Abstract
The genus Leucheria Lag. (Asteraceae Bercht. and J. Presl, tribe Nassauvieae Cass.) comprises 45 species and three infraspecific taxa distributed in the Andean region from southern Chile and Argentina to Peru. Six species are annual herbs. The genus has had a long taxonomic history involving the transference of species described originally under many different genera. The main objectives of this paper were to determine the phylogenetic relationships of species of Leucheria, examine the hypothesis that the ancestor of Leucheria would have originated in a forested habitat and examine the validity of nine morphologically defined evolutionary lines recognized in earlier work on the genus. Additionally we investigated whether the annual species of Leucheria are derived. We extracted DNA from leaf material for 45 taxa (94%) of Leucheria. We used Bayesian inference and plastid and nuclear genes to construct a phylogenetic hypothesis. Results show that Leucheria is monophyletic and is comprised of two main clades. One clade comprises perennial acaulescent/subacaulescent species, all with a solitary capitulum. We recognized three lineages in the second clade comprised of caulescent species that exhibit multiple capitula. Optimization of life-form over the phylogeny showed that five of the six annual species studied are derived in our tree. We conclude that the appearance of the annual habit is associated with the colonization of arid conditions in the winter rainfall coastal desert of northern Chile. Our result shows that species of Leucheria from forested habitats are derived. Discrepancies with previously recognized morphologically defined evolutionary lines were detected.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Southern South America houses many genera and tribes of Asteraceae, the second most species rich and a widely cosmopolitan family of angiosperms (Stevens 2001; Panero and Crozier 2016). A Middle Eocene fossil from northwestern Patagonia and climatic reconstruction of the fossil-bearing locality suggest that Asteraceae may have formed part of a diverse tropical to subtropical vegetation assemblage in southern South America (Wilf et al. 2005; Barreda et al. 2010). The fossil documents the divergence of Mutisioideae s.l. and Carduoideae from Barnadesioideae at least 47.5 Ma (Barreda et al. 2010). Here we undertake a molecular phylogenetic reconstruction of the genus Leucheria Lag. belonging to the tribe Nassauvieae Cass. of the large predominantly South America subfamily Mutisioideae (Cass.) Lindl. Tribe Nassauvieae contains 25 genera (Katinas et al. 2008b). However, to date, molecular phylogenies for the tribe Nassauvieae have only been constructed for Perezia (Simpson et al. 2009) and Nassauvia (Maraner et al. 2012).
Leucheria is exclusively South American, occurring from 11°N in Peru to 54°S (55) in Tierra del Fuego (Fig. 1), where it is found in forests, Mediterranean-type climate vegetation, winter rainfall coastal desert, Andean vegetation, Magellanic tundra and Patagonian steppe. The great majority of the species occur in the high Andes and in the Mediterranean-type climate area of central Chile. All Leucheria species are herbaceous, with both annual (six species) and perennial (39 species) species represented. Species are acaulescent/subacaulescent with a single large capitulum or caulescent with numerous, small capitula disposed in cymose or paniculate-like cymose inflorescences (Crisci 1976).
The genus Leucheria has a long history involving transferences of numerous currently unrecognized genera. In his comprehensive monograph of Leucheria, Crisci (1976) recognized 46 species and one subspecies. Katinas et al. (2008b, c) recognized 47 species, and Moreira-Muñoz et al. (2012) recognized 43 species and two subspecies for Chile. Currently 45 species, two subspecies and one variety are recognized in the flora of the Southern Cone (Zuloaga et al. 2008, and it is including Ratto et al. 2014).
The ancestor of Leucheria according to Crisci (1976) would have been a large caulescent forest-dwelling species, with the leaves distributed along the entire stem and bearing a paniculate-cymose inflorescence with large capitula. This ancestor gave rise to smaller herbaceous plants that subsequently colonized a wide range of drier habitats in South America. Crisci (1976) assigned species of the genus to nine evolutionary lines based mainly on characteristics of the stem, inflorescence morphology, capitulum size and distributional area.
Phylogenetic studies show that annual species are derived from a perennial ancestor (Datson et al. 2008; Cruz-Mazo et al. 2009). However, transitions in the reverse direction have also been reported. For example, Bena et al. (1998) showed that perennial species of Medicago (Fabaceae) were derived from an annual lineage, Tank and Olmstead (2008) showed that the perennial clade of Castilleja (Orobanchaceae) with 160 species was derived from an annual ancestor, and Eastwood et al. (2008) and Drummond (2008) showed the perennial species of Lupinus were derived. If Leucheria arose in a forested habitat as suggested by Crisci (1976), the annual species of Leucheria are likely to have evolved later as the genus spread northward into more arid conditions and thus be derived.
The main objectives of this paper were to determine the phylogenetic relationships of species of Leucheria as presently circumscribed, test the hypothesis that the ancestor of Leucheria evolved in a forested habitat as proposed by Crisci (1976) and examine whether the nine lineages proposed by this author based on morphological characters are monophyletic. To answer these questions, we constructed a molecular phylogeny of Leucheria based on the ITS of the rDNA and the trnL intron and trnL-trnF spacer and rpl32 intron of the cpDNA, identified clades within the genus and compared the results with Crisci’s (lines) informal infrageneric phylogeny. We investigated where the perennial and annual species are placed in the phylogeny.
Methodology
Taxon sampling
We collected material of Leucheria species and outgroups for DNA extraction in the field. Samples were stored in silica. Vouchers are deposited in the herbaria HULS, CONC and SGO. For some species of Leucheria, we also sampled herbarium specimens in the collections of the herbaria listed above. We also downloaded sequences for some species from GenBank. The total number of Leucheria taxa considered was 45, representing 94% of all currently recognized taxa (Table 1). We also sampled 18 representatives of the tribe Nassauvieae found in other studies to be closely related to Leucheria (Kim et al. 2002; Katinas et al. 2008a; Panero and Funk 2008; Gruenstaeudl et al. 2009). Given the sister relationship to Asteraceae with Calyceraceae (Kim and Jansen 1995; Lundberg and Bremer 2003), Nastanthus scapigerus was chosen as outgroup.
DNA extraction, amplification and sequencing
Genomic DNA was extracted with the DNeasy Plant Kit (Qiagen, Valencia, CA, USA). We amplified the DNA using the primers listed in Table 2. PCR used a final volume of 30 µL, which contained 4 μl DNA (25 ng/μl), 8.35 μl distilled water, 3 μl MgCl2 (25 mM), 6 μl buffer, 2.4 μl of dNTP (1 mM), 1.8 μl of each primer (10x), 2.4 μl BSA (25 mM) and 0.25 μl GoTaq (5 U/μl). DNA was denatured at 95 °C for 5 min, followed by 35 amplification cycles of 45 s at 94 °C, annealing for 1 min at 50 °C, elongation for 1.5 min at 72 °C and a final extension of 7 min at 72 °C. Samples were sent to Macrogen (Seoul, South Korea) for purification and sequencing. Sequences were loaded, edited and aligned using ChromasPro 2.33 (Technelysium, Brisbane, Australia) and BioEdit 7.0 (Hall 1999) and have been deposited in GenBank (Table 1).
Phylogenetic analysis, character reconstruction and evolutionary lines
We performed a combined analysis for sequences of the nuclear gene ITS (White et al. 1990) and the two chloroplast genes, trnL-trnF (Taberlet et al. 1991) and rpl32 (Shaw et al. 2007) (Table 2). Bayesian inference analyses were performed with MrBayes (Ronquist and Huelsenbeck 2003). For the combined analysis with Bayesian inference, three partitions were used corresponding for each genes (ITS, trnL-trnF and rpl32), in which evolutionary models for each one were: GTR+G in ITS; GTR+I+G in trnL-trnF; and GTR+I in rpl32. The Tracer program (v1.6, Rambaut et al. 2003–2013) was used to visualize output parameters in order to prove stationarity and if there are duplicated runs or not to converged on the same mean likelihood. Runs appeared stationary prior to 206 generations, and we conservatively excluded the first 2.0 × 106 generations of each run as burn-in. The effective sample size (ESS) value was greater than 200 in a range between 8076 and 32,250. Nodes with ≥0.95 were considered to be supported for posterior probabilities (Ronquist and Huelsenbeck 2003).
Life-form reconstruction was performed on the combined Bayesian tree using parsimony and maximum likelihood methods as implemented in Mesquite 2.74 (Maddison and Maddison 2008). Life-form character was scored as annual or perennial according to information given in Crisci (1976) (Table 1). To analyze the evolutionary lines proposed by Crisci (1976), the nine evolutionary lines were compared with the molecular phylogenetic relationships obtained in this study (Online Resource 1).
Results
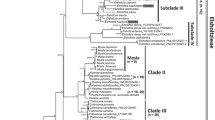
The data matrix contained 3631 nucleotide characters (945 ITS, 1139 trnL-trnF and 1547 rpl32). The pattern indicated in this study was maintained in all cases. The few differences with the combined analysis are due to: (a) genes with different evolutionary history (maternal and bi-parental inherence); (b) not all trees were constructed with the same number of species, since not all the genes could be amplified in some samples; (c) the robustness level of many nodes in each of the trees was low, but these strengthened when information from genes was combined; and (d) in the combined analysis secondary signals appeared that were not observed in the analysis of the genes separately. Topologies based on individual markers are shown in online resources 2, 3 and 4. The combined analysis shows that the genus Leucheria is monophyletic with respect to the groups considered (Fig. 2). Consequently, all species belonging to genera synonymized under Leucheria are part of Leucheria as originally circumscribed by Crisci (1976). Included here are L. floribunda and L. nutans originally described in Eizaguirrea and Clybatis, respectively, and which according to Crisci (1976) are somewhat atypical in the genus.
Bayesian inference phylogenetic tree based on combined analysis (rDNA and cpDNA) for taxa of the genus Leucheria and 19 taxa belong to the Calyceraceae and the tribe Nassauvieae. Posterior probability values are found above the branches. Asterisks indicate nodes without support (<0.90). Gray lines indicate the external group, and black lines the internal group
The topology revealed a primary division between acaulescent/subacaulescent species with solitary capitula (Clade A) and caulescent species with numerous capitula (Clade B). The only exception to this observation is L. achillaeifolia, which although caulescent and its inflorescence has multiple capitula, is part of Clade A. Clade A contains 16 taxa of perennial herbs. Three well-supported lineages were identified in Clade B. Subclade I comprises two species (L. coerulescens and L. floribunda), both of which are perennial herbs; Subclade II contains 13 taxa, of which three are annuals; Subclade III contains 14 taxa, including two annual species (Fig. 3). These last three lineages unlike A and B cannot be separated by any obvious morphological characteristic.
Bayesian inference phylogenetic tree based on combined analysis (rDNA and cpDNA) for 45 taxa of the genus Leucheria and taxa belong to the Calyceraceae and the Tribe Nassauvieae. Posterior probability values are found above the branches. Asterisks indicate nodes without support (<0.90). Gray lines indicate the external group, and black lines the internal group. The principal clades are denoted by letter A (acaulescent/subacaulescent species with solitary capitula) and B (caulescent species with numerous capitula). Subclades (Sc) are denoted by roman numerals (I, II, III). The colors of the lines indicate the nine evolutionary lines recognized by Crisci (1976): light green Line L. thermarum; yellow Line L. glacialis; red Line L. amoena; blue Line L. cerberoana; dark green Line L. candidisima; orange Line L. salina; cyan Line L. achillaeifolia; violet Line L. nutans, pink Line L. floribunda and black taxa not considered by Crisci (1976)
Table 1 shows the vegetation type for each species of Leucheria. The three forest species are found in different subclades (Subclade I: L. coerulescens; Subclade III: L magna and L. thermarum) and not sister to all other species of Leucheria. Species sister to the forest dwellers are found predominantly in non-forested habitats. Thus, it is unlikely that the Leucheria clade arose from an ancestor that inhabited forest.
We found incongruences between the evolutionary lines proposed by Crisci (1976) and our phylogenetic results (Fig. 3). Species of the L. thermarum line are dispersed throughout Subclade I and III. Species in each of the L. glacialis, L. amoena and L. cerberoana lines are found in Subclade II and III. Species of the L. salina and L. candidissima line are all found in Clade A, but are not monophyletic. The L. achillaeifolia and L. nutans lines, each with a single species, are also in Clade A, whereas the L. floribunda line, also with a single species, is part of Clade B, Subclade I (Fig. 3).
Reconstruction of life-form showed that the annual herbs in Leucheria, found in Subclade II and III are derived (Figs. 3, 4). Two of the five annuals included in the phylogeny form a single clade within Subclade II, indicating that while the annual habit arose from the perennial habit, some annuals in Leucheria have given rise to other annual species. These particular annual species inhabit the coastal winter rainfall desert in northern Chile (L. cerberoana and L. cumingii) as L. menana. The remaining annuals (L. tenuis y L. oligocephala) form a clade in Subclade III and occur at low to mid-elevations in the Mediterranean-type climate area of central Chile (Figs. 3, 4).
Discussion
According to our results, the sister group to Leucheria according to the outgroups used, is a clade formed by monotypic Marticorenia and Moscharia (with two closely related species). Leucheria’s closest relatives according to Crisci (1976) are Holocheilus and Macrachaenium. Kim et al. (2002) using cpDNA concluded that the sister group of Leucheria was Jungia. Gruenstaeudl et al. (2009) using cpDNA placed Leucheria in a clade with Perezia and Nassauvia. According to Katinas et al. (2008a), based on cpDNA, the sister group to Leucheria is Polyachyrus. We were unable to extract quality DNA from Polyachyrus, which prevented us from verifying Katinas et al. (2008a) findings. Panero and Funk (2008) using cpDNA concluded that Leucheria is sister to a clade formed by Acourtia, Dolichlasium, Jungia, Nassauvia, Perezia and Trixis. Resolution of this important issue for understanding the evolutionary history of tribe Nassauvieae Cass., subfamily Mutisioideae clearly requires consideration of all genera recognized in the Nassauvieae tribe.
Crisci (1976) proposed that Leucheria descended from a forest-dwelling ancestor. In the topology presented here, the three forest species of Leucheria (L. magna, L. coerulescens, L. thermarum) appear in two derived subclades. Thus, it is unlikely that the ancestor of Leucheria grew in a forested habitat. The great majority of species in Leucheria are found in open, non-forested habitats. From there, species have evolved into forests on at least two occasions.
Our results suggest Leucheria underwent a major split between acaulescent plants with solitary capitula (Clade A) and caulescent plants with multiple capitula (Clade B comprised of three subclades). In Leucheria, the acaulescent habitat is probably primitive, but more work and dating are required to ascertain this conclusion. Species in Clade A are found principally in high Andean vegetation from the extreme southern part of the South American continent to southern Peru. The acaulescent habitat is common in high elevation species (Fabbro and Körner 2004), where plants of short stature are favored under the harsh environmental conditions. Species belonging to the three caulescent lineages (Subclades I, II, III) are distributed principally in sub-Andean vegetation, Mediterranean-type vegetation and coastal desert (Table 1). Species found at relatively lower elevations, as are the species in these three subclades are likely to have evolved well-developed stems because of competition for light with other species in taller vegetation.
Molecular analysis of cpDNA suggests that the caulescent state is basal in the genus Rheum (Sun et al. 2012). In the genus Streptocarpus, the basal species are caulescent species, but shifts to the acaulescent habit have also occurred (Möller and Cronk 2001). In Viola, derived clades can include both caulescent and acaulescent species (Ballard and Sytsma 2000). Evolutionary transitions between the caulescent and acaulescent state will depend on the sequence of habitats colonized in evolutionary time. For example, acaulescent species are common in high alpine environments. When a lineage descends from high elevation, it is likely to acquire a caulescent habit; to the contrary, if a linage colonizes into the high altitude environment from lower elevation, the direction of evolution is likely to be from caulescent to acaulescent.
The discrepancies found between the nine morphologically defined evolutionary lines recognized by Crisci (1976) and the lineages identified in this study are mainly due to the types of characters that were used to categorize the nine lines. Morphological characteristics, such as amount of pubescence or plant size, can be highly homoplastic (Álvarez et al. 1999) and show similarities without an ancestor–descendent relationship. Consequently, morphological and molecular characters commonly show incongruences (Hillis and Wiens 2000). Moreover, characteristics such as plant size can be affected by environmental factors (Goyenechea and Contreras-Ramos 2007). Crisci (1976) omitted two species from his nine evolutionary lines (L. papillosa and L. glandulosa). In the topology presented here, these species are found in Clade A and Subclade III, respectively.
The results showed that the annual habit in Leucheria is a derived condition that evolved on two independently occasions in Subclades II and III. The three annual herbs in Subclade II (L. cerberoana, L. cumingii and L. menana) inhabit the Chilean coastal winter rainfall desert characterized by extremely low and unpredictable rainfall. Rainfall varies from 100 to 120 mm/year in the southern part of the winter rainfall desert to 14 mm/year in the northern sector near Antofagasta. Annuals in Subclade III (L. tenuis and L. oligocephala) are found in the northern part of central Chile with a Mediterranean-type climate, where rainfall is less than 250–700 mm/year. In general, annuals are favored in desert and Mediterranean-type climates. Annuals can avoid long dry seasons and unpredictable rainfall as seed banks and have the capacity for high growth rates during the humid season (Schaffer and Gadgil 1975; Evans et al. 2005; Jara et al. 2006; Rivero et al. 2007). Annuals are favored in deserts also because of the abundance of open space, which enhances seedling survival (Schaffer and Gadgil 1975; Crawley 1997; Dimmitt 2000; Silvertown and Charlesworth 2001; Evans et al. 2005). In central Chile, the frequency of annual species in the vascular plant flora increases with the degree of aridity (Arroyo et al. 1995), and it is known that the annual species have longer lived seed banks than perennial species in Chaetanthera (Arroyo et al. 2006). Although no experimental work has been undertaken to detect seed banks in Leucheria, it is very likely that the annual species will turn out to have persistent seed banks.
References
Álvarez Y, Juste J, Tabares E, Garrido-Pertierra A, Ibáñez C, Bautista JM (1999) Molecular phylogeny and morphological homoplasy in fruitbats. Molec Biol Evol 16:1061–1067
Arroyo MTK, Zedler PH, Fox MD (eds) (1995) Ecology and biogeography of mediterranean ecosystems in Chile, California and Australia, California and Australia. Springer, New York. doi10.1007/978-4612-2490-7
Arroyo MT, Chacon P, Cavieres LA (2006) Relationship between seed bank expression, adult longevity and aridity in species of Chaetanthera (Asteraceae) in Central Chile. Ann Bot (Oxford) 98:591–600. doi:10.1093/aob/mcl134
Ballard HE, Sytsma KJ (2000) Evolution and biogeography of the woody Hawaiian violets (Viola, Violaceae): arctic origins, herbaceous ancestry and bird dispersal. Evolution 54:1521–1532. doi:10.1111/j.0014-3820.2000.tb00698.x
Barreda VD, Palazzesi L, Tellería MC, Katinas L, Crisci JV, Bremer K, Passalia MG, Corsolini R, Rodríguez Brizuela R, Bechis F (2010) Eocene patagonia fossils of the daisy Family. Science 329:1621. doi:10.1126/science.1193108
Bena G, Lejeune B, Prosperi JM, Olivieri I (1998) Molecular phylogenetic approach for studying life-history evolution: the ambiguous example of the genus Medicago L. Proc R Soc Lond B 265:1141–1151. doi:10.1098/rspb.1998.0410
Crawley MJ (1997) Plant ecology, 2nd edn. Blackwell Science Ltd, Oxford. doi:10.1002/9781444313642
Crisci JV (1976) Revisión del género Leucheria (Compositae: Mutisieae). Darwiniana 20:9–126
Cruz-Mazo G, Buide ML, Samuel R, Narbona E (2009) Molecular phylogeny of Scorzoneroides (Asteraceae): evolution of heterocarpy and annual habit in unpredictable environments. Molec Phylogen Evol 53:835–847. doi:10.1016/j.ympev.2009.08.001
Datson PM, Murray BG, Steiner KE (2008) Climate and the evolution of annual/perennial life-histories in Nemesia (Scrophulariaceae). Pl Syst Evol 270:39–57. doi:10.1007/s00606-007-0612-4
Dimmitt MA (2000) Plant ecology of the Sonoran Desert region. In: Phillips SJ, Comus W (eds) A natural history of the Sonoran Desert. Arizona-Sonora Desert Museum Press, University of California Press, Tucson, pp 158–167
Drummond CS (2008) Diversification of Lupinus (Leguminosae) in the western New World: derived evolution of perennial life history and colonisation of montane habitats. Molec Phylogen Evol 48:408–421. doi:10.1016/j.ympev.2008.03.009
Eastwood RJ, Drummond CS, Schifino-Wittmann MT, Hughes CE (2008) Diversity and evolutionary history of Lupinus—insights from new phylogenies. In: Palta JA, Berger JB (eds) Lupins for health and wealth. International Lupin Association Canterbury, New Zealand, pp 346–354
Evans MEK, Hearn DJ, Hahn WJ, Spangle JM, Venable DL (2005) Climate and life-history evolution in evening primroses (Oenothera, Onagraceae): a phylogenetic comparative analysis. Evolution 59:1914–1927. doi:10.1111/j.0014-3820.2005.tb01061.x
Fabbro T, Körner C (2004) Altitudinal differences in flower traits and reproductive allocation. Flora Morphol Distrib Funct Ecol Pl 199:70–81. doi:10.1078/0367-2530-00128
Goyenechea I, Contreras-Ramos A (2007) Controversias en Sistemática Filogenética. In: Contreras-Ramos A, Cuevas Cardona C, Goyenechea I, Iturbe U (eds) La sistemática: base del conocimiento de la biodiversidad. Universidad Autónoma Estado de Hidalgo, Mexico, pp 75–83
Gruenstaeudl M, Urtubey E, Jansen RK, Samuel R, Barfuss MHJ, Stuessy TF (2009) Phylogeny of Barnadesioideae (Asteraceae) inferred from DNA sequence data and morphology. Molec Phylogen Evol 51:572–587. doi:10.1016/j.ympev.2009.01.023
Hall TA (1999) BioEdit: a user-friendly biological sequence alignment editor and analysis program for windows 95/98/NT. Nucleic Acids Symp Ser 41:95–98
Hillis DM, Wiens JJ (2000) Molecules versus morphology in systematics: conflicts, artifacts, and misconceptions. Phylogenetic analysis of morphological data. Smithsonian Inst Press, Washington
Jara PA, Arancio G, Moreno R, Carmona MR (2006) Factores abióticos que influencian la germinación de seis especies herbáceas de la zona árida de Chile. Revista Chilena Hist Nat 79:309–319. doi:10.4067/S0716-078X2006000300003
Katinas L, Crisci JV, Schmidt-Jabaily R, Williams C, Walker J, Drew B, Bonifacino JM, Sytsma KJ (2008a) Evolution of secondary heads in Nassauviinae (Asteraceae, Mutisieae). Amer J Bot 95:229–240
Katinas L, Pruski J, Sancho G, Tellería MC (2008b) The subfamily Mutisioideae (Asteraceae). Bot Rev 74:469–716. doi:10.1007/s12229-008-9016-6
Katinas L, Tellería MC, Crisci JV (2008c) A new species of Leucheria (Asteraceae, Mutisieae) from Chile. Novon 18:366–369. doi:10.3417/2006108
Kim KJ, Jansen RK (1995) ndhF sequence evolution and the major clades in the sunflower family. Proc Natl Acad Sci USA 92:10379–10383
Kim HG, Loockerman DJ, Jansen RK (2002) Systematic implications of ndhF sequence variation in the Mutisieae (Asteraceae). Syst Bot 27:598–609. doi:10.1043/0363-6445-27.3.598
Lundberg J, Bremer K (2003) A phylogenetic study of the order Asterales using one morphological and three molecular data sets. Int J Pl Sci 164:553–578
Maddison WP, Maddison DR (2008) Mesquite. http://mesquiteproject.org/mesquite/mesquite.html
Maraner F, Samuel R, Stuessy TF, Crawford DJ, Crisci JV, Pandey A, Mort ME (2012) Molecular phylogeny of Nassauvia (Asteraceae, Mutisieae) based on nrDNA ITS sequences. Pl Syst Evol 298:399–408. doi:10.1007/s00606-011-0553-9
Möller M, Cronk QCB (2001) Evolution of morphological novelty: a Phylogenetic analysis of growth patterns in Streptocarpus (Gesneriaceae). Evolution 55:918–929. doi:10.1111/j.0014-3820.2001.tb00609.x
Moreira-Muñoz A, Morales V, Muñoz-Schick M (2012) Actualización sistemática y distribución geográfica de Mutisioideae (Asteraceae) de Chile. Gayana Bot 69:9–29
Panero JL, Crozier BS (2016) Macroevolutionary dynamics in the early diversification of Asteraceae. Molec Phylogen Evol 99:116–132. doi:10.1016/j.ympev.2016.03.007
Panero JL, Funk VA (2008) The value of sampling anomalous taxa in phylogenetic studies: major clades of the Asteraceae revealed. Molec Phylogen Evol 47:757–782. doi:10.1016/j.ympev.2008.02.011
Ratto F, Bello M, Bartoli A (2014) Novedades en Leucheria (Asteraceae, Mutisieae). Bol Soc Argent Bot 49:91–92
Rivero RM, Kojima M, Gepstein A, Sakakibara H, Mittler H, Gepstein S, Blumwald E (2007) Delayed leaf senescence induces extreme drought tolerance in a flowering plant. Proc Natl Acad Sci USA 104:19631–19636. doi:10.1073/pnas.0709453104
Ronquist F, Huelsenbeck JP (2003) MrBayes 3: bayesian phylogenetic inference under mixed models. Bioinformatics 19:1572–1574. doi:10.1093/bioinformatics/btg180
Schaffer WM, Gadgil M (1975) Selection for optimal life histories in plants. In: Cody M, Diamond J (eds) The ecology and evolution of communities. Belknap, Cambridge, pp 142–157
Shaw J, Lickey EB, Schilling E, Small RL (2007) Comparison of whole chloroplast genome sequences to choose noncoding regions for phylogenetic studies in angiosperms: the tortoise and the hare III. Amer J Bot 94:275–288. doi:10.3732/ajb.94.3.275
Silvertown J, Charlesworth D (2001) Introduction to plant population biology, 4th edn. Blackwell Science Ltd, Oxford
Simpson BB, Arroyo MTK, Sipe S, Dias de Morales M, McDill J (2009) Phylogeny and evolution of Perezia (Asteraceae: Mutisieae: Nassauviinae). J Syst Evol 47:431–443. doi:10.1111/j.1759-6831.2009.00039.x
Stevens PF (2001) Angiosperm Phylogeny Website. Missouri Botanical Garden, University of Missouri, St Louis. Available at: http://www.mobot.org/MOBOT/research/Apweb
Sun Y, Wangb A, Wana D, Wang Q, Liu J (2012) Rapid radiation of Rheum (Polygonaceae) and parallel evolution of morphological traits. Molec Phylogen Evol 63:150–158. doi:10.1016/j.ympev.2012.01.002
Taberlet P, Gielly L, Pautou G, Bouvet J (1991) Universal primers for amplification of three non-coding regions of chloroplast DNA. Pl Molec Biol 17:1105. doi:10.1007/BF00037152
Tank DC, Olmstead RG (2008) From annuals to perennials: phylogeny of subtribe Castillejinae (Orobanchaceae). Amer J Bot 95:608–625. doi:10.3732/ajb.2007346
White TJ, Bruns T, Lee S, Taylor J (1990) Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics. In: Innis M, Gelfand S, Sninsky J, White T (eds) PCR protocols: a guide to methods and applications. Academic Press, San Diego, pp 315–322
Wilf P, Johnson KR, Cúneo NR, Smith ME, Singer BS, Gandolfo MA (2005) Eocene plant diversity at Laguna del Hunco and Río Pichileufú, Patagonia, Argentina. Amer Naturalist 165:634–650. doi:10.1086/430055
Zuloaga FO, Morrone O, Belgrado ML (2008) Catálogo de las Plantas Vasculares del Cono Sur (Argentina, Sur de Brasil, Chile, Paraguay y Uruguay). Missouri Botanical Garden Press, St. Louis
Acknowledgements
We thank ULS, CONC, SGO, AGUCH, SI, LPB for their disposition to supply herbarium material for DNA extraction. Andrew Stanworth of Falklands Conservation, Marcela Nicola of the Institute of Botany Darwinion and Francisco Ratto of the Faculty of Agriculture University of Buenos Aires are especially thanked for sending herbarium material and locating specimens in the field. Work was funded by FONDECYT Initiation 11130299. Grants ICM-MINECON P05-002-IEB, PBF-23 to the Institute of Ecology and Biodiversity supplied materials and equipment.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Handling editor: Hanna Schneeweiss.
Information on electronic supplementary material
Below is the link to the electronic supplementary material.
Information on Electronic Supplementary Material
Information on Electronic Supplementary Material
Online Recourse 1. Evolutionary lines in Leucheria recognized by Crisci (1976).
Online Recourse 2. Bayesian Inference phylogenetic tree based on rDNA ITS.
Online Recourse 3. Bayesian Inference phylogenetic tree based on cpDNA trnL–trnF.
Online Recourse 4. Bayesian Inference phylogenetic tree based on cpDNA rpl32.
Online Recourse 5. Alignment used to produce phylogenie.
Rights and permissions
About this article
Cite this article
Jara-Arancio, P., Vidal, P.M., Panero, J.L. et al. Phylogenetic reconstruction of the South American genus Leucheria Lag. (Asteraceae, Nassauvieae) based on nuclear and chloroplast DNA sequences. Plant Syst Evol 303, 221–232 (2017). https://doi.org/10.1007/s00606-016-1366-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00606-016-1366-7