Abstract
In Central Europe, the genus Platanthera traditionally comprised two species, P. chlorantha Cust. ex Rchb. (Pc) and P. bifolia (L.) Rich. (Pb). They are morphologically characterized by a wide and narrow separation of anthers, respectively. However, a third form with intermediate anther distance has repeatedly been hypothesized but only hesitantly accepted. In addition, intermediate morphology has been also used as the main character of P. × hybrida. However, the status of some purported hybrid populations is challenged by the local lack of parental species, their successful reproduction and non-intermediate traits. Despite this unclear situation, detailed genetic and morphological analyses are lacking. Here, we studied morphology and molecular markers within the P. chlorantha/bifolia group in Central Europe. Three morphological groups emerged representing Pc, Pb and a third form, here informally referred to as non-hybrid intermediates (Pn). The latter is characterized, among other trait differences, by intermediate distance between anthers [(0.7)–1–2.2 mm] and long spurs (28–40 mm). Three gene pools were identified, which largely corresponded to the three morphological groups. The Pn gene pool had several high-frequency private alleles substantiating its genetic independence. Some of the Pn populations were previously interpreted as P. × hybrida suggesting that Pn was overlooked hitherto and mistaken to represent hybrids. The non-perfect fit between morphological and genetic groups highlights the potential for fast morphological evolution. Overall, the finding of three distinct lineages within the bifolia/chlorantha group necessitates a thorough reanalysis of reported taxa and a reevaluation of our understanding of their distribution, ecology and evolution.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The genus Platanthera has been coined a small-scale model for understanding the role of floral specialization on the adaptive radiation of Orchidaceae as a whole, because of the diversity of pollination syndromes found (Hapeman and Inoue 1997). Here, the best studied pollination syndromes, i.e. the suites of flower traits selected for by particular types of pollinators, are those of butterfly and moth pollination, because most Platanthera species are pollinated by Lepidoptera (van der Pijl and Dodson 1966; Hapeman and Inoue 1997) which feed on nectar that is presented in spurs. During flower visits pollinaria are attached to the pollinators’ head, eyes or proboscis and are thus transferred between flowers (Darwin 1877; Nilsson 1978; Hapeman and Inoue 1997). On the genus level, gynostemium and pollinaria show large variation, are responsive to selection and thus are involved in diversification of Platanthera (Efimov 2011). Nilsson (1978, 1983) proved that the distance between anthers is a major trait determining the attachment to, and delivery of, pollinaria by lepidopteran pollinators. Therefore, both the length of the spur and the distance between anthers are decisive for the success of plant–pollinator interaction in Platanthera and have been shown to be under strong selection by moth pollinators (Maad 2000; Boberg and Ågren 2009; Boberg et al. 2014). Thus, gynostemium morphology is an essential trait affecting reproductive isolation.
In Central Europe, the genus Platanthera traditionally comprises two widespread species, P. chlorantha Cust. ex Rchb. and P. bifolia (L.) Rich. The two species are morphologically reasonably well characterized by large and small flowers, respectively. A more particular distinction is a wide separation and downward divergence of anthers in P. chlorantha and narrow separation of mostly parallel anthers in P. bifolia (Table 1). However, beyond the chlorantha-bifolia dichotomy, large variation of flower traits within P. bifolia s.l. has been repeatedly documented (e.g. Müller 1868). Such observations also fostered several attempts to further subdivide P. bifolia s.l. taxonomically (Wallroth 1822; Reichenbach 1831; Babington 1836; Drejer 1843; Müller 1868; Bisse 1963; Løjtnant 1978; Buttler 2011). However, despite these attempts, only recently the later taxonomic concepts have become accepted in some local floras. Thus, if accepted at all, besides P. bifolia s.str., characterized as small-flowered, short-spurred with narrow anthers, a second form is currently referred to either as P. bifolia subsp. latiflora (Drejer) Løjtnant (e.g. Pedersen and Faurholdt 2010; Jäger 2011; Krok et al. 2013), or as P. fornicata (Bab.) Buttler (e.g. AHO Thüringen 2014), which is characterized as large-flowered, long-spurred and with the distance between anthers being intermediate between those of P. bifolia subsp. bifolia and P. chlorantha (Table 1).
The delayed recognition and the hesitant acceptance of taxa beyond the chlorantha-bifolia dichotomy may be due to three non-mutually exclusive reasons. First, other taxa might simply be non-existent or very rare. Such a view dates back to Darwin who had studied the chlorantha-bifolia system and discovered the contrasting and incompatible placement of pollinia on eyes and probosces of pollinators, respectively, and asserted “Should these two forms [P. chlorantha and P. bifolia] be hereafter proved to graduate into each other, independently of hybridization, it would be a remarkable case of variation; and I, for one, should be as much pleased as surprised at the fact….” Moreover, he hypothesized that deviations from these two morphological forms are unlikely to persist: “Variations in the structure of the flower …, unless they led to the viscid discs touching some part of the body of an insect where they would remain firmly attached, would be of no service, […] and consequently such variations would not be preserved and perfected” Darwin (1877, p. 73). Thus, Darwin conferred an inviolable status to the chlorantha-bifolia dichotomy. However, considerable variation of gynostemium morphology within P. bifolia has long been known. For example, Müller (1868) pointed out that what German botanists generally considered to be P. bifolia differed from Darwin’s description in particular by a wider separation of anthers. Moreover, recent studies showed that within P. bifolia spur length is responsive to selection by local pollinator communities varying in proboscis length and shows a bimodal distribution (Boberg et al. 2014) suggestive of considerable intraspecific variation and differentiation.
Second, Platanthera morphotypes deviating from the chlorantha-bifolia dichotomy might have been mistaken to be hybrids. In sympatric populations of P. chlorantha and P. bifolia hybridization can take place. Morphologically, intermediate hybrids typically occur in low numbers and have reduced reproductive success relative to parental species (Nilsson 1983; Baum and Baum 2011). Although P. × hybrida had been described as overall intermediate between the parental species (Bruegger 1882), distinctive diagnostic traits were not defined. Thus, the intermediate distance between anthers has often been used as diagnostic trait of hybrids (Jäger 2011; Krok et al. 2013). Interestingly, a number of reports exist about “stable P. × hybrida populations” (Perko 1997), in which both parents are absent (e.g. Schulze 1894; Perko 1997; Künkele and Baumann 1998; Claessens and Kleynen 2006). However, in contrast to the expectation of suboptimal pollination, fruit set in such populations is reported to be high (Perko 1997; Künkele and Baumann 1998) and successful pollinators have been observed (Claessens et al. 2008). In addition, although anther distance is intermediate, other traits, e.g. spur and labellum length of such plants have not been found to be intermediate but to be outside the range of both putative parents (Claessens and Kleynen 2006). Thus, it may be questioned whether such populations are actually hybrids.
Third, as far as molecular phylogenetic analyses of the bifolia/chlorantha group are concerned, genetic variation was found to be extremely low. Sequence divergence at the nuclear ribosomal internal transcribed spacer region locus typically used for phylogenetic analyses did not even allow to separate bifolia from chlorantha (Bateman et al. 2009). The lack of genetic variation at that locus likely discouraged the search for further intraspecific differentiation. However, genetic variation in the group was detected in allozymes (Brzosko et al. 2009) and chloroplast genes (Pavarese et al. 2011), suggesting that other genetic markers may be more informative.
Thus overall, the hesitant acceptance of changes to the chlorantha-bifolia dichotomy, the obscurity of putative hybrid populations and the lack of meaningful genetic data, warrants independent evidence that is lacking hitherto. Here, we studied general and floral morphology and molecular markers to assess the morphological and genetic variation within the P. chlorantha/bifolia group in Central Europe with particular focus on intermediate morphotypes and putative P. × hybrida. We expect hybrids between P. bifolia and P. chlorantha to show both intermediate phenotypes and admixed genotypes. In contrast, any independent evolutionary line is expected to display a unique trait combination and an independent gene pool.
As will be shown below, the intermediate morphotype broadly matching P. bifolia subsp. latiflora or P. fornicata is an independent genetic group not of hybrid origin. However, because the diagnostic traits, morphological characterization and habitat affiliation of these taxa do not match the range of our observations, we informally refer to this form as non-hybrid intermediates.
Materials and methods
Studied species and sampled populations
We investigated P. chlorantha (Custer) Rchb.f. [Pc], P. bifolia L. (Rich.) s.str. [Pb] and non-hybrid intermediates [Pn] (see Online Resource 1 for photos). All individuals were categorized based on their flower traits (Table 1). We treated plants characterized by a trait combination of intermediate distance between anthers [(0.7)–1–2.2 mm] which are either parallel, slightly diverging downwards or, very rarely, slightly diverging upwards, long spur (28–40 mm) and long labellum (10–18 mm) as a separate informal taxon non-hybrid intermediates (Pn). All Pn populations consisted solely of this taxon and neither Pc nor Pb did occur in these sites or their vicinity. Note that two of the investigated Pn populations (sites Wrakelberg and Wylre Akkers) were previously referred to as “P. × hybrida” (Claessens and Kleynen 2006; Claessens et al. 2008). In addition, we collected what we considered to be early generation hybrids (P. bifolia × P. chlorantha = P. × hybrida [Px]), i.e. morphologically intermediate plants occurring in a site (Sistig) with sympatric populations of both parental species, henceforth referred to as putative hybrids. In the second site with sympatric Pc and Pb (Kuttenberg), no intermediate plants were found. Although recently P. × hybrida Brügger was reduced to a synonym of Platanthera × graebneri (M.Schulze) Domin by Efimov (2016), we, for the sake of simplicity, stick to the former. In total we studied 14 populations in western and eastern Germany, the Netherlands and Belgium (Table 2). Within sites, which were either open grassland, woody vegetation or a mosaic thereof, we applied a stratified random sampling, i.e. we selected plants from all parts of the habitat (e.g. open, woody, sunny, shady, moist and dry), collecting 8–10 plants per site.
Morphological analysis
For each selected plant we took the following measurements in the field: total height, height of the stem, length of inflorescence, number of leaves, leaf angle (1:0°–30°, 2:30°–70°, 3:>70°), length of longest leaf, width of longest leaf and number of flowers. We collected the uppermost and lowest complete flower and measured the following flower traits (acronyms) in mm (see Online Resource 1 for detailed trait explanation): spur length (sp_l), labellum length (lab_l), dorsal sepal length (s1_l), dorsal sepal width (s1_w), lateral sepal length (s2_l), lateral sepal width (s2_w), petal length (p_l), petal width (p_w), distance between anthers at the top (ant_dt) and at the bottom (ant_db, equalling the distance between viscidia) and anther length (ant_l). For each sampled plant, we calculated the average of the two collected flowers and calculated anther orientation (AO = ant_db/ant_dt) with AO = 1 indicating parallel anthers, >1 downward divergence and >1 upward divergence. For a subset of flowers (n = 7, 5, 17, 4 for Pb, Pc, Pn, Px, respectively) we furthermore excised a pollinarium from one anther and measured the length of the caudicle and of the pollinium, which, however, were not used in the statistical analyses (but see Table 1).
To visualize the variation of flower traits among individuals and to depict the relevant traits we used principle component analysis (PCA) on scaled data using the function prcomp. In order to test whether and how floral traits per se are structured, we applied k-means clustering with Mclust (Fraley et al. 2015) on scaled data. K-means clustering determines, without any a priori classification, both an optimal statistical model and an optimal number of clusters in a dataset based on the Bayesian information criterion (BIC). To test how confident Pb, Pc and Pn can be distinguished by floral morphology and to identify the most relevant traits, we used linear discriminant analysis using the function lda on scaled flower data disregarding hybrids. To test whether trait means differed significantly between taxa we performed analyses of variance and subsequent Tukey’s HSD tests. All statistical analyses were performed in the software environment R (R Core Team 2015).
Molecular marker analysis
Genomic DNA was extracted from leaf tissue using DNeasy 96 kits (Qiagen). We generated amplified fragment length polymorphism (AFLP) markers following Durka et al. (2017). After screening of 16 primer combinations, we used the fluorescent-labelled primer combinations with selective bases AAC (FAM)-CTGG, ACC (NED)-CTGA, AGC (PET)-CACC and ACA (VIC)-CAGT. Fragment separation was performed on an ABI 3130 genetic analyser with GenScan500-LIZ as size standard. Genotyping was performed by manually defining bins in GenMapper 5.0 and setting band-specific peak-height thresholds. Genotyping error rates were calculated based on 64 replicate samples. Markers with individual error rates >5% were discarded. This procedure resulted in a total of 148 loci in the range of 50–500 bp, 124 of which were polymorphic (FAM 39, NED 22, PET 17, VIC 46). Of these, 26 were rare, i.e. occurred or were lacking only once (n = 8), twice (n = 6) or three times (n = 12), respectively.
In addition, we sequenced the ITS region from ribosomal DNA (including partial 18S rRNA, ITS1, 5.8S rRNA and ITS2) for selected samples of P. chlorantha (n = 5), P. bifolia s.str. (n = 8), non-hybrid intermediates (n = 10) and a putative hybrid of P. bifolia × P. chlorantha (n = 1) using primers ITS5 and ITS4 (White et al. 1990) and standard sequencing protocols as described in Stark et al. (2011) (GenBank accession codes KY007621-KY0078627).
We used principal coordinate analysis (PCoA) to display genetic relationships among individual samples based on Euclidian genetic distance using GenAlex (Peakall and Smouse 2012). To identify genetically differentiated gene pools we used Bayesian cluster analysis with STRUCTURE (Falush et al. 2007). We used default options for ancestry and allele frequency models, used only genetic data and did not use population origin as prior. Because PcoA indicated a low number of genetic groups, the number of clusters was run from K = 1–10 with 50,000 burnin and 100,000 diagnostic MCMC iterations and 10 replicate runs per K. Identification of the most probable number of clusters followed Evanno et al. (2005). We also applied Bayesian cluster analysis as implemented in BAPS (Corander et al. 2008) and k-means clustering as implemented in DAPC (Jombart et al. 2010). However, we do not show these results because they were very similar to those of STRUCTURE and resulted in the same K. We identified the number and frequency of private markers, i.e. markers that only occurred in particular taxa or gene pools. Genetic distance between gene pools was quantified with Nei’s genetic distance in AFLPSurv (Vekemans 2002).
Results
Morphology
A comparative view on trait values of the Platanthera taxa Pc, Pb and Pn is given in Fig. 1 and Online Resource 2. As expected, most vegetative traits did not differ among the three taxa, only stem height was significantly higher in Pn and leaf width was lower in Pb than in the other taxa (Online Resource 2). Variation of floral traits was more pronounced as seven out of 12 traits differed significantly between all three taxa and 11 out of 12 traits differed between Pn and Pb (Fig. 1). A number of traits differed strongly between Pb and Pc with Pn showing intermediate values, such as distance between anthers (top, bottom and anther orientation), anther length and sepal width. However, for other traits Pn had trait values outside the range of Pb and Pc, in particular for length of spur, labellum and dorsal sepal and for width and length of petals. Putative hybrid individuals were quite variable morphologically but had intermediate values for most traits, none of which differed significantly from both parental species (Fig. 1). Across the whole dataset, some of the floral traits were strongly correlated, e.g. spur length and labellum length (r = 0.844), anther distance top and bottom (r = 0.906), anther distance and anther length (r = 0.825) and the widths of the two sepals (r = 0.812).
Floral traits of investigated Platanthera taxa P. chlorantha (Pc, n = 28), P. bifolia subsp. bifolia (Pb, n = 38), non-hybrid intermediates (Pn, n = 24) and P. × hybrida (Px, n = 6) displayed as boxplots where the thick line indicates the median, boxes range from lower to upper quartile, whiskers indicate 1.5 times the interquartile range, and single points are outliers. Similar letters above boxes indicate non-significant differences among groups tested by ANOVA and post hoc test
Similarity of floral traits among individuals as depicted by principle component analysis showed that the three taxa formed reasonably well defined and largely separated clusters (Fig. 2a). The three taxa were more clearly separated along the diagonal running from bottom-left to top-right indicating differences in anther distances, anther length and anther orientation. Here, Pn was intermediate between Pb and Pc although the separation from Pn was not very pronounced. In contrast, each of the three clusters extended along the other diagonal indicating considerable within-taxon variation particularly in spur and labellum length. However, Pn did not encompass the lower range of spur and labellum length present in Pb and Pc. Px individuals did not form a clear cluster, although they had intermediate positions between Pb and Pc.
Principle component analysis of 104 individuals based on 12 flower traits. Dots show individuals along the first two principle components representing 71.3% of total floral trait variation. Ellipses envelop 68% of points within a group. a Individuals are coloured by morphologically defined taxon, symbols denote populations. Traits are represented by arrows which point in the direction of increasing trait values, the angle between arrows reflecting their correlation; orthogonal arrows are unrelated. b Individuals are coloured by affiliation to morphological groups as identified by k-means clustering. In c, individuals are coloured by affiliation to gene pools as identified by Bayesian cluster analysis of AFLP data with samples showing admixture of gene pools considered hybrids (see Fig. 5). Note that a, b and c show the same PCA analysis
K-means clustering of floral traits identified an optimal number of four clusters (VVE model, BIC: −2360) which showed large but not complete overlap with the taxonomic groups (Table 3; Fig. 2b). Clusters 1 and 2 both consisted mainly of Pb, clusters 3 largely matched Pc and 4 matched Pn. The two Pb clusters represented different groups of source populations that differed particularly in spur and labellum length. Again, the Px individuals did not form a separate cluster but were affiliated with clusters 1, 2 and 3, indicating both their morphological heterogeneity and their similarity to the parental species.
Discriminant analysis on flower data of the three taxa, allowed us to correctly classify 98.9% of samples. The first discriminant axis separated Pc perfectly from the rest with anther distance bottom (r = 0.984), anther distance top (r = 0.907), anther orientation (r = 0.806) and anther length (r = 0.784) being most strongly correlated to LD scores (Online Resource 3). The second discriminant axis separated Pb from Pn with spur length (r = 0.907), labellum length (r = 0.669) and petal length (r = 0.605) being the most important traits. However, one sample of Pn was predicted to be Pb.
Thus, distance between anthers and spur length are the two most influential of the investigated flower traits to distinguish the taxa (Fig. 3).
Molecular marker analysis
The DNA sequences of the nuclear ITS region (727 bp) showed very low levels of variation. Only a single position was found to be polymorphic (nrITS 1: A/C) within all taxa.
AFLP markers were highly polymorphic within all taxa. The PCoA of AFLP genotypes showed three major well-separated clusters, largely corresponding to P. chlorantha, P. bifolia and non-hybrid intermediates (Fig. 4). However, in contrast to all other Pc, those from one site (Sistig) clustered with Pn. Putative hybrids (Px) that originated from this particular site took intermediate positions between the two parental species of the same site, strongly corroborating their hybrid origin.
Principle coordinate analysis displaying genetic relationships among individual plants based on AFLP genotypes of Platanthera taxa from different sites. Colours correspond to morpho-taxa (Fig. 2a). PCoA axes 1 + 2 explained 66% of variation of the 6 extracted axes
Bayesian cluster analysis with STRUCTURE identified K = 3 gene pools. High ∆K values were obtained for both K = 2 and K = 3. However, at K = 2, P. bifolia together with most P. chlorantha formed one gene pool, while Pn together with the Pc population from Sistig formed the second gene pool. Separate analyses of the first gene pool revealed again two groups representing Pb and Pc. Overall, this strongly suggests the existence of three gene pools (Fig. 5, Online resource 4). The three gene pools are referred to as bifolia-, chlorantha- and non-hybrid intermediates-gene pools, because they largely corresponded to P. chlorantha, P. bifolia and non-hybrid intermediates, respectively (Fig. 2c). However, similar to the PCoA analysis, Pc from site Sistig was part of the non-hybrid intermediates-gene pool. The putative hybrids from this site were clearly identified as hybrids because they showed genotypes admixed from both the bifolia- and non-hybrid intermediates-gene pools (mean admixture proportion: 34 and 62%, respectively).
Bayesian cluster analysis based on AFLP genotypes with STRUCTURE at K = 3. Each narrow bar represents an individual and displays its affiliation (posterior probability q) to three identified gene pools, largely corresponding to P. bifolia subsp. bifolia (blue), non-hybrid intermediates (green) and P. chlorantha (red). For details see text. Sequence of populations from left to right corresponds to sequence in Figs. 2a and 4
Analysis of individual AFLP loci revealed that the differentiation patterns between taxa were largely due to differences in allele frequencies rather than to private alleles. Only seven, four and three AFLP markers were private to the taxa Pc, Pb and Pn, respectively, and had low marker frequencies (mean 10.7%, maximum 31%). However, when the gene pools identified by STRUCTURE were considered, six, four and seven private markers were found in the chlorantha-, bifolia- and non-hybrid intermediates-gene pools. Moreover, the non-hybrid intermediates-gene pool had three private markers with high, nearly fixed allele frequency (AAC_CTGG_343: 71%, ACC_CTGA_215: 87%, AGC_CACC_421: 91%), substantiating its genetic independence. Mean Nei’s genetic distance was more than twice as large between the non-hybrid intermediates-gene pool and the two other gene pools (Pn–Pb: mean d = 0.125; Pn–Pc: d = 0.130) than between the chlorantha and bifolia gene pools (Pb–Pc: d = 0.046).
Discussion
We found in Central European Platanthera a clear separation into three independent gene pools largely representing P. chlorantha, P. bifolia and plants informally referred to here as non-hybrid intermediates, the latter, although morphologically intermediate, being not of hybrid origin. The genetic differentiation was largely, but not completely, matched by morphological differentiation of flower traits. P. chlorantha was characterized by a large distance between anthers and downward divergence of anthers, P. bifolia by short distance between parallel anthers and on average short spurs and non-hybrid intermediates by an intermediate distance of anthers but long spurs. As a notable exception, one Pc population (Sistig) with typical morphology of P. chlorantha was part of the non-hybrid intermediates-gene pool and gave rise to hybrids with sympatric P. bifolia.
Non-hybrid intermediates are an independent group
Setting aside for the moment the P. chlorantha population in site Sistig (discussed below), we will first focus on the morphotypes referred to here as non-hybrid intermediates. Genetically, all plants of this morphologically defined group belonged to the non-hybrid intermediates-gene pool. This gene pool is clearly different from Pc and Pb and not of hybrid origin of these two. Its genetic distance from Pb and Pc is larger than that between Pb and Pc. In addition, it is characterized by several private AFLP markers in contrast to both Pb and Pc. Also morphologically, members of the non-hybrid intermediates-gene pool are different from Pc and Pb and are characterized by intermediate anther separation, but,—in general—longer spurs. Almost all flower traits differed significantly between Pb and Pn. Thus, the non-hybrid intermediates-gene pool is as much an own group as Pb and Pc.
Flower morphology of Pn as defined here largely corresponds to trait values given for P. bifolia subsp. latiflora (Løjtnant 1978) and P. fornicata (Table 1; Buttler 2011). However, both these taxa have explicitly been described as woodland forms in contrast to Pb, reportedly growing outside of forests (Løjtnant 1978; Buttler 2011). We consider these attributions as incomplete, as our samples of Pn inhabited either mostly calcareous grasslands or woodlands and thus seem to have a rather broad habitat affiliation. Similarly, our Pb populations were not restricted to open sites. Thus, because a simple attribution to a habitat may mislead biologists to premature species identification, we stress that a detailed inspection of flower traits is indispensable irrespective of the habitat.
Previously, populations of non-hybrid intermediates were obviously either neglected, i.e. they were included in a broadly defined P. bifolia s.l. It is an open question, how Pn is related to Scandinavian plants referred to as P. b. subsp. latiflora which seem to be widespread there (Krok et al. 2013) and to the long-spurred plants studied by Boberg et al. (2014). Alternatively, Pn were interpreted as P. × hybrida, as in Claessens and Kleynen (2006) and Claessens et al. (2008) who investigated the same sites as we did. Similar cases of “stable P. × hybrida “populations in which both parents are absent are known from Austria and Germany (Perko 1997; Künkele and Baumann 1998) and might also belong to Pn. Thus, the distribution of Pn is largely unknown, but potentially it is widely distributed. Field ecologists and botanical monitoring programmes therefore need to pay attention to these two forms. Obviously, a range wide analysis of morphological and genetic relationships in the bifolia/chlorantha group is urgently needed to resolve uncertainties.
Platanthera × hybrida
We have clearly shown that two populations previously designated as “stable” P. × hybrida (sites Wrakelberg and Wylre Akkers) which totally lack the parental species are not hybrids. However, in one sympatric population of Pb and Pc, where both were abundant (>1500 flowering plants), we found a small number, i.e. about 1% of each of the parental species’ abundance, of individuals with admixed genotype typical for early generation hybrids. In contrast, in another site with small sympatric populations of Pb and Pc, hybrids could not be found. This is consistent with the expectation that hybrids can be found in sympatric populations of Pb and Pc only (Nilsson 1985) and the finding that hybrids suffer from reduced reproductive fitness and thus likely are very rare (Foley and Clarke 2005; Baum and Baum 2011). Although it might be possible that hybrids can build-up populations in the absence of the parental species through colonization of new habitats by seed, this is quite unlikely given the rarity of the hybrids within established populations.
Whether P. × hybrida can morphologically be confidently differentiated from non-hybrid intermediates is difficult to assess, because our sample size of P. × hybrida was small and morphologically very variable and thus allows only preliminary conclusions. Both taxa have intermediate distance of anthers (1–2 mm), but Pn has longer spurs and labella, which might allow their distinction from P. × hybrida, should they co-occur. Moreover, our hybrid individuals derived from a cross between Pb and a Pc population that was part of the non-hybrid intermediates-gene pool and thus no typical Pc. Clearly, many more samples of genetically verified P. × hybrida need to be considered for a thorough assessment.
Evolutionary dynamics, pollination and reproductive isolation
Since Darwin the distance between anthers, and in particular viscidia, in European Platanthera has been considered to represent a dichotomy with either narrow (Pb) or widely separated (Pc) viscidia. As Darwin has shown, this system is driven by lepidopteran pollinators to which anthers are considered to be not perfectly attached when viscidia have intermediate distances. This scenario has been validated for P. × hybrida (Nilsson 1983) which suffers a large disadvantage in pollen receipt relative to parents as a result of both anther separation and spur length. Anther separation in Pn is also intermediate between Pb and Pc but still relatively narrow (1–2 mm). However, as evidenced by high seed set in the investigated Pn populations (pers. obs.) and documented lepidopteran pollinators carrying pollinaria on their proboscis similar to Pb (Claessens et al. 2008), pollination seems not to be hindered in non-hybrid intermediates. Still, differences in anther separation between the taxa, particularly between Pc and Pn, will contribute to reproductive isolation. However, it is an open question whether the difference of anther separation between Pb and Pn is sufficiently large to lead to incompatible placement of pollinia.
Spur length in the Platanthera bifolia/chlorantha group has been of considerable interest as it varies widely ranging from 15 to >50 mm. It has been shown to be under strong selection by pollinators via the length of their proboscis (Maad 2000; Maad and Alexandersson 2004; Maad and Nilsson 2004; Boberg and Ågren 2009; Boberg et al. 2014). In his studies, Boberg and co-workers (Boberg and Ågren 2009; Boberg et al. 2014) investigated both late flowering, short-spurred grassland populations and early flowering, long-spurred woodland populations of P. bifolia s.l. Although it is unknown how their long-spurred relates to our Pn, there is no reason to doubt that similar selective plant–pollinator interactions will act. Thus, lepidopteran pollinators with different proboscis length might be an additional selective force contributing to reproductive isolation and genetic separation between the Pb and Pn gene pools. However, comprehensive data on the moth species, and respective proboscis length, that pollinate Pb and Pn in Central Europe are lacking. Moreover, spur length has been shown to be affected by other factors, too. For example, it increases during anthesis and may be affected by resource status, and thus trait values depend on the timing and locality and may be biased between studies (Bateman and Sexton 2008). However, overall, the studies that considered two taxa within P. bifolia s.l. report highly consistent ranges for spur length (Table 1). In this study, spur length similarly varied widely and average spur length increased from Pb to Pc to Pn. The three genetic groups showed large intragroup variation in spur length, particularly Pb, in which spur length differed strongly between particular source populations. However, although some overlap among groups was found, spur length had diagnostic value in the discriminant analysis where it was the main trait that allowed to distinguish Pb from Pn. Thus, both, anther distance and spur length seem to be central flower traits that need to be considered simultaneously when studying differentiation and selection in the P. bifolia/chlorantha group.
Temporal and spatial isolation represents additional mechanisms of reproductive isolation which additionally need to be considered. Flowering is reported to commence 2–3(–4) weeks earlier in Pn compared to Pb (Müller 1868; Løjtnant 1978; Buttler 2011). Thus, although individual plants flower for several weeks allowing for some overlap of flowering periods, some phenological isolation is highly likely. However, flowering times of both Pb and Pn overlap with that of Pc. Spatial isolation may exist first on a large scale, i.e. concerning species ranges, which was hypothesized by Løjtnant (1978) who suggested that Pb has an Atlantic distribution, whereas P. latiflora is supposed to be more continentally distributed in Central Europe. Second, on a local scale affiliation to different habitats or to different soil chemistry may contribute to reproductive isolation between Pb and Pn. However, as discussed above, a simple contrast of forest versus non forest is a misleading oversimplification.
Incongruence of phenotype and genotype of populations in Sistig
One of the populations examined, Sistig, which represents morphologically typical P. chlorantha, belonged to the Pn gene pool. There is no doubt on these results as it was exactly this population where we detected hybrids that showed both intermediate morphology and intermediate, i.e. admixed, genotypes. This affiliation of some Pc to the Pn gene pool impairs the congruence between genotype and phenotype of both Pc and Pn. We can only hypothesize on scenarios leading to this conflicting situation. In Platanthera, floral traits are labile and/or sensitive to selection (Efimov 2011). Recently, Bateman et al. (2012) even speculated about the genetic basis underlying floral variation in the P. bifolia/chlorantha group suspecting that a single gene might be responsible for variation in gynostemium width. Thus, assuming that a very limited number of genes are involved, first, spontaneous mutation within Pn in site Sistig may bring about the observed morphological switch to a Pc morphotype. Second, as the genetic affiliation to the Pn gene pool is unequivocal, another potential scenario might be that within the genetic background of the Pn gene pool recent selection, acting on a genetic polymorphism, has driven floral morphology from Pn to Pc. Pollinator shifts have been shown to lead to fitness gain and drive floral morphology from P. bifolia towards P. chlorantha (Maad and Nilsson 2004), which may be an underlying ecological mechanism. Lastly, other mechanisms might be involved in the switch from one morphotype to another, like mycorrhizal associates (Stark et al. 2009; Bateman et al. 2014), or epigenetic modifications (Paun et al. 2010, 2011) which may affect phenotypes in orchids. The incongruence of phenotype and genotype in this case may finally, however, question the taxonomic concept of the bifolia/chlorantha group. Therefore, it is important to assess how common the observed phenomenon actually is.
Conclusions
This study clearly shows that the P. bifolia/chlorantha group consists of at least three gene pools which largely also represent three morphological groups, namely P. chlorantha, P. bifolia s.str. and a taxon informally referred to here as non-hybrid intermediates. To further consolidate the status of the latter we suggest analysing variable molecular markers, flower morphology including traits like anther separation and caudicula length, and pollination biology. In doing so, we advocate to investigate the whole geographic range of the P. bifolia/chlorantha group and advise against only regional assessments, particularly if restricted only to peripheral areas, which are unable to yield a comprehensive picture. The fact that the non-hybrid intermediates previously were either neglected and included in a larger P. bifolia s.l. or had been mistaken as P. × hybrida, necessitates a reevaluation of our understanding of the distribution and ecology of these taxa. In addition, the large body of studies on the evolution of floral morphology and pollinator-driven selection in the group need to be reevaluated in the light of the strong genetic differentiation of three groups shown here.
References
AHO Thüringen eV (2014) Thüringens Orchideen, p 864. Arbeitskreis Heimische Orchideen Thüringen, Uhlstädt-Kirchhasel
Babington CC (1836) On several new or imperfectly understood British and European Plants. Trans Linn Soc Lond 17:451–464
Bateman RM, Sexton R (2008) Is spur length of Platanthera species in the British Isles adaptively optimized of an evolutionary red herring? Watsonia 27:1–21
Bateman RM, James KE, Luo YB, Lauri RK, Fulcher T, Cribb PJ, Chase MW (2009) Molecular phylogenetics and morphological reappraisal of the Platanthera clade (Orchidaceae: Orchidinae) prompts expansion of the generic limits of Galearis and Platanthera. Ann Bot (Oxford) 104:431–445. doi:10.1093/aob/mcp089
Bateman RM, James KE, Rudall PJ (2012) Contrast in levels of morphological versus molecular divergence between closely related Eurasian species of Platanthera (Orchidaceae) suggests recent evolution with a strong allometric component. New J Bot 2:110–148
Bateman RM, Rudall PJ, Bidartondo MI, Cozzolino S, Tranchida-Lombardo V, Carine MA, Moura M (2014) Speciation via floral heterochrony and presumed mycorrhizal host switching of endemic butterfly orchids on the Azorean archipelago. Amer J Bot 101:979–1001. doi:10.3732/ajb.1300430
Baum A, Baum H (2011) Zweiblättrige Waldhyazinthe, Platanthera bifolia (L.) Rich., ein Beitrag zur Orchidee des Jahres 2011 in Deutschland. J Eur Orch 43:15–34
Bisse J (1963) Ein Beitrag zur Kenntnis der deutschen Orchideenflora. Feddes Repert 67:181–189
Boberg E, Ågren J (2009) Despite their apparent integration, spur length but not perianth size affects reproductive success in the moth-pollinated orchid Platanthera bifolia. Funct Ecol 23:1022–1028
Boberg E, Alexandersson R, Jonsson M, Maad J, Ågren J, Nilsson LA (2014) Pollinator shifts and the evolution of spur length in the moth-pollinated orchid Platanthera bifolia. Ann Bot (Oxford) 113:267–275. doi:10.1093/aob/mct217
Bruegger CG (1882) Platanthera hybrida. Jahresb Naturf Ges Graub 25:107–108
Brzosko E, Wróblewska A, Tałałaj I, Adamowski W (2009) Patterns of genetic diversity in Platanthera bifolia (Orchidaceae) with respect to life history traits and recent range expansion. Folia Geobot 44:131–144
Buttler KP (2011) Revision von Platanthera bifolia sensu lato. Jahresber Wetterauischen Ges Gesammte Naturk 159–161:93–108
Claessens J, Kleynen J (2006) Anmerkungen zur Hybridbildung bei Platanthera bifolia und P. chlorantha. J Eur Orch 38:3–28
Claessens J, Gravendeel B, Kleynen J (2008) Cucullia umbratica L. als Bestäuber von Platanthera x hybrida Bruegg. in Süd-Limburg (Niederlande). J Eur Orch 40:73–84
Corander J, Marttinen P, Siren J, Tang J (2008) Enhanced Bayesian modelling in BAPS software for learning genetic structures of populations. BMC Bioinform 9:539. doi:10.1186/1471-2105-9-539
Darwin C (1877) The various contrivances by which orchids are fertilised by insects, 2nd edn. John Murray, London
Drejer S (1843) Critiske Bemærkninger om nogle danske Orchideer. Naturhistor Tidskr 4:45–70
Durka W, Michalski SG, Berendzen KW, Bossdorf O, Bucharova A, Hermann JM, Hölzel N, Kollmann J (2017) Genetic differentiation within multiple common grassland plants supports seed transfer zones for ecological restoration. J Appl Ecol 54:116–126. doi:10.1111/1365-2664.12636
Efimov P (2011) An intriguing morphological variability of Platanthera s.l. Eur J Environ Sci 1:125–136
Efimov PG (2016) A revision of Platanthera (Orchidaceae, Orchidinae, Orchideae) in Asia. Phytotaxa 254:1–233
Evanno G, Regnaut S, Goudet J (2005) Detecting the number of clusters of individuals using the software STRUCTURE: a simulation study. Molec Ecol 14:2611–2620
Falush D, Stephens M, Pritchard JK (2007) Inference of population structure using multilocus genotype data: dominant markers and null alleles. Mol Ecol Notes 7:574–578
Foley MSY, Clarke S (2005) Orchids of the British Isles. Griffin Press, Cheltenham
Fraley C, Raftery AE, Scrucca L (2015) Package ‘mclust’, normal mixture modelling for model-based clustering, classification and density estimation. Technical Report no. 597, Department of Statistics, University of Washington
Hapeman JR, Inoue K (1997) Plant-pollinator interactions and floral radiation in Platanthera (Orchidaceae). In: Givnish TJ, Sytsma KJ (eds) Molecular evolution and adaptive radiation. Cambridge University Press, Cambridge, pp 433–454
Jäger EJ (2011) Rothmaler—Exkursionsflora von Deutschland. Gefäßpflanzen: Grundband. Elsevier, Heidelberg
Jombart T, Devillard S, Balloux F (2010) Discriminant analysis of principal components: a new method for the analysis of genetically structured populations. BMC Genet 11:94
Krok T, Almquist S, Jonsell L, Jonsell B (2013) Svensk flora: Fanerogamer och kärlkryptogamer. Liber, Stockholm
Künkele S, Baumann H (1998) Platanthera bifolia (L.) Rich. × P. chlorantha (Custer) Rchb. = Platanthera × hybrida Brügger 1882. In: Sebald O, Seybold S, Philippi G, Wörz A (eds) Die Farn- und Blütenpflanzen Baden-Württembergs, vol 8. Ulmer, Stuttgart, pp 456–458
Løjtnant B (1978) Nomenclatural notes upon Scandinavian orchids. Feddes Repert 89:13–18
Maad J (2000) Phenotypic selection in hawkmoth-pollinated Platanthera bifolia: targets and fitness surfaces. Evolution 54:112–123
Maad J, Alexandersson R (2004) Variable selection in Platanthera bifolia (Orchidaceae): phenotypic selection differed between sex functions in a drought year. J Evol Biol 17:642–650
Maad J, Nilsson LA (2004) On the mechanism of floral shifts in speciation: gained pollination efficiency from tongue- to eye-attachment of pollinia in Platanthera (Orchidaceae). Biol J Linn Soc 83:481–495. doi:10.1111/j.1095-8312.2004.00406.x
Müller H (1868) Beobachtungen an westfälischen Orchideen. Verhandl. Naturhist. Verein der Preußischen Rheinlande u Westfalen 25:1–62
Nilsson LA (1978) Pollination ecology and adaptation in Platanthera chlorantha (Orchidaceae). Bot Not 131:35–51
Nilsson LA (1983) Processes of isolation and introgressive interplay between Platanthera bifolia (L.) Rich. and P. chlorantha (Custer) Reichb. (Orchidaceae). Bot J Linn Soc 87:325–350
Nilsson LA (1985) Characteristics and distribution of intermediates between Platanthera bifolia and P. chlorantha (Orchidaceae) in the Nord countries. Nordic J Bot 5:407–419. doi:10.1111/j.1756-1051.1985.tb01670.x
Paun O, Bateman RM, Fay MF, Hedrén M, Civeyrel L, Chase MW (2010) Stable epigenetic effects impact adaptation in allopolyploid orchids (Dactylorhiza: Orchidaceae). Molec Biol Evol 27:2465–2473. doi:10.1093/molbev/msq150
Paun O, Bateman RM, Fay MF, Luna JA, Moat J, Hedren M, Chase MW (2011) Altered gene expression and ecological divergence in sibling allopolyploids of Dactylorhiza (Orchidaceae). BMC Evol Biol. doi:10.1186/1471-2148-11-113
Pavarese G, Tranchida-Lombardo V, Cogoni A, Cristaudo A, Cozzolino S (2011) Where do Sardinian orchids come from: a putative African origin for the insular population of Platanthera bifolia var. kuenkelei? Bot J Linn Soc 167:466–475. doi:10.1111/j.1095-8339.2011.01190.x
Peakall R, Smouse PE (2012) GenAlEx 6.5: genetic analysis in excel. Population genetic software for teaching and research—an update. Bioinformatics 28:2537–2539. doi:10.1093/bioinformatics/bts460
Pedersen HAE, Faurholdt N (2010) Danmarks vilde orkidéer. Gyldendal, Kopenhagen
Perko M (1997) Beobachtungen zu einige Hybriden aus der Familie der Orchideen (Orchidaceae) in Kärnten/Österreich inkl. Dactylorhiza x juennensis M. PERKO, nothosp. nat. nov. Carinthia II 187:89–101
R Core Team (2015) R: a language and environment for statistical computing. R foundation for statistical computing, Vienna. Available at: http://www.R-project.org
Reichenbach HGL (1831) Iconographica Botanica. Hofmeister, Leipzig
Schulze M (1894) Die Orchidaceen Deutschlands, Deutsch-Österreichs und der Schweiz [reprint 1993] F. Eugen Köhlers, Gera
Stark C, Babik W, Durka W (2009) Fungi from the roots of the common terrestrial orchid Gymnadenia conopsea. Mycol Res 113:952–959
Stark C, Michalski SG, Babik W, Winterfeld G, Durka W (2011) Strong genetic differentiation between Gymnadenia conopsea and G. densiflora despite morphological similarity. Pl Syst Evol 293:213–226. doi:10.1007/s00606-011-0439-x
van der Pijl L, Dodson CH (1966) Orchid flowers: their pollination and evolution. University of Miami Press, Miami
Vekemans X (2002) AFLP-SURV version 1.0. Distributed by the author. Laboratoire de Génétique et Ecologie Végétale, Université Libre de Bruxelles, Belgium
Wallroth FW (1822) Schedulae criticae de plantis florae Halensis selectis. Kümmeli, Halle
White TJ, Bruns T, Lee S, Taylor J (1990) Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics. In: Innis MA, Gelfand DH, Sninsky JJ, White TJ (eds) PCR protocols: a guide to methods and applications. Academic Press, New York, pp 315–322
Acknowledgements
We thank J. and M. Kleynen, J. Claessens and W. Hahn for their support for our field studies, and H. Baumgartner, K. Kergel, B. Margenburg, K. Heyde, V. Kögler, P. Müller for their help in collecting samples. We also wish to thank B. v. d. Vijver for constructive discussions. We are grateful to the authorities of the district governments Euskirchen, Steinfurt and Coesfeld and to the relevant authorities in Belgium and the Netherlands for sampling permissions.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Handling editor: Andreas Tribsch.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Information on Electronic supplementary material
Information on Electronic supplementary material
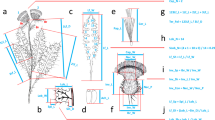
Online Resource 1 Photos of flowers and pollinaria of non-hybrid intermediates, P. chlorantha and P. bifolia with indication of measured traits.
Online Resource 2 Box plots of vegetative characters for investigated Platanthera taxa.
Online Resource 3 Linear discriminant analysis of P. chlorantha, P. bifolia and non-hybrid intermediates based on flower traits.
Online Resource 4 Details of the STRUCTURE analysis of AFLP data of Platanthera species, including results at K = 2.
Online Resource 5 The morphological dataset.
Online Resource 6 The AFLP dataset.
Rights and permissions
About this article
Cite this article
Durka, W., Baum, A., Michalski, S.G. et al. Darwin’s legacy in Platanthera: are there more than two species in the Platanthera bifolia/chlorantha group?. Plant Syst Evol 303, 419–431 (2017). https://doi.org/10.1007/s00606-016-1381-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00606-016-1381-8