Abstract
Gamochaeta (tribe Gnaphalieae, Asteraceae) is composed of ca. 60 species primarily distributed in tropical and subtropical America. Within the tribe Gnaphalieae, the genus is characterized by capitula arranged in spikes or head-like clusters, few hermaphroditic central florets, truncate style branches with apical sweeping trichomes, pappus bristles connate at the base into a ring falling as a unit, and achenes with globose twin trichomes. Previous molecular phylogenetic studies have suggested the paraphyly of the genus, but have not provided a basis for redefining generic limits due to incomplete taxon sampling. To address this problem, DNA sequences from the plastid (trnL-F) and nuclear (ETS and ITS) genomes were analyzed from a broad taxon sample representing the full range of morphological variation known in the genus. Our results affirm that Gamochaeta is paraphyletic as presently circumscribed. Two clades can be recognized: one clade that includes the majority of the species currently assigned to Gamochaeta and a second clade that includes Gamochaetopsis, Stuckertiella and seven species of Gamochaeta. We present here a new circumscription of Gamochaeta, including two new combinations, Gamochaeta alpina and Gamochaeta peregrina, and the resurrection of Gamochaeta capitata. Our results also show Omalotheca supina, O. norvegica and O. sylvatica, which were placed by some authors in Gamochaeta or in Gnaphalium, form a monophyletic group distantly related to both genera.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Gamochaeta (tribe Gnaphalieae, Asteraceae) is represented by ca. 60 species characterized by their capitula arranged in spikes or head-like clusters, pistillate florets outnumbering the hermaphroditic disk florets, truncate style branches with apical sweeping trichomes, pappus bristles connate at the base into a ring that falls as a unit, and achenes with globose twin trichomes (e.g., Cabrera 1961; Anderberg 1991; Bayer et al. 2007; Ward et al. 2009). The greatest diversity is in South America, with ca. 45 species (Cabrera 1963, 1971, 1974, 1978; Aristeguieta 1964; Marticorena and Quezada 1985; Dillon and Sagástegui-Alva 1991a, b; Freire 1998; Deble and Marchiori 2007; Freire and Iharlegui 2008, 2014; Freire et al. 2011; Hind 2011). A few species are native to Central America, e.g., G. irazuensis, G. standleyi (Pruski 2011), and North America, e.g., G. argyrinea and G. ustulata (Nesom 1990a, 2004, 2006), and a few species are adventive or naturalized in Asia, Australia, Hawaii, New Zealand, Southern Africa, and Europe, e.g., Gamochaeta calviceps and G. pensylvanica (Drury 1971; Hilliard 1983; Chen and Bayer 2011; Alford 2012).
Gamochaeta was first proposed as a genus by Weddell (1855) to accommodate five species of Gnaphalium L. diagnosed by the presence of pappus bristles connate at the base into a ring, hence the name of the genus (from the Greek gamos “connate” and xaite “hairs”). In the following years, many authors considered Gamochaeta as a section of Gnaphalium (Bentham and Hooker 1873; Hoffmann 1890; Wagenitz 1965; Drury 1970, 1971; Merxmüller et al. 1977; Hilliard and Burtt 1981; Webb 1988), whereas others recognized it as a distinct genus (Cabrera 1961; Nesom 1990a, 2004, 2006; Anderberg, 1991; Dillon and Sagástegui-Alva 1991a, b; Freire and Iharlegui 1997, 2014; Bayer et al. 2007; Deble and Marchiori 2007; Chen and Bayer 2011; Hind 2011).
In the first morphological cladistic study of generic relationships in the Gnaphalieae, Anderberg (1991) included a cladogram in which Gamochaeta was sister to the “Lucilia group” (e.g., Belloa J.Rémy, Chevreulia Cass., Facelis Cass.) and made numerous combinations in Gamochaeta. Anderberg’s work demonstrated the heterogeneity of Gnaphalium as it was circumscribed in many floras (e.g., Rémy 1849; Aristeguieta 1964), as well as by Merxmüller et al. (1977). Anderberg (1991) also recognized many of the segregate genera from Gnaphalium, i.e., Anaphaloides (Benth.) Kirp., Euchiton Cass., Gamochaeta, Gnaphaliothamnus Kirp., Homognaphalium Kirp., and Pseudognaphalium Kirp., but still left a rather heterogeneous Gnaphalium including Omalotheca Cass. and Synchaeta Kirp. Different authors have variously recognized the genus Omalotheca (including Synchaeta) or placed Omalotheca species in Gamochaeta or Gnaphalium. Nesom (1990b) excluded Omalotheca from Gnaphalium and mentioned that Omalotheca was superficially similar to Gamochaeta in its spiciform capitulescences. In their treatment for Flora of China, Chen and Bayer (2011) placed some species of Omalotheca in Gamochaeta (O. nanchuanensis (Y.Ling & Y.Q.Tseng) Holub, O. sylvatica (L.) Sch.Bip. & F.W.Schultz, O. norvegica (Gunnerus) Sch.Bip. & F.W.Schultz) but placed the type species of the genus (O. supina (L.) DC.) in Gnaphalium. However, Blöch et al. (2010) considered Omalotheca hoppeana (W.D.J.Koch) Sch.Bip. & F.W.Schultz, O. norvegica, O. supina and O. sylvatica as Gnaphalium species.
More recently, phylogenetic analyses utilizing molecular data have been used to address relationships within the tribe Gnaphalieae. Based on the plastid marker trnL-F, Blöch et al. (2010) presented a molecular phylogeny of the Asian-European Leontopodium R.Br. ex Cass. and found a relationship between Gamochaeta (G. pensylvanica), Antennaria Gaertn., Ewartia Beauverd, Leucogenes Beauverd and Leontopodium. Based on both nuclear (ETS, ITS) and plastid markers (rpL32-trnL intergeneric spacer + trnL intron + trnL-F), Galbany-Casals et al. (2010) recognized the “FLAG clade” including Filago Loefl., Leontopodium, Antennaria and Gamochaeta. In a study focused on ancient polyploidy in Gnaphalieae, Smissen et al. (2011) also recovered Gamochaeta [G. coarctata , G. subfalcata] in the “FLAG clade.” More recently, Freire et al. (2015) using DNA sequences from plastid (rpl32-trnL, trnL-F) and nuclear (ITS and ETS) markers, together with morphological characters, presented a cladogram of the South American “Lucilia group s.l.” in which Gamochaeta (three species) formed a well-supported clade together with Gamochaetopsis and Stuckertiella, hence providing initial evidence that Gamochaeta, as currently circumscribed, might not be monophyletic.
The aim of this study is to extend the sampling of Gamochaeta to provide a better supported and more reliable phylogenetic hypothesis for generic realignments using one plastid (trnL-F) and two nuclear DNA regions (ETS and ITS).
Materials and methods
Ingroup and outgroup
This study includes 33 species of Gamochaeta representing ca. 60 % of the species of the genus, all major morphological forms (concolorous and discolorous leaves, spicate and head-like arrangement of capitula) and almost the entire distributional and elevational range of the genus. In order to test the monophyly of Gamochaeta, we included 22 species of 13 other genera of Gnaphalieae, ten of them belonging to the FLAG clade and two outside of the FLAG clade. The choice of these genera and species was based on results of previous investigations: (1) Based on Freire et al. (2015), representatives of the sister group of the Gamochaeta-Stuckertiella clade such as Belloa chilensis (Hook. & Arn.) J.Rémy, Berroa gnaphalioides (Less.) Beauverd, Facelis plumosa (Wedd.) Sch.Bip., Jalcophila boliviensis Anderb. & S.E.Freire, Jalcophila ecuadoriensis M.O.Dillon & Sagást. and Lucilia acutifolia (Poir.) Cass. were included; (2) based on Galbany-Casals et al. (2010), Smissen et al. (2011), and Freire et al. (2015), other related Gnaphalieae species such as Antennaria chilensis J. Rémy, Filago fuscescens Pomel, F. lutescens Jord., F. pyramidata L., Gnaphalium austroafricanum Hilliard, G. declinatum L.f., G. uliginosum L., Leontopodium alpinum Cass., L. microphylum Hayata, Omalotheca norvegica, O. supina, and O. sylvatica were included. Trees were rooted with Achyrocline tomentosa Rusby, selected from Freire et al. (2015). DNA samples were obtained from silica-preserved leaves and from herbarium specimens. Vouchers are deposited in CONC, LP, LPB, MNCS, MO, SI, SZU, USMS, and W. When no plant material was available, sequences were obtained from GenBank (“Appendix”).
Morphological characters and distribution of the species of Gamochaeta in Tables 1, 2 and 3 are from the literature and own observations.
DNA extraction, amplification, sequencing, sequence alignment and editing
DNA extraction used the modified CTAB protocol by Doyle and Dickson (1987), adapted for small amounts of plant material. When material preserved in silica gel was not available, DNA was extracted from herbarium specimens using the DNeasy Plant Mini Kit (QIAGEN Inc., Hilden, Germany).
The plastid intergenic spacer trnL-F (primers C and F; Taberlet et al. 1991) and nuclear regions ITS (primers ITS4 and ITS5; White et al. 1990) and ETS (primers ETS1 and 18S-ETS; Bayer et al. 2002 and Baldwin and Markos 1998, respectively) were selected for this study. Reactions were performed in a final volume of 25 μl or rarely in 50 μl. Each reaction contained 50–100 ng of DNA, 1.5 units of Taq polymerase (Invitrogen Life Technologies, São Paulo, Brazil or TaKaRa ExTaq, Otsu, Shiga, Japan), 1 × PCR Buffer, 5 mM MgCl2, 0.2 pmol of each primer and 0.025 mM of each dNTP. In species for which these protocols were unsuccessful, BSA 0.4 % and DMSO 1.6 % or a mixture of trehalose, BSA and polysorbate-20 (Samarakoon et al. 2013) were included to increase the yield of PCR. The annealing temperatures ranged between 48 and 52 °C for the plastid markers and 56–60 °C for the nuclear markers. Final extension at 72 °C for 6 min terminated the reactions. The quality of the PCR products was estimated by electrophoresis and visualized with ethidium bromide under UV light. A negative control with no template was included for each series of amplifications to test for contamination. PCR products were sequenced by Macrogen Inc. (Korea) or Eurofins MWG Operon (Louisville, KY).
Sequences were assembled and edited using the program ChromasPro version 1.34 (Technelysium Pty, Ltd, Tewantin, Australia). Matrices were edited using the program BioEdit (Hall 1999), and sequences were aligned using the application ClustalW, using multiple alignment with the option run ClustalW. Data matrices are deposited at TreeBase (TB2: S19294).
Phylogenetic analyses
Analyses of nuclear and plastid sequences were performed separately and combined. Parsimony analyses were conducted using the program TNT version 1.1 (Goloboff et al. 2008), with all characters equally weighted and considered unordered. Gaps were scored as missing data. In all analyses, parsimony-uninformative characters were omitted. Heuristic searches were performed using 1000 random addition replicates and tree bisection–reconnection (TBR) branch swapping, saving ten trees per replicate. Branch support was assessed with 10,000 parsimony jackknifing replicates (JK; Farris et al. 1996), using ten series of random addition sequences, swapped using TBR and holding two optimal trees per series. Consistency index (CI; Kluge and Farris 1969) and retention index (RI; Farris 1989) were calculated as measures of homoplasy.
For each of the four markers, an appropriate model of evolution was selected with JModeltest v.2.1.1 (Darriba et al. 2012) based on the Akaike information criterion, AIC (Akaike 1973; Sugiura 1978; Hurvich and Tsai 1989). The best models were GTR + I+G for ITS; HKY + G for ETS and GTR + G for trnL-F. Bayesian inference was performed as implemented in Beast version 1.8.1 (Drummond et al. 2012). With BEAUti v.1.6.2 (Drummond and Rambaut 2007) we created the input file with the nucleotide substitution models mentioned above, empirical base frequencies, four gamma categories, under an uncorrelated lognormal relaxed-clock model (Drummond et al. 2006), and a Yule process of speciation as prior. The MCMC analysis was performed for 10,000,000 generations and sampled every 1000th generation. Convergence of the chains was checked using Tracer v.1.5 (Drummond and Rambaut 2007). All trees obtained prior to convergence were discarded, and trees were summarized in a maximum clade credibility tree in TreeAnnotator v.1.6.2 (Drummond and Rambaut 2007). Trees were visualized and edited using FigTree version 1.4.2 (Rambaut 2014).
Analyses were performed for (1) plastid data only (trnL-F), (2) nuclear data only (ITS + ETS), and (3) combined nuclear + plastid data.
Results
Matrices
Sequences of the plastid trnL-F region were obtained for 47 taxa, including 27 Gamochaeta species, Gamochaetopsis alpina, Stuckertiella capitata (Wedd.) Beauverd, and ten other genera, Achyrocline tomentosa serving as the outgroup. The total length of the sequence ranged from 599 bp in Gamochaeta grazielae to 754 bp in G. andina, G. serpyllifollia, and Gamochaetopsis alpina. The aligned matrix consisted of 779 characters, of which 24 were parsimony informative.
Sequences of the nuclear ETS region were obtained for 55 taxa, including 33 Gamochaeta species, Gamochaetopsis alpina and Stuckertiella capitata and 20 other species, including the outgroup. The total length of the sequence ranged from 356 bp in Leontopodium to 474 bp in Omalotheca norvegica and Antennaria chilensis. The aligned matrix consisted of 482 characters, of which 112 were parsimony informative.
Sequences of the nuclear ITS region were obtained for 45 taxa, including 25 Gamochaeta species, Gamochaetopsis alpina and Stuckertiella capitata and 17 other species plus Achyrocline tomentosa. The total length of the sequence ranged from 516 bp in Gamochaeta americana to 527 bp in Gnaphalium uliginosum. The aligned matrix consisted of 532 characters, of which 92 were parsimony informative.
Parsimony and Bayesian analyses showed highly congruent results, with the latter providing more resolved nodes. Consensus trees obtained from the parsimony analyses of nuclear and nuclear + plastid data are illustrated, but some of the more resolved nodes from Bayesian analysis are also shown.
Relationships
The analysis of the plastid marker resulted in a highly polytomized consensus tree (CI 0.87, RI 0.95) due to low character state variability, showing little relevant information. Furthermore, the relationships do not correspond to any proposed hypotheses and even though they are poorly supported we decided to show the data obtained (Fig. 1). The combined analysis of the nuclear markers (hereafter referred to as nuclear analysis) resulted in 175 most parsimonious trees (CI 0.60, RI 0.79), and the combined analysis of the three markers (hereafter referred to as the combined analysis) resulted in 209 most parsimonious trees (CI 0.61, RI 0.79).
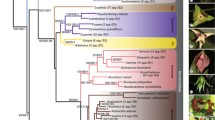
The hypothesis of relationships obtained from the nuclear and combined analyses shows that Gamochaeta, as currently circumscribed, is paraphyletic, since Gamochaetopsis and Stuckertiella also appear nested within Gamochaeta with 98/97 Jackknife support and a posterior probability of 1.00 (Figs. 2, 3).
Strict consensuses tree from combined molecular (ETS + ITS +trnL-F) data. Numbers above branches are JK values from the parsimony analysis, and numbers below branches Bayesian posterior probabilities (PP, asterisk indicates lack support). a–c Bayesian topologies. Bold lines indicate Gamochaeta and related genera
The combined data, under Bayesian analysis, show the pair Omalotheca norvegica-O. sylvatica (maximum values) distant from Gamochaeta, forming a group with Omalotheca supina (Fig. 3) but with low support (54/0.91).
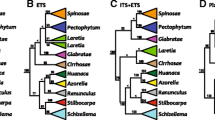
In the nuclear and combined analyses, Gamochaeta species are divided into two major groups: clade A (99/1.00) including Gamochaetopsis alpina, Gamochaeta affinis Gamochaeta affinis , Gamochaeta andina, Gamochaeta depilata, Gamochaeta nivalis , Gamochaeta procumbens, Gamochaeta serpyllifolia, and Gamochaeta spiciformis; and clade B (*/0.86, 65/1.00) including all the remaining Gamochaeta species (Figs. 2, 3).
Clade A (Fig. 3) is principally characterized by having capitula arranged in head-like clusters (vs. clade B with capitula usually arranged in spikes). In the nuclear analysis Stuckertiella is placed as a basal member in the clade B (*/0.84, Fig. 2), and in the combined analysis Stuckertiella is placed as a basal member in the clade A (52/0.99, Fig. 3).
In both analyses, clade B showed some internal resolution, with the following clades present: Gamochaeta ambatensis + G. falcata (85/1.00 Fig. 2; 85/1.00 Fig. 3), principally characterized by having capitula with 50–70 marginal florets, and linear-oblanceolate or oblanceolate leaves (Table 2); G. filaginea + G. pensylvanica (61/1.00 Fig. 2, 61/1.00 Fig. 3), principally characterized by having capitula with 70–80 marginal florets, acute or subacute to obtuse inner phyllaries, and oblanceolate or spathulate leaves (Table 2); G. argyrinea + G. chionesthes (61/0.98 Fig. 2; 62/0.99 Fig. 3b), principally characterized by having capitula with up 120 marginal florets, and discolorous, oblanceolate leaves (Table 2); G. humilis + G. longipedicellata + G. lulioana (63/0.93 Fig. 2, 63/0.94 Fig. 3c), principally characterized by having caespitose or subcaespitose habit, capitula with 27–40 marginal florets, acute inner phyllaries, and oblanceolate or oblong-obovate to oblong-spathulate leaves (Table 2); and G. camaquaensis + G. erecta + G. grazielae + G. stachydifolia (75/1.00 Fig. 2, 71/1.00 Fig. 3), principally characterized by having capitula with 50–100 marginal florets, acute to acuminate inner phyllaries, and concolorous, oblanceolate to spathulate leaves (Table 2). Other small clades were also present within clade B in each analysis (Figs. 2, 3), e.g., G. americana clade (Fig. 3), which comprises G. americana, G. coarctata and G. ustulata, all with strongly discolorous and broadly obovate to spathulate leaves.
The clade Belloa chilensis, Berroa gnaphalioides, Facelis plumosa and Lucilia acutifolia is placed as sister group of clades A and B (53/0.88 Fig. 2) in both the nuclear and combined analyses (51/0.88 Fig. 3). They also conform to two morphologically well-defined groups. The genera of the clade of Belloa chilensis, Berroa gnaphalioides, Facelis plumosa and Lucilia acutifolia are defined by solitary or few together capitula and elongated (rarely clavate) twin hairs. Conversely, the clade A + B is defined by capitula arranged in head-like clusters or in spiciform inflorescences, and globose (sometimes clavate) twin hairs.
Finally, Gamochaetopsis alpina and Stuckertiella capitata appear nested within Gamochaeta in both analyses with Bayesian posterior probabilities and jackknife of 98 and 97, respectively (Figs. 2 and 3).
Discussion
The analyses affirm that Gamochaeta as currently circumscribed is paraphyletic, indicating the need of a revised circumscription at the generic level in the group.
Taxonomic position of Stuckertiella and Gamochaetopsis (Figs. 2, 3, 6)
Stuckertiella was described by Beauverd (1913) with two species: Stuckertiella capitata transferred from Gamochaeta and S. peregrina. The monotypic genus Gamochaetopsis was established by Anderberg and Freire (1991) to include Gamochaetopsis alpina from southern Chile and Argentina, which was principally diagnosed by its achenes with short clavate twin hairs and capitula arranged in head-like clusters. The position of Stuckertiella and Gamochaetopsis in our analyses is congruent with morphological evidence. All the morphological characters that define Stuckertiella and Gamochaetopsis are also found in species of Gamochaeta (Table 1) with the exception of the autoapomorphy in Stuckertiella of the presence of functionally male central florets with four anthers (three with a small obtuse apical appendage and one with a long, acute apical appendage). The close similarity between Gamochaetopsis and Gamochaeta, and between Stuckertiella and Gamochaeta had previously been noted by Cabrera (1971) and Anderberg (1991), respectively. Furthermore, Stuckertiella shares with Gamochaetopsis clade its capitula arranged in head-like clusters, and with the remaining species of Gamochaeta its capitula with many florets (Table 1).
Unfortunately, we were unable to obtain living material of Stuckertiella peregrina, and herbarium specimens were not of sufficient quality for DNA extractions. However, we predict that this species will also group with Gamochaeta, given that it has character states like Stuckertiella capitata that unite this group with Gamochaeta.
Redefinition of Gamochaeta boundaries (Figs. 2, 3, 4, 5, 6)
Gamochaeta is taxonomically difficult due to the fact that most species exhibit considerable morphological and ecological variability (Table 2). In this work, we included a broad geographical-taxonomic sampling, including species that grow at middle elevation (less than 3000 m), especially in dry hills, grasslands, sand hills, and disturbed habitats characterized by having ascending or erect stems, or more rarely, cespitose habit (e.g., G. alpina, G. depilata, G. nivalis, G. procumbens, G. serpyllifolia, and G. spiciformis). A small number of species (e.g., G. deserticola, G. erythractis , G. meridensis , G. meridensis, G. paramora) grow at high altitudes (3000–4000 m) in paramo, jalca and puna; and a few prostrate and acaulescent species (e.g., G. cabrerae , G. humilis, G. longipedicellata, G. lulioana) are adapted to life at even higher elevations between 3500–4500 m.
Achenial hairs in Gamochaeta. a globose achenial hairs (G. peregrina, Cabrera 8009, SI), b clavate achenial hairs (G. hiemalis, Deble and Deble 11875, SI), c globose myxogenic achenial hairs (G. americana, Urtubey 187, SI), d globose achenial hairs (G. girardiana, Deble and Deble 4501, SI), e style branches with pollen of disk floret (G. americana, Urtubey 187, SI), f clavate achenial hairs (G. alpina, Cabrera 5902, LP). Scale bars a 20 µm, b 20 µm, c 20 µm, d 40 µm, e 40 µm, f 20 µm
Morphological diversity in Gamochaeta and allied genera: a G. lulioana, capitula solitary (photo S. Beck), b G. longipedicellata, capitula solitary (photo E. Urtubey), c G. stachydifolia, capitula arranged in spikes (photo F.O. Zuloaga), d Spike basally discontinuous of G. stachydifolia (photo F.O. Zuloaga), e G. coarctata, capitula arranged in spikes (photo Pensiero), f G. filaginea, capitula arranged in spikes (photo F.O. Zuloaga)
In order to have Gamochaeta monophyletic, we propose that the two species of Stuckertiella and the single species of Gamochaetopsis are transferred to Gamochaeta (Table 3). Synapomorphies for this large clade include the following: multistemmed perennial herbs, oblanceolate leaves, small capitula arranged in spikes or head-like clusters, marginal female florets outnumbering the hermaphroditic central florets, style branches truncate and penicillate, short-pilose achenes with globose twin hairs, and pappus bristles basally connate.
The low sequence divergence and lack of resolution within the Gamochaeta clade is probably due to a rapid and recent diversification in the Andes, as was postulated by Hughes and Eastwood (2006). In this way, two centers of diversity in the Andes could be suggested, one in the southern portion of South America and the second one mainly in the Andean-Brazilian region, with few species reaching North America.
Omalotheca (Figs. 2, 3)
Cassini published Omalotheca in Cuvier´s Dictionnaire des Sciences Naturelles in 1828 to include the Eurasian Gnaphalium supinum L. and distinguished it from Gnaphalium based on a uniseriate pappus and obovoid, compressed achenes. Drury (1970) also distinguished Omalotheca from Gnaphalium and Gamochaeta based on terminal inflorescences in a leafy spikes and elongate twin hairs not emitting mucilage in water.
The molecular phylogeny we present here shows Omalotheca supina, O. norvegica and O. sylvatica forming a monophyletic group (Figs. 2, 3) distantly related to Gamochaeta and to Gnaphalium uliginosum (which is the generic type of Gnaphalium). This was pointed out by Galbany-Casals et al. (2010), who showed that Gnaphalium supinum was neither closely related to Gnaphalium s.str. nor to Gamochaeta. Similarly, Blöch et al. (2010) indicated that Omalotheca (sub Gnaphalium) was distantly related to Gamochaeta. The diagnostic characters for Omalotheca are the presence of connate pappus bristles (vs. free in Gnaphalium), lack of myxogenic twin hairs on the fruits (vs. usually myxogenic in Gamochaeta), spiciform capitulescence (vs. corymbs in Gnaphalium) and pistillate flowers with corollas filiform-tubular (vs. filiform in Gamochaeta and Gnaphalium). Further, as was pointed out by Nesom (1990b), Gamochaeta is strictly a New World genus.
Gamochaeta nanchuanensis (Y.Ling & Y.Q.Tseng) Y.S.Chen & R.J.Bayer was not included in our present analyses, so its phylogenetic position remains untested, although the original description of Gnaphalium nanchuanense Y.Ling & Y.Q.Tseng (Ling and Tseng 1978) states that it is very similar to G. sylvaticum, thus indicating that it, too, likely belongs in Omalotheca.
Taxonomic treatment
Gamochaeta Wedd., Chlor. And. 1(4–6): 151. 1856. Gnaphalium L. subgen. Gamochaeta (Wedd.) Gren., Fl. Jurass. 2: 427. 1869. ≡ Gnaphalium L. sect. Gamochaeta (Wedd.) Benth. & Hook. f., Gen. Pl. 2(1): 306. 1873. ≡ Gnaphalium L. sect. Gamochaeta (Wedd.) O.Hoffm., Nat. Pflanzenfam. 4(5): 188. 1894, comb. illeg. (Art. 53)—LECTOTYPE (designated by Cabrera 1961: 362): G. americana (Mill.) Wedd.
= Stuckertiella Beauverd, syn. nov. Bull. Soc. Bot. Genève sér. 2, 5: 205. 1913. —LECTOTYPE (designated by Cabrera, 1978: 128): S. capitata (Wedd.) Beauverd.
= Gamochaetopsis Anderb. & S.E.Freire, syn. nov. Bot. J. Linn. Soc. 106(2): 186. 1991—TYPE: G. alpina (Poepp.) Anderb. & S.E.Freire.
Sixty-one species distributed from the USA to Argentina: G. affinis Cabrera [Gnaphalium affine d’Urv., nom. illeg. (Art. 53); G.malvinense H.Koyama, nom. illeg. (Art. 52); Gamochaeta malvinensis (H.Koyama) T.R.Dudley, comb. illeg. (Art. 53)]; G. aliena (Hook. & Arn.) Cabrera (Gnaphalium alienum Hook. & Arn.); Gamochaeta alpina (Poepp.) S.E.Freire & Anderb., comb. nov. ≡ Laennecia alpina Poepp., Nov. Gen. Sp. Pl. 3: 56, tab. 262. 1845 [= Lucilia alpina (Poepp.) Cabrera, Gamochaetopsis alpina (Poepp.) Anderb. & S.E.Freire]; G. ambatensis Ariza; G. americana (Mill.) Wedd. [Gnaphalium americanum Mill.; G. purpureum L. var. americanum (Mill.) Klatt; G. consanguineum Gaudich.; G. guatemalense Gand.; Gamochaeta guatemalensis (Gand.) Cabrera]; G. andina (Phil.) Cabrera (Gnaphalium andinum Phil.); G. antarctica (Hook.f.) Cabrera (Gnaphalium antarcticum Hook.f.); G. antillana (Urb.) Anderb. (Gnaphalium antillanum Urb.); G. argentina Cabrera; G. argyrinea G.L.Nesom; G. axillaris (J.Rémy) Cabrera (Gnaphalium axillaris J.Rémy); G. badillana (Aristeg.) Anderb. (Gnaphalium badillanum Aristeg.); G. beckii Urtubey & S.E.Freire; G. berteroana (DC.) Cabrera [Gnaphalium berteroanum DC.; G. stachydifolium Lam. var. berteroanum (DC.) DC.]; G. cabrerae Anderb. [Gamochaeta monticola M.O.Dillon & Sagástegui, nom. illeg. (Art. 53); G. oreophila M.O.Dillon & Sagástegui, nom. illeg. (Art. 53)]; G. calviceps (Fernald) Cabrera (Gnaphalium calviceps Fernald); G. camaquaensis Deble; Gamochaeta capitata Wedd., Chlor. And. 1: 153. 1855 [= Stuckertiella capitata (Wedd.) Beauverd, Gnaphalium capitatum (Wedd.) Griseb., comb. illeg. (Art. 53), non Lamarck, 1786, nec Thunberg, 1799, Gnaphalium weddellianum Rusby, Gamochaeta weddelliana (Rusby) Anderb., nom. illeg. (Art. 52)]; G. chamissonis (DC.) Cabrera [Gnaphalium chamissonis DC.; G. fernandezianum Phil.; G. julietii Phil.; G. polybotryum Phil.; G. purpureum L. var. julietii (Phil.) Reiche; G. serranoi Phil.; Gamochaeta. fernandeziana (Phil.) Anderb.; G. julietii (Phil.) Anderb.; G. polybotrya (Phil.) Cabrera; G. serranoi (Phil.) Cabrera]; G. chionesthes G.L.Nesom; G. coarctata (Willd.) Kerguélen [Gnaphalium coarctatum Willd.; G. purpureum L. var. spicatum Klatt; G. spicatum Lam., nom. illeg. (Art. 53); Gamochaeta spicata Cabrera]; G. depilata (Phil.) Cabrera (Gnaphalium depilatum Phil.; G. obscurum Phil.); G. deserticola Cabrera; G. diffusa Deble & Marchiori; G. erecta Deble; G. erythractis (Wedd.) Cabrera [Merope erythractis Wedd.; Belloa erythractis (Wedd.) Cabrera); Gnaphalium erythractis (Wedd.) Griseb.]; G. falcata (Lam.) Cabrera [Gnaphalium falcatum Lam., G. purpureum L. var. falcatum (Lam.) Torr. & A.Gray; G. stachydifolium Lam. var. falcatum (Lam.) Klatt]; G. filaginea (DC.) Cabrera (Gnaphalium filagineum DC.); G. foliosa (Phil.) Anderb. [Gnaphalium foliosum Phil.; Gamomochaeta chilensis Deble, nom. illeg. (Art. 53)]; G. girardiana Deble & A.S.Oliveira; G. grazielae (Rizzini) Deble (Gnaphalium grazielae Rizzini); G. hiemalis Cabrera [Gnaphalium hiemale Rizzini, nom. illeg. (Art. 53); Gamochaeta brasiliana Deble]; G. humilis Wedd.; G. irazuensis G.L.Nesom; G. longipedicellata Cabrera; G. lulioana S.E.Freire & Iharl.; G. meridensis V.M.Badillo; G. monticola (Phil. ex Reiche) Cabrera (Gnaphalium monticola Phil. ex Reiche); G. neuquensis Cabrera; G. nigrevestis Deble & Marchiori; G. nivalis Cabrera [Gnaphalium affine d’Urv. var. pusillum Speg.; G. nivale Phil., nom. illeg. (Art. 53); Gamochaeta spiciformis (Sch.Bip.) Cabrera var. subaffinis Cabrera]; G. oligantha (Phil.) L.E.Navas (Gnaphalium oliganthum Phil.); G. paramora (S.F.Blake) Anderb. [Gnaphalium paramorum S.F.Blake; Lucilia paramora (S.F.Blake) V.M.Badillo]; G. pensylvanica (Willd.) Cabrera [Gnaphalium pensylvanicum Willd.; G. peregrinum Fernald; G. purpureum L. subsp. pensylvanica (Willd.) O.Bolòs & Vigo; G. purpureum L. var. spathulatum Baker; G. spathulatum Lam., nom. illeg. (Art. 53); G. platense Cabrera; Gamochaeta platensis (Cabrera) Cabrera]; Gamochaeta peregrina (Beauverd) S.E.Freire & Anderb., comb. nov. = Stuckertiella peregrina Beauverd, Bull. Soc. Bot. Genève sér. 2, 5: 208. 1913. [= Stuckertiella peregrina Beauverd var. fusca Beauverd, Stuckertiella peregrina Beauverd var. albida Beauverd]; G. procumbens (Phil.) Cabrera (Gnaphalium procumbens Phil.); G. purpurea (L.) Cabrera [Gnaphalium purpureum L.; G. rosaceum I.M. Johnst.; Gamochaeta rosacea (I.M.Johnst.) Anderb.]; G. rizzini Cabrera; G. serpyllifolia Wedd. [Gnaphalium serpyllifolium J.Rémy, nom. illeg. (Art. 53); Gamochaeta munnozii Cabrera, nom. illeg. (Art. 52)]; G. simplicicaulis (Willd. ex Spreng.) Cabrera [Gnaphalium simplicicaule Willd. ex Spreng.; G. purpureum L. var. simplicicaule (Willd. ex Spreng.) Klatt]; G. sphacelata (Kunth) Cabrera [Gnaphalium sphacelatum Kunth; G. purpureum L. var. sphacelatum (Kunth) Speg.]; G. spiciformis (Sch.Bip.) Cabrera [Gnaphalium spiciforme Sch.Bip.; G. affine d´Urv. var. medium Speg.; G. mucronatum Phil.; G. peteroanum Phil.; Gamochaeta peteroana (Phil) Anderb.]; G. stachydifolia (Lam.) Cabrera [Gnaphalium stachydifolium Lam.; G. purpureum L. var. stachydifolium (Lam.) Baker]; G. stagnalis (I.M.Johnst.) Anderb. (G. stagnale I.M.Johnst.); G. standleyi (Steyerm.) G.L.Nesom (Gnaphalium standleyi Steyerm.); G. subfalcata (Cabrera) Cabrera (Gnaphalium subfalcatum Cabrera); G. suffruticosa (Phil.) Anderb. (Gnaphalium suffruticosum Phil.); G. thouarsii (Spreng.) Anderb. (Gnaphalium thouarsii Spreng.); G. ustulata (Nutt.) Holub [Gnaphalium ustulatum Nutt.; G. pannosum Gand.; G. purpureum L. var. ustulatum (Nutt.) B.Boivin; Gamochaeta ustulata (Nutt.) G.L.Nesom]; G. valparadisea (Phil.) Anderb. (Gnaphalium valparadiseum Phil.); G. villarroelii (Phil.) Cabrera (Gnaphalium villarroelii Phil.).
Concluding remarks
Our study, using a plastid marker (trnL-F) and two nuclear markers (ETS and ITS), suggested that the genus Gamochaeta is paraphyletic and that Stuckertiella and the monotypic genus Gamochaetopsis should be included for the establishment of the monophyly in the genus. This American genus Gamochaeta is diagnosed by having slender pappus bristles that are basally connate, central florets hermaphroditic (rarely functionally male), capitula arranged in terminal head-like clusters or more usually crowed into spiciform inflorescences, and achenes pilose (occasionally glabrous), with short globose or sometimes clavate, duplex hairs. Two new combinations are proposed under Gamochaeta: Gamochaeta alpina (Poepp.) S.E.Freire & Anderb. and Gamochaeta peregrina (Beauverd) S.E.Freire & Anderb., and Gamochaeta capitata Wedd. is resurrected from synonymy of Stuckertiella capitata (Wedd.) Beauverd.
References
Akaike H (1973) Information theory and an extension of the maximum likelihood principle. In: Petrov BN, Csaki F (eds) Second international symposium on information theory. Akademiai Kiado, Budapest, pp 267–281
Alford MH (2012) New records of Gamochaeta (Asteraceae) in the Hawaiian Archipelago. In: Evenhuis NL, Eldredge LG (eds), Records of the Hawaii Biological Survey for 2011. Bishop Mus Occas Pap 113:1–6
Anderberg AA (1991) Taxonomy and phylogeny of the tribe Gnaphalieae (Asteraceae). Opera Bot 104:1–195
Anderberg AA, Freire SF (1991) A cladistics and biogeographic analysis of the Lucilia group (Asteraceae, Gnaphalieae). Bot J Linn Soc 106:173–198
Aristeguieta L (1964) Compositae. In: Lasser T (ed) Flora de Venezuela 10(1). Dirección de Recursos Naturales Renovables MAC, Caracas, pp 1–483
Baldwin BG, Markos S (1998) Phylogenetic utility of the external transcribed spacer (ETS) of 18S–26S rDNA: congruence of ETS and ITS trees of Calycadenia (Compositae). Molec Phylogen Evol 10:449–463
Bayer RJ, Greber DG, Bagnall NH (2002) Phylogeny of Australian Gnaphalieae (Asteraceae) based on four sequences, the trnL intron, trnL/trnF intergenic spacer, matK, and ETS. Syst Bot 27:801–814
Bayer RJ, Breitwieser I, Ward J, Puttock C (2007) Tribe Gnaphalieae. In: Kadereit JW, Jeffrey C (eds) The families and genera of vascular plants, flowering plants—Eudicots: Asterales, vol 8. Springer, Berlin, pp 246–283
Beauverd G (1913) Contribution à l’ètude des Composées 8(1). Le genre Stuckertiella Beauverd. Bull Soc Bot Genève 2:205–209
Bentham G, Hooker JD (1873) Compositae. In: Bentham G, Hooker JD (eds) Genera plantarum 2. Lovell Reeve & Co, London, pp 163–533
Blöch C, Dickoré WB, Samuel R, Stuessy TF (2010) Molecular phylogeny of the edelweiss (Leontopodium, Asteraceae-Gnaphalieae). Edinburgh J Bot 67:235–264
Cabrera AL (1961) Observaciones sobre las Inuleae-Gnaphalineae (Compositae) de América del Sur. Bol Soc Argent Bot 9:359–386
Cabrera AL (1963) Compuestas: Gamochaeta. In: Cabrera AL (ed), Flora de la Provincia de Buenos Aires, Colecc Ci Inst Nac Tecnol Agropecu 4(6), Buenos Aires, pp 166–178
Cabrera AL (1971) Compuestas: Gamochaeta. In: Correa MN (ed), Flora Patagónica, Colecc Ci Inst Nac Tecnol Agropecu 8(7), Buenos Aires, pp 117–127
Cabrera AL (1974) Compuestas: Gamochaeta. In: Burkart A (ed), Flora Ilustrada de Entre Ríos, Colecc Ci Inst Nac Tecnol Agropecu 6(6), Buenos Aires, pp 319–327
Cabrera AL (1978) Compuestas: Gamochaeta. In: Cabrera AL (ed), Flora de la Provincia de Jujuy, Colecc Ci Inst Nac Tecnol Agropecu 13(10), Buenos Aires, pp 302–311
Chen YS, Bayer RA (2011) Gamochaeta. In: Wu ZY, Raven PH, Hong DY (eds), Flora of China, Fam 276 Asteraceae, vol. 20–21, Science Press and Missouri Bot. Gard. Press, Beijing, & St. Louis, pp 776–778
Darriba D, Taboada GL, Doallo R, Posada D (2012) jModelTest 2: more models, new heuristics and parallel computing. Nature Meth 9:772. doi:10.1038/nmeth.2109
Deble LP, Marchiori JNC (2007) Sinopse do género Gamochaeta Weddell (Asteraceae-Gnaphalieae) no Brasil. Balduinia 10:21–31
Dillon MO, Sagástegui-Alva A (1991a) Sinopsis de los géneros de Gnaphaliinae (Asteraceae-Inuleae) de Sudamérica. Arnaldoa 1:5–91
Dillon MO, Sagástegui-Alva A (1991b) Gamochaeta. In: Macbride JF & collab (eds), Flora of Peru, Asteraceae: Part V. Fieldiana, Bot, n.s. 26:27–32
Doyle JJ, Dickson EE (1987) Preservation of plant samples for DNA restriction endonuclease analysis. Taxon 36:715–722
Drummond AJ, Rambaut A (2007) BEAST: Bayesian evolutionary analysis by sampling trees. BMC Evol Biol 7:214. doi:10.1186/1471-2148-7-214
Drummond AJ, Ho SYW, Phillips MJ, Rambaut A (2006) Relaxed phylogenetics and dating with confidence. PLoS Biol 4:e88. doi:10.1371/journal.pbio.0040088
Drummond AJ, Suchard MA, Xie D, Rambaut A (2012) Bayesian phylogenetics with BEAUti and the BEAST 1.7. Molec Biol Evol 29:1969–1973
Drury DG (1970) A fresh approach to the classification of the genus Gnaphalium with particular reference to the species present in New Zealand (Inuleae-Compositae). New Zealand J Bot 8:222–248
Drury DG (1971) The American spicate cudweeds adventive to New Zealand (Gnaphalium Section Gamochaeta-Compositae). New Zealand J Bot 9:157–185
Farris JS (1989) The retention index and the rescaled consistency index. Cladistics 5:417–419. doi:10.1111/j.1096-0031.1989.tb00573.x
Farris JS, Albert V, Källersjö M, Lipscomb D, Kluge A (1996) Parsimony jackknifing outperforms neighbor-joining. Cladistics 12:99–124
Freire SE (1998) Inuleae. Compositae. In: Bocquet GF, Crosby MR (eds) Flora del paraguay, vol 27. Conservatoire et Jardin botaniques de la Ville de Genéve-Missouri Botanical Garden, Geneve & St Louis, pp 1–100
Freire SE, Iharlegui L (1997) Sinopsis preliminar del género Gamochaeta (Asteraceae, Gnaphalieae). Bol Soc Argent Bot 33:23–35
Freire SE, Iharlegui L (2008) Asteraceae: Gamochaeta. In: Zuloaga FO, Morrone, O, Belgrano, MJ (eds), Catálogo de las Plantas Vasculares del Cono Sur, Monogr Syst Bot Miss Bot Gard, Missouri Botanical Garden, St Louis 107:1307–1312
Freire SE, Iharlegui L (2014) Gamochaeta. In: Anton, AM, Zuloaga, FO, Belgrano, MJ (eds), Freire, SE (coord), Flora Vascular Argentina, Asteraceae: Anthemideae-Gnaphalieae, 7(1). Estudio Sigma, Buenos Aires, pp 463–482
Freire SE, Paz Deble L, Iharlegui L (2011) Tribe Inuleae. In: Reis A (ed) Flora Ilustrada Catarinense. Herbário Barbosa Rodrigues, Itajai, pp 1067–1197
Freire SE, Chemisquy MA, Anderberg AA, Beck SG, Meneses RI, Loeuille B, Urtubey E (2015) The Lucilia group (Asteraceae, Gnaphalieae): phylogenetic and taxonomic considerations based on molecular and morphological evidence. Pl Syst Evol 301:1227–1248. doi:10.1007/s00606-014-1147-0
Galbany-Casals M, Andres-Sanchez S, Garcia-Jacas N, Susanna A, Rico E, Martinez-Ortega MM (2010) How many of Cassini anagrams should there be? Molecular systematics and phylogenetic relationships in the Filago group (Asteraceae, Gnaphalieae), with special focus on the genus Filago. Taxon 59:1671–1689
Goloboff PA, Farris JS, Nixon KC (2008) TNT, a free program for phylogenetic analysis. Cladistics 24:774–786
Hall TA (1999) BioEdit: a user-friendly biological sequence alignment editor and analysis program for Windows 95/98/NT. Nucleic Acids Symp Series 41:95–98
Hilliard OM (1983) Gamochaeta (sub Gnaphalium). In: Leistner OA (ed) Flora of southern Africa 33(7) Asteraceae-Inuleae 2-Gnaphaliinae 1. Botanical Research Institute, Pretoria, pp 27–29
Hilliard OM, Burtt BL (1981) Some generic concepts in Compositae–Gnaphaliinae. Bot J Linn Soc 82:181–232
Hind DJN (2011) An annotated preliminary checklist of the Compositae of Bolivia. version 2. Available at: http//www.kew.org/science/tropamerica/boliviacompositae. Accessed 11 May 2012
Hoffmann O (1890) Compositae-Inuleae. In: Engler A, Prantl K (eds) Die Naturlichen Pflanzenfamilien 4(5). Wilhelm Engelmann, Leipzig, pp 172–210
Hughes CE, Eastwood RJ (2006) Island radiation on a continental scale: exceptional rates of plant diversification after uplift of the Andes. Proc Natl Acad Sci USA 103:10334–10339. doi:10.1073/pnas.0601928103
Hurvich CM, Tsai CL (1989) Regression and time series model selection in small samples. Biometrika 76:297–307. doi:10.1093/biomet/76.2.297
Kluge AG, Farris SJ (1969) Quantitative phyletics and the evolution of anurans. Syst Zool 18:1–32. doi:10.2307/2412407
Ling Y, Tseng YQ (1978) Miscellaneous notes on Chinese compositae. Acta Phytotax Sin 16:82–86
Marticorena C, Quezada M (1985) Catálogo de la flora vascular de Chile. Gayana, Bot 42:5–157
Merxmüller H, Leins P, Roessler H (1977) Inuleae-systematic review. In: Heywood VH, Harborne JB, Turner BL (eds) The Biology and chemistry of the Compositae. Academic Press, London, pp 577–602
Nesom GL (1990a) Taxonomic status of Gamochaeta (Asteraceae: Inuleae) and the species of the United States. Phytologia 68:186–198
Nesom GL (1990b) Taxonomic summary of Omalotheca (Asteraceae: Inuleae). Phytologia 68:241–246
Nesom GL (2004) New species of Gamochaeta (Asteraceae: Gnaphalieae) from the eastern United States and comments on similar species. Sida 21:717–742
Nesom GL (2006) Anaphalis, euchiton, facelis, gamochaeta, gnaphalium, omalotheca, pseudognaphalium, xerochrysum (Gnaphalieae). In: Flora of North America Editorial Committee (ed) 1993+. Flora of North America North of Mexico, vol 19. Oxford University Press, New York, pp 415–442
Pruski JF (2011) Asteraceae: Gamochaeta. In: Davidse G, Sousa Sánchez M, Knapp S, Chiang Cabrera F (eds), Flora Mesoamericana 5(2). Missouri Botanical Garden Press, México DF, pp 598–610
Rambaut A (2014) Tree Figure Drawing Tool, version 1.4.2, Institute of Evolutionary Biology, University of Edinburgh
Rémy EJ (1849) Compuestas. In: Gay C (ed), Historia física y política de Chile. Botánica 4, en casa del autor, Paris, pp 5–317
Samarakoon T, Wang SY, Alford MH (2013) Enhancing PCR amplification of DNA from recalcitrant plant specimens using a trehalose-based additive. Appl Plant Sci 1:1200236. doi:10.3732/apps.1200236
Smissen RD, Galbany-Casals M, Breitwieser I (2011) Ancient allopolyploidy in the everlasting daisies (Asteraceae: Gnaphalieae): complex relationships among extant clades. Taxon 60:649–662
Sugiura N (1978) Further analysis of the data by Akaike’s information criterion and the finite corrections. Commun Stat- Theory Methods A7:13–26
Taberlet P, Gielly L, Pautou G, Bouvet J (1991) Universal primers for amplification of three non-coding regions of chloroplast DNA. Pl Molec Biol 17:1105–1109. doi:10.1007/BF00037152
Wagenitz G (1965) Compositae (Korbblutler) In: Hegi G (ed), lllustrierte Flora von Mitteleuropa 2. Augflage, 6(3) Lieferung 2, Carl Hanser Verlag, Munchen
Ward J, Bayer RJ, Breitwieser I, Smissen R, Galbany-Casals M, Unwin M (2009) Gnaphalieae. In: Funk VA, Susanna A, Stuessy TF, Bayer RJ (eds) Systematics, evolution, and biogeography of compositae. International Association for Plant Taxonomy, Vienna, pp 539–588
Webb CJ (1988) Asteraceae: Gnaphalium. In: Webb CJ, Sykes WR, Garnock-Jones PJ (eds) Flora of New Zealand, vol. 4, Botany Division D.S.I.R., Christchurch, pp 234–249
Weddell HA (1855) Gamochaeta pars 4–6, pp 151–154 in Chloris Andina, P. Bertrand, Paris, pp 473–484
White TJ, Bruns T, Lee S, Taylor J (1990) Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics. In: Innis MA, Gelfand DH, Shinsky JJ, White TJ (eds) PCR protocols: a guide to methods and applications. Academic Press, San Diego, pp 315–322
Acknowledgments
We express our thanks to the reviewers for critical reading of the manuscript; to S. Beck (LPB, Bolivia) for his assistance during field work in Bolivia; to F. Zuloaga (SI, Argentina) and A. Tribsch (SZU, Austria) who provided material from their own collections; to L. Aagesen for comments on early drafts of the manuscript; to the curators of CONC, LP, LPB, MNCS, MO, SI, SZU, USMS and W for providing materials; to F. Zuloaga and S. Beck for permission to use their photographs included in Fig. 6; J. Hurst for technical assistance. This work was supported by Consejo Nacional de Investigaciones Científicas y Técnicas for financial support (PIP 112-200801-02196) to S. E. Freire and FONDECYT No. 1150425 to A. Moreira Muñoz.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Handling editor: Christoph Oberprieler.
Appendix
Appendix
Species used in the molecular studies with voucher information and GenBank accession numbers (ETS, ITS and trnL-F). Accession number in bold indicates data obtained from GenBank. * indicates sequence not obtained for that marker.
Achyrocline tomentosa. KM091358, KM091389, KM091420. Antennaria chilensis var. magellanica. KM091373, KM091395, *. Antennaria dioica. FN645610, FJ639964, FN645790. Belloa chilensis KM091349, KM091388, KM091430. Berroa gnaphalioides. KM091355, KM091386, KM091418. Facelis plumosa. KM091372, KM091394, KM091428. Filago fuscescens. FN645579, FN645846, FN645764. Filago lutescens. FN645596, FN645883, FN645779. Filago pyramidata. FN645589, FN645872, HM364535. Gamochaeta affinis. Argentina. Tierra del Fuego: Ushuaia, Bahía Valentín, 16 Oct 1971, Dudley et al. 303 (MO). KX078016, *, *. Gamochaeta alpina. (under Gamochaetopsis alpina). KM091356, KM091390, KM091417. Gamochaeta ambatensis. Argentina. Jujuy: Valle Grande, de San Francisco a Alto de Calilegua, 21 Feb 2008, Zuloaga et al. 10324 (SI). KX078017, *, KX078049. Gamochaeta americana. KM091382, KM091411, KM091437. Gamochaeta andina. Chile. Maule: Maule, Lueberg and Teiller 2290 (CONC). KX078018, KX077990, KX078050. Gamochaeta antillana. USA. Mississippi: Lamar County, Alford 3892 (USMS). KX078019, KX077991, KX078051. Gamochaeta argyrinea. USA. Nesom WMGT8 (LP). KX078020, *, *. Gamochaeta beckii. Bolivia. La Paz: Nor Yungas, camino a Coroico, entrada del camino viejo hacia la Mina Lourdes, 30 Mar 2010, Urtubey et al. 508 (SI). KX078021, KX077992, KX078052. Gamochaeta calviceps. Argentina. Entre Ríos: Colón, camino al arroyo El Palmar, 51 m a. s. l., 7 Dec 2008, Urtubey and Baztarrica 384 (SI). KX078022, *, *. Gamochaeta camaquaensis. Brasil. Rio Grande do Sul: Cacapava do Sul, 30 Sep 2009, Deble and Deble 11879 (SI). KX078023, KX077993, KX078053. Gamochaeta capitata (under Stuckertiella capitata) KM091369, KM091398, KM091412. Gamochaeta chamissonis. Chile. Metropolitana: El Yeso, 1283 m a. s. l., 26 Jan 2012, Urtubey and Freire 713 (SI). KX078024, KX077994, KX078054. Gamochaeta chionesthes. USA. Giorgia: 15 Apr 2001, Nesom GASC04-26 (LP). KX078025, KX077995, KX078055. Gamochaeta coarctata. Argentina. Neuquén: Minas, de Chos Malal a Andacollo, ca. 40 km junto al arroyo El Manzanito, 1443 m a. s. l., 12 Jan 2009, Urtubey et al. 409 (SI). KX078026, KX077996, KX078056. Gamochaeta depilata. Chile. Biobío: Mihoc et al. 4092 (CONC). KX078027, KX077997, KX078057. Gamochaeta deserticola. Bolivia. La Paz: Murillo, 4100 m a. s. l., 4.8 km al NE del autopista por el camino subiendo el Valle del Río Kaluyo 28 Feb 1987, Solomon 16209 (LPB). KX078028, *, KX0780580. Gamochaeta erecta. Brasil. Rio Grande do Sul: Cacapava do Sul, 28 Oct 2009, Deble and Deble 11874 (SI). KX078029, KX077998, KX078060. Gamochaeta erythractis. Argentina. Jujuy: Humahuaca, camino a la Mina Aguilar, 3940 m a. s. l., 23 Mar 2009, Urtubey and Freire 442 (SI). KX078030, KX077999, KX078061. Gamochaeta falcata. Argentina. Córdoba: Calamuchita, desde Yacanto de Calamuchita al Champaquí, ca. 2100 m a. s. l., 18 Jan 2006, Urtubey and Baztarrica 197 (SI). *, KX078000, *. Uruguay. Colonia: Conchillares, playa, 18 Nov 2010, Urtubey 520 (SI). KX0780310, *, *. Gamochaeta filaginea. Argentina. Buenos Aires: Isla Martín García, 24 Sep 2000, Hurrell 4363 (LP). KX078032, *, *. Gamochaeta grazielae. Brasil. Río de Janeiro: Nov 2006, Deble and Deble 6018 (SI). KX078033, KX078001, KX078062. Gamochaeta humilis Bolivia. La Paz: Nor Yungas, camino a Coroico, bajando de la ruta en la entrada del camino viejo hacia la Mina Lourdes, 3919 m a. s. l., 30 Mar 2010, Urtubey et al. 506 (SI). KX078034, KX078002, KX078059. Gamochaeta longipedicellata. Bolivia. La Paz: Murillo, 24 Mar 2010, Urtubey et al. 473 (SI). KM091381, KM091410, KX078063. Gamochaeta lulioana. Bolivia. La Paz: Murillo, en el borde de bofedal, 4661 m a. s. l., 24 Mar 2010, Urtubey et al. 480 (SI). KX078035, KX078003, KX078064. Gamochaeta nivalis. Argentina. Río Negro: Bariloche, Cerro Catedral, 16 Jan 2009, Urtubey et al. 415 (SI). KX078036, *, KX078065. Gamochaeta pensylvanica. Argentina. Buenos Aires: La Plata, ciudad Vieja, 6 Oct 2012, Urtubey 755 (SI). *, *, KX078066. USA. Mississippi: Lamar County, Alford 4156 (USMS). KX078037, KX078004, *. Gamochaeta procumbens. Argentina. Tierra del Fuego: Rio Grande, Estancia Cullén, 6 Jan 1971, Goodall 3118 (Herb. Goodall). KX078038, KX078005, *. Gamochaeta purpurea. USA, Nesom WMGT13 (LP) KX078039, KX078006, KX078067. Gamochaeta serpyllifolia Chile. Los Ríos: Valdivia, Comuna de Panguipulli, faldeos Volcán Chosuenco, 39º55’/71º59’, 1558 m a. s. l., 5 Feb 2012, Baeza 4357 (CONC). KM091380, KX078010, KX078068. Gamochaeta simplicicaulis. Argentina. Jujuy: Tilcara, camino de Molulo a Huairahuasi, 3100 m a. s. l., 11 Feb 2010, Zuloaga et al. 11702 (SI). KX078040, KX078007, KX078069. Gamochaeta sphacelata. Argentina. Salta: Santa Victoria, Nazareno, 3308 m a. s. l., Adler 4 (MNCS 1665). KX078041, *, KX078070. Gamochaeta spiciformis. Chile. Magallanes: Domínguez 984 (CONC). *, KX078011, KX078071. Argentina. Tierra del Fuego: Ushuaia, Brown Sawmill, Goodall 286 (LP). KX078042, *, *. Gamochaeta stachydifolia. Argentina. Buenos Aires: Tandil, hacia sierras de Las Animas desde el Hotel Elegance, 12 Nov 2005, Urtubey and Baztarrica 179 (SI) KX078043, *, *. Ibid. Frente al hotel Elegance, 1 Oct 2010, Urtubey 513 (SI). *, KX078008, KX078072. Gamochaeta subfalcata. Uruguay. Canelones: La Floresta, 19 Nov 2010, Urtubey 524 (SI). KX078044, KX078009, KX078073. Gamochaeta ustulata USA. Oregon: Lincoln County, Shannon Straub 201 (USMS). KX078045, KX078012, KX078074. Gnaphalium austroafricanum. FN645630, FN645830, FN645756. Gnaphalium declinatum FR821617, *, FR822634. Gnaphalium uliginosum Austria. Upper Austria: Innviertel, Reichersberg am Inn, Hübing, 22 Aug 2010, Pflugbeil 936 (SZU). KX078048, KX078015, KX07807077. Jalcophila boliviensis. KM091370, KM091402, KM091429. Jalcophila ecuadorensis. KM091383*,*. Leontopodium alpinum FM173135, GU943413, AF141821 + AF141733. Leontopodium microphyllum. FJ640015, FJ639947, FJ640047 + FJ639977. Lucilia acutifolia. KM091374, KM091396, KM091432. Omalotheca norvegica (Gunnerus) Sch.-Bip. & F.W.Schultz. Russia. Republic Altai: surroundings of Zeminsky (Zeminskij) pass to area NE of the pass, ca. 105 km S Gorno Altaysk (Altajsk), subalpine Siberian pine forest, 1700-1730 m a. s. l., 11 Aug 2003, Tribsch and Essl 10383 (W). KX078047, KX078013, KX078075. Omalotheca supina FN645558, AY445230, FN645789. Omalotheca sylvatica. Austria. Carinthia: Lavanttal, Koralpe, Hartelsberg SE Wolfsberg, 1345 m a. s. l., Riegler-Hager 921 (W). KX078046, KX078014, KX078076.
Rights and permissions
About this article
Cite this article
Urtubey, E., López, A., Chemisquy, M.A. et al. New circumscription of the genus Gamochaeta (Asteraceae, Gnaphalieae) inferred from nuclear and plastid DNA sequences. Plant Syst Evol 302, 1047–1066 (2016). https://doi.org/10.1007/s00606-016-1316-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00606-016-1316-4