited value in investigating the phylogeny of the Oncidiinae. The genera with highest number of species anatomically studied are Gomesa and Oncidium and give us evidence that characteristics of the elaiophores are variable at a generic level, and therefore, this is indicative of homoplasy. Renner and Schaefer (2010) mentioned that convergence on a stereotypical syndrome of floral traits, associated with pollination by oil-collecting bees, has been strong and that genera such as Oncidium s.l., which were defined by floral characteristics, turn out to be grossly polyphyletic (Chase et al. 2009). Thus, in some genera (e.g., Gomesa, Oncidium, and Trichocentrum) the selection pressure exerted by pollinators seems to have played a key role in the evolution. For some species of these genera initially Muellerian, later probably Batesian, mimicry of Malpighiaceae could be explained by evolutionary acquisitions (Renner and Schaefer 2010). Even though some of these species can be considered putative mimics of Malpighiaceae, the presence of elaiophores in orchid flowers represents that these species are legitimate oil reward (Neubig et al. 2012).
On the other hand, genera as Zygostates, Ornithocephalus and Phymatidium are homogeneous in the characteristics associated with floral oil secretion. These species, which are included in the monophyletic Ornithocephalinae clade (Neubig et al. 2012), seem to have acquired elaiophores only one time along their evolution, as a synapomorphy for this group. Regarding the genus Lockhartia, we observed that the species considered in our cluster analysis belonged in the majority in subcluster B3, but are also represented in subclusters B1 and B2; although with some differences, the genus is totally positioned in cluster B. The phylogeny of Neubig et al. (2012) demonstrated the monophyly of Lockhartia; this suggests that oil flower was acquired in some ancestral lineage and then advanced with some differences in some branches. Blanco et al. (2013) performed a detailed study of floral elaiophores in Lockhartia and affirmed that the common ancestor may as well have had relatively simple, food-deceptive flowers and that the more elaborate type of elaiophore, found in species of the so-called Imbricata-group, is a derived characteristic.
Based on reports of pollinators and/or floral visitors in Oncidiinae and their correlation with the types of oil flowers here recognized (see Table 2), different species of Centris were associated with species of Oncidiinae within type A, with few exceptions such as O. cheirophorum, for which was cited Paratetrapedia sp. (Silvera 2002; Pacek and Stpiczyńska 2007); and Trichocentrum ascendens and T. stipitatum (presumably type A), where Trigona nigra was reported, a non-oil-collecting bee species (Parra-Tabla et al. 2000) and Paratetrapedia sp. (Silvera 2002), respectively. However, species of Centris sp. were also observed visiting these two species. On the other hand, the different subtypes of the type B were preferentially associated with species of Paratetrapedia s.l. (including Lophopedia) and Tetrapedia sp.
Species of cluster A are principally pollinated by bees of genus Centris. In most species of these bees, the females possess anteroventral (or inner) basitarsal combs on the fore- and midbasitarsi in a “four legged” pattern of oil-collecting organs (Neff and Simpson 1981). These bees land at the flower, grasp the tabula infrastigmatica with the mandibles and scrape the callus (and lateral lobes) to obtain the floral oil (Vogel 1974). On the other hand, species belonging to cluster B (all subclusters) are principally pollinated by species of Paratetrapedia s.l. and Tetrapedia. In females and males of Paratetrapedia s.l. (Michener and Moure 1957; Neff and Simpson 1981; Roig-Alsina 1997; Cocucci et al. 2000) and Tetrapedia (Michener and Moure 1957; Neff and Simpson 1981), the combs are present in position posteroventral (or outer) only on the forebasitarsi, in a “two-legged” pattern of the oil-collecting organs. These bees either grasp the tabula infrastigmatica (e.g., Singer and Cocucci 1999) grip to the labellum with midlegs (Neff and Simpson 1981) or support their body on labellum (Vogel 1974) while working on the callus with the forelegs. The different positions (anteroventral/inner or posteroventral/outer) of the oil-collecting apparatus of different bees force these pollinators to forage differently.
Chase et al. (2015) commented that the parallel evolution of oil-bee pollination arised in many independent linajes (Papadopulos et al. 2013) and shifts away from this pollination syndrome to other pollinators in different clades. The results obtained in this work support this idea, therefore to continue with the study of elaiophores in members of Oncidiinae (especially in groups [i.e., genera, clades] not or few studied), and help to understand the manner that this particular pollination system has evolved in the subtribe Oncidiinae.
References
Aliscioni SS, Torretta JP, Bello ME, Galati BG (2009) Elaiophores in Gomesa bifolia (Sims) M.W. Chase and N.H. Williams (Oncidiinae: Cymbidieae: Orchidaceae): structure and oil secretion. Ann Bot (Oxford) 104:1141–1149. doi:10.1093/aob/mcp199
Anderson WR (1979) Floral conservatism in neotropical Malpighiaceae. Biotropica 11:219–223
Blanco MA, Davies KL, Stpiczyńska M, Carlsward BS, Ionta GM, Gerlach G (2013) Floral elaiophores in Lockhartia Hook. (Orchidaceae: Oncidiinae): their distribution, diversity and anatomy. Ann Bot (Oxford) 112:1775–1791. doi:10.1093/aob/mct232
Buchmann SL (1987) The ecology of oil flowers and their bees. Annual Rev Ecol Evol Syst 18:343–369. doi:10.1146/annurev.es.18.110187.002015
Carmona-Díaz G, García-Franco JG (2009) Reproductive success in the Mexican rewardless Oncidium cosymbephorum (Orchidaceae) facilitated by the oil-rewarding Malpighia glabra (Malpighiaceae). Pl Ecol 203:253–261. doi:10.1007/s11258-008-9543-6
Chase MW, Williams NH, Faria AD, Neubig KM, Amaral MCE, Whitten WM (2009) Floral convergence in Oncidiinae (Cymbidieae; Orchidaceae): an expanded concept of Gomesa and a new genus Nohawilliamsia. Ann Bot (Oxford) 104:387–402. doi:10.1093/aob/mcp067
Chase MW, Cameron KM, Freudenstein JV, Pridgeon AM, Salazar G, Berg C, Schuiteman A (2015) An updated classification of Orchidaceae. Bot J Linn Soc 177:151–174. doi:10.1111/boj.12234
Cocucci AA, Sérsic A, Roig-Alsina A (2000) Oil-collecting structures in Tapinotaspidini: their diversity, function and probable origin. Mitt Münch Entomol Ges 90:51–74
Damon AA, Cruz-López L (2006) Fragrance in relation to pollination of Oncidium sphacelatum and Trichocentrum oerstedii (Orchidaceae) in the Soconusco region of Chiapas, Mexico. Selbyana 27:186–194
Darwin C (1862) The various contrivances by which orchid are fertilized. John Murray, London
Davies KL, Stpiczyńska M (2008) The anatomical basis of floral, food-reward production in Orchidaceae. In: Teixeira da Silva J (ed) Floriculture, ornamental and biotechnology: advances and topical issues, vol 5. Global Science Books, Isleworth, pp 392–407
Davies KL, Stpiczyńska M (2009) Comparative histology of floral elaiophores in the orchids Rudolfiella picta (Schltr.) Hoehne (Maxillariinae sensu lato) and Oncidium ornithorhynchum HBK (Oncidiinae sensu lato). Ann Bot (Oxford) 104:221–234. doi:10.1093/aob/mcp119
Davies KL, Stpiczyńska M, Rawski M (2014) Comparative anatomy of floral elaiophores in Vitekorchis Romowicz and Szlach., Cyrtochilum Kunth and a florally dimorphic species of Oncidium Sw. (Orchidaceae: Oncidiinae). Ann Bot (Oxford) 113:1155–1173. doi:10.1093/aob/mcu045
Dodson CH (1962) The importance of pollination in the evolution of the orchids of tropical America. Amer Orchid Soc Bull 31:525–534
Dressler RL (1990) The major clades of the Orchidaceae-Epidendroideae. Lindleyana 5:117–125
Dressler RL (1993) Phylogeny and classification of the orchid family. Dioscorides Press, Oregon
Gomiz NE, Torretta JP, Aliscioni SS (2013) Comparative anatomy of elaiophores and oil secretion in the genus Gomesa (Orchidaceae). Turkish J Bot 37:859–871. doi:10.3906/bot-1209-6
Gomiz NE, Torretta JP, Aliscioni SS (2014) Zygostates alleniana (Orchidaceae: Epidendroideae: Cymbidieae: Oncidiinae): estructura floral relacionada a la polinización. Anales Jard Bot Madrid 7:1–9. doi:10.3989/ajbm.2378
Michener CD, Moure JC (1957) A study of the classification of the more primitive non-parasitic anthophorine bees (Hymenoptera, Apoidea). Bull Amer Mus Nat Hist 112:395–452
Neff JL, Simpson BB (1981) Oil-collecting structures in the Anthophoridae (Hymenoptera): morphology, function, and use in systematic. J Kansas Entomol Soc 54:95–123
Neubig KM, Whitten WM, Williams NH, Blanco MA, Endara L, Burleigh JG, Silvera K, Cushman JC, Chase MW (2012) Generic recircumscriptions of Oncidiinae (Orchidaceae: Cymbidieae) based on maximum likelihood analysis of combined DNA datasets. Bot J Linn Soc 168:117–146. doi:10.1111/j.1095-8339.2011.01194.x
Pacek A, Stpiczyńska M (2007) The structure of elaiophores in Oncidium cheirophorum Rchb. f. and Ornithocephalus kruegeri Rchb. f. (Orchidaceae). Acta Agrobot 60:9–14. doi:10.5586/aa.2007.024
Pacek A, Stpiczyńska M, Davies KL, Szymczak G (2012) Floral elaiophore structure in four representatives of the Ornithocephalus clade (Orchidaceae: Oncidiinae). Ann Bot (Oxford) 110:809–820. doi:10.1093/aob/mcs158
Pansarin EM, Pansarin LM (2011) Reproductive biology of Trichocentrum pumilum: an orchid pollinated by oil-collecting bees. Pl Biol (Stuttgart) 13:576–581. doi:10.1111/j.1438-8677.2010.00420.x
Pansarin LM, De Moraes Castro M, Sazima M (2009) Osmophore and elaiophores of Grobya amherstiae (Catasetinae, Orchidaceae) and their relation to pollination. Bot J Linn Soc 159:408–415. doi:10.1111/j.1095-8339.2009.00953.x
Papadopulos AST, Powell MP, Pupulin F, Warner J, Hawkins JA, Salamin N, Chittka L, Williams NH, Whitten WM, Loader D, Valente LM, Chase MW, Savolainen V (2013) Convergent evolution of floral signals underlies the success of Neotropical orchids. Proc Roy Soc London Ser B Biol Sci 280:20130960. doi:10.1098/rspb.2013.0960
Parra-Tabla V, Vargas CF, Magaña-Rueda S, Navarro J (2000) Female and male pollination success of Oncidium ascendens (Orchidaceae) in two contrasting habitat patches: forest vs agricultural field. Biol Conservation 94:335–340. doi:10.1016/S0006-3207(99)00187-1
Pemberton RW (2008) Pollination of the ornamental orchid Oncidium sphacelatum by the naturalized oil-collecting bee (Centris nitida) in Florida. Selbyana 29:87–91
Proctor M, Yeo P, Lack A (1996) The natural history of pollination. Timber Press, Oregon
Reis MG, de Faria AD, Bittrich V, Amaral MCE, Marsaioli AJ (2000) The chemistry of flower rewards–Oncidium (Orchidaceae). J Brazil Chem Soc 11:600–608. doi:10.1590/S0103-50532000000600008
Reis MG, de Faria AD, dos Santos IA, Amaral MDE, Marsaioli AJ (2007) Byrsonic acid—the clue to floral mimicry involving oil-producing flowers and oil-collecting bees. J Chem Ecol 33:1421–1429. doi:10.1007/s10886-007-9309-y
Renner SS, Schaefer H (2010) The evolution and loss of oil-offering flowers: new insights from dated phylogenies for angiosperms and bees. Philos Trans Ser B 365:423–435. doi:10.1098/rstb.2009.0229
Rohlf F (1998) NTSYS-Pc: numerical taxonomy and multivariate analysis system version 20. Department of Ecology and Evolution. State University of New York, Stony Brook. Available at: http://www.exetersoftware.com/downloads/ntsysguide21.pdf
Roig-Alsina A (1997) A generic study of the bees of the tribe Tapinotaspidini, with notes on the evolution of their oil-collecting structures (Hymenoptera, Apidae). Mitt Münchner Entomol Ges 87:3–21
Silvera K (2002) Adaptive radiation of oil-reward compounds among Neotropical orchid species (Oncidiinae). MSc Thesis, University of Florida, Gainesville
Singer RB, Cocucci AA (1999) Pollination mechanisms in four sympatric southern Brazilian Epidendroideae orchids. Lindleyana 14:47–56
Singer RB, Marsaioli AJ, Flach A, Reis MG (2006) The ecology and chemistry of pollination in Brazilian orchids: recent advances. In: Teixeira da Silva J (ed) Floriculture, ornamental and plant biotechnology, vol 4. Global Science Books, Isleworth, pp 569–582
Stpiczyńska M, Davies KL (2008) Elaiophore structure and oil secretion in flowers of Oncidium trulliferum Lindl. and Ornithophora radicans (Rchb.f.) Garay and Pabst (Oncidiinae: Orchidaceae). Ann Bot (Oxford) 101:375–384. doi:10.1093/aob/mcm297
Stpiczyńska M, Davies KL, Gregg A (2007) Elaiophore diversity in three contrasting members of Oncidiinae (Orchidaceae). Bot J Linn Soc 155:135–148. doi:10.1111/j.1095-8339.2007.00681.x
Stpiczyńska M, Davies KL, Pacek-Bieniek A, Kamińska M (2013) Comparative anatomy of the floral elaiophore in representatives of the newly re-circumscribed Gomesa and Oncidium clades (Orchidaceae: Oncidiinae). Ann Bot (Oxford) 112:839–854. doi:10.1093/aob/mcu045
Thiers B (2016-continuously updated) Index herbariorum: a global directory of public herbaria and associated staff. New York Botanical Garden’s virtual herbarium, New York. http://sweetgum.nybg.org/science/ih/
Torretta JP, Gomiz NE, Aliscioni SS, Bello ME (2011) Biología reproductiva de Gomesa bifolia (Orchidaceae, Cymbidieae, Oncidiinae). Darwiniana 49:16–24
Tremblay RL, Ackerman JD, Zimmerman JK, Calvo RN (2005) Variation in sexual reproduction in orchids and its evolutionary consequences: a spasmodic journey to diversification. Biol J Linn Soc 84:1–54. doi:10.1111/j.1095-8312.2004.00400.x
Vale A, Navarro L, Rojas D, Álvarez JC (2011) Breeding system and pollination by mimicry of the orchid Tolumnia guibertiana in Western Cuba. Pl Spec Biol 26:163–173. doi:10.1111/j.1442-1984.2011.00322.x
van der Cingel NA (2001) Atlas of orchid pollination: orchids of America, Africa, Asia and Australia. AA Balkema, Rotterdam
van der Pijl L, Dodson CH (1966) Orchid flowers: their pollination and evolution. University of Miami Press, Coral Gables
Vinson SB, Frankie GW, Williams HJ (1996) Chemical ecology of bees of the genus Centris (Hymenoptera: Apidae). Florida Entomol 79:109–129
Vogel S (1969) Flowers offering fatty oil instead of nectar. In: Abstracts of the papers presented at the XI International Botanical Congress, University of Washington, Seattle, USA, 24 August–2 September, 1969, p 229
Vogel S (1974) Ölblumen und ölsammelnde Bienen. Trop Subtrop Pflanzenwelt 7:285–547
Zarlavsky GE (2014) Histología Vegetal: técnicas simples y complejas. Sociedad Argentina de Botánica, Buenos Aires
Acknowledgements
This work was supported by FONCYT PICT 2013-1867 and CONICET PIP 2012-2014 (11220110100312). We thank to B. Galati and G. Zarlavsky for technical assistance, G. Sanchez for image processing, A. Torretta for the revision of manuscript and M. Bello and M. Lovisolo for providing plant material, and two anonymous reviewers for their constructive comments on this manuscript.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declared that they have no conflict of interest.
Additional information
Handling editor: Louis P. Ronse De Craene.
Electronic Supplementary Material
Below is the link to the electronic supplementary material.
Information on Electronic Supplementary Material
Information on Electronic Supplementary Material
Online Resource 1. Data base used in the cluster analysis with the corresponding bibliography.
Rights and permissions
About this article
Cite this article
Gomiz, N.E., Torretta, J.P. & Aliscioni, S.S. New evidence of floral elaiophores and characterization of the oil flowers in the subtribe Oncidiinae (Orchidaceae). Plant Syst Evol 303, 433–449 (2017). https://doi.org/10.1007/s00606-016-1382-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00606-016-1382-7