Abstract
Paullinia weinmanniifolia (Sapindaceae) is an endemic Brazilian climber found in the Atlantic Forest. The flowers are arranged in synflorescences with approximately 34 thyrses. In the studied material, each thyrse produced 85.8 male flowers and 15.8 female flowers. The small, diclinous and zygomorphic flowers present a sophisticated morphology, described here in detail. They are visited by many insects, with bees being the main pollinators. Sex expression was investigated by monitoring one population of restinga in Maricá Environmental Protection Area, Rio de Janeiro State, Brazil, during 2–4 years. Experiments were conducted taking into account thyrse, synflorescence, plant and population. Most individuals showed a duodichogamic sequence of flowering (male–female–male). However, the population overall had a more complex flowering pattern: some individuals were protogynous, others protandrous; a few individuals produced flowers of only one sex, and some individuals changed sex expression in the second year of the study, either from male to female or from female to male. This was the first ever labile sex expression recorded for the tribe Paullinieae. Anthetic female and male flowers were never found simultaneously in the same thyrse or synflorescence. Nevertheless, the two flower morphs overlapped, though rarely, within the same individual. The pattern of flowering observed in this species maximizes the level of outcrossing, since the temporal separation of male and female flowers on the same plant is precise enough for the species to be regarded as (obligatorily) xenogamous.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Sapindaceae are a monophyletic family (Acevedo-Rodríguez 1993; Gadek et al. 1996; Harrington et al. 2005; Buerki et al. 2009) and most species are tropical and subtropical (Judd et al. 2009). The family includes trees, shrubs and lianas with tendrils (Acevedo-Rodríguez et al. 2010). Paullinieae are a largely Neotropical tribe that comprises one-third of the species in the family (Harrington et al. 2005) and are characterized by lianas with tendrils. All Paullinieae species, except for Thinouia Triana and Planchon, exhibit zygomorphic highly elaborate flowers with appendages related to the signalling, protection and maintenance of nectar quality (Endress and Matthews 2006), but despite the complexity of the flowers little is known about the floral biology and pollination in this group.
Flowers of Sapindaceae are seemingly bisexual but functionally unisexual (Acevedo-Rodríguez et al. 2010), although frequently described as polygamous (Radlkofer 1931–1934; Croat 1976; Aluri et al. 1998; Judd et al. 2009). However, dioecy, monoecy with dichogamous (sequence of male–female or female–male flowers) or duodichogamous flowering (sequence of male–female–male flowers), monoecy with heterodichogamous flowering (two reciprocal morphs: male-first and female-first flowering individuals), or labile sex expression (individuals reverse their sex allocation depending on environment/phenotype interactions) have been reported for Sapindaceae species (Lloyd and Webb 1986; Verdú and Gleiser 2005; Renner et al. 2007; Acevedo-Rodríguez et al. 2010). Some monoecious species, as Cupania guatemalensis Radlk. (Bawa 1977) and Allophylus serratus Kurz (Aluri et al. 1998), exhibit synchronized dichogamy (after Lloyd and Webb 1986) with little or no overlap of male and female flowers within an individual.
Among Sapindaceae, Acer L. is the best studied genus, showing a large diversity in sex expression among and within species (reviewed by Renner et al. 2007). Phylogenetic analysis in the genus allowed inferences on the evolution of sexual systems, suggesting that the most frequent transitions were those from monoecy with duodichogamous flowering to dioecy or to monoecy with heterodichogamous flowering; returns from dioecy to other systems are unlikely (Renner et al. 2007). Nevertheless, the temporal polymorphisms and sexual expression in Paullinieae are poorly known, except for references or some data about duodichogamous flowering for Paullinia coriacea Casar. and P. weinmanniifolia Mart. from sandy coastal plains (restingas) at Rio de Janeiro (Ormond et al. 1991), and for P. cupana Kunth, the popular guaraná (Escobar et al. 1984), and P. rugosa Radlk. (Paula 1989), from the Amazon region. Thus, all the information on sexual systems of Sapindaceae is based on studies with trees (as Acer, Allophylus, Cupania, and Xerospermum), and there is a knowledge gap on mating strategies of lianas, the more derived group in the family (Buerki et al. 2009).
We here report on a 2–4 years study of the sexual expression of P. weinmanniifolia, showing the sequence of male and female flowers in the inflorescences and individuals, in addition to aspects of floral morphology, floral biology and possible pollinators in a population of the sandy coastal plains from the southeast of Brazil. This is the first study that presents a thorough monitoring of sexual expression in a Sapindaceae liana population and we aimed to answer the following questions: (1) is there a high synchronization between the male and female flowers at different levels of floral organization (thyrse, synflorescence and individual plant)? (2) In Sapindaceae, is the complex sexual expression described for trees also found in lianas?
Materials and methods
Studied species
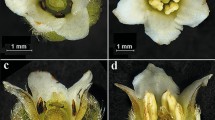
Paullinia weinmanniifolia Mart., suberect shrub (up to 1 m tall, forming isolated thickets), erect arching shrub (approximately 2.5 m tall) or liana (supported by other individuals). Synflorescences bracteate and frondose-bracteate (Fig. 1), up to 34 loosely arranged or condensed thyrses that may or may not have a pair of opposite tendrils, coiled at base (all thyrses with tendrils—49 %; all thyrses without tendrils—31 %; thyrses with and without tendrils—20 %). Thyrses with 25–43 paraclades of subwhorled or crossed-opposite cincinni, each one with 4–7 unisexual flowers. Flowers zygomorphic, tetramerous, nectariferous, and scented (Figs. 2a–c, n, 3a, b). Calyx light green, glandular and pubescent, with sepals cucullate in two whorls, outer with two lateral sepals (1.2 ± 0.3 mm long), and inner with two sepals (2.3 ± 0.5 mm long), the posterior entire and the anterior emarginated (Fig. 2a–c, d–g). Corolla white, with four membranaceous petals (2.5 ± 0.5 mm long), bearing a basal hood-shaped petaloid appendage (Fig. 2h, i), each appendage connivent with densely villose margin and a fleshy yellowish crest at the apex; posterior petals bifurcate and lateral petals simple (Fig. 3a, b, e, g), posterior petal appendages with a deflected ligule projected under the crest (Fig. 2h). Nectary unilateral, 4-lobed, two posterior ovoid with obtuse apex and two lateral smaller (Figs. 2c, n, 3c); petals and appendages covered by the nectary lobes (Fig. 2c, n). Androecium with eight stamens, five long (2.4 ± 0.3 mm long) and three short (1.6 ± 0.3 mm long), arranged in a single whorl, around a pistillode (Fig. 2a–c, j–k, m, m′), carried by an androgynophore (Fig. 2c, j); filaments pubescent (Fig. 2j, m, m′), anthers rimose and introrse; pollen grains white, dry, triangular, triporate, with a rugulose, perforate exine. Gynoecium with a superior trigonous-ellipsoid ovary, 3-carpellate, with a single ovule per carpel; single style with three papillate stigmatic lobes (Fig. 2n, o, p); staminodes arranged around the ovary, the smallest 1.3 ± 0.3 mm long, the largest 1.7 ± 0.3 mm long (Fig. 2l, l′, n).
Flowers of Paullinia weinmanniifolia a–c, j male flowers. d–e External lateral sepals. f Anterior emarginate sepal. g Posterior sepal. h–i Posterior and lateral petals, showing appendage with crest at the apex. k Pistillode. l–l′ Staminodes. m–m′ Stamens. n Female flower. o Ovary cross section. p Gynoecium longitudinal section. an androgynophore, lat se lateral sepal, post se posterior sepal, ant se anterior sepal, post pet posterior petal, lat pet lateral petal, st stamens, lat nec lob lateral nectar lobe, post nec lob posterior nectar lobe, cr crest, p pistillode, stg stigma (G.V. Somner 930 and H.A. Lima)
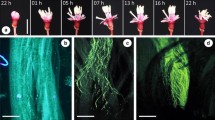
Paullinia weinmanniifolia at the Maricá Environmental Protection Area, Rio de Janeiro State, Brazil. a Male flower. b Female flower. c Detail of the posterior nectar lobes of a male flower. d Xylocopa (Neoxylocopa) ordinaria visiting the flowers. e Apis mellifera visiting the flowers. f Thyrse with all flowers in female phase
Studied area
The study was carried out along four flowering seasons (1999, 2000, 2009 and 2010), in the Maricá Environmental Protection Area, Maricá municipality, State of Rio de Janeiro, Southeastern Brazil (22°53′–22°52′S; 42°52′–42°51′W). Paullinia weinmanniifolia occurs on the sandy coastal plains, specifically in scrub dunes (Menezes and Araújo 2005).
Floral morphology was studied in the field, with the naked eye or with the help of a pocket magnifying glass, and also in the laboratory using a stereoscopic microscope. Fifty synflorescences from ten plants were randomly selected to classify thyrse type in relation to the presence or absence of tendrils. The number of paraclades of cincinni and buds were determined for 20 thyrses from ten plants. Flower measurements were obtained from both sexes (n = 25 males and 25 females; average ± standard deviation), and averages were compared using Student’s t test.
The period of anthesis was observed in four plants, on six different days, from 8 p.m. to 6 a.m., including sex type, petal and sepal modifications, time of stigmatic receptivity (female flowers), time of anther dehiscence (male flowers) and beginning of scent emission and nectar secretion. Stigmatic receptivity was tested using an aqueous solution of 20 % hydrogen peroxide (Kearns and Inouye 1993). The neutral-red test (Vogel 1990) was used to identify possible scent-producing glands (osmophores). Pollen grain viability was estimated using acetocarmine (Radford et al. 1974) and the presence of pollenkitt was tested with Sudan III (Johansen 1940).
The diversity and behaviour of flower visitors were studied through 30 h of direct observations, made in 10 days, covering the period from 6:00 a.m. to 4:00 p.m. The insect visitors were collected with a net, fixed for identification, and included in the Costa Lima (CECL) entomological collection, located at the Departamento de Entomologia e Fitopatologia, Universidade Federal Rural do Rio de Janeiro and at the Departamento de Entomologia, Museu Nacional, Universidade Federal do Rio de Janeiro. To find the frequency of flower visitors, during 6 days in 2010, parallel to the observations on sexual expression, we identified and counted all insects visiting each flower of the 31 plants, during 3–10 min within a period from 8:00 a.m. to 2:00 p.m.
The study of reproductive phenology and sexual system was performed during the longest blooming episode of P. weinmanniifolia (March to June), taking into account four levels of organization: thyrse, synflorescence, plant and population. To verify the patterns of production of male and female flowers in individual thyrses and the average of male and female flowers per thyrse, we randomly sampled 27 thyrses, at bud stage, from 5 plants. These thyrses were bagged with cotton bags to observe the alternating cycles of male and female flowers. At intervals of 3–9 days, all the open or fallen flowers inside the bags were collected, observed and counted, and afterwards the thyrses were re-bagged. These flowers were analysed in relation to sex and morphological conditions of senescence that allowed us to differentiate anthesis, newly fallen (white with a few brown spots), and old flowers (totally brown and dried). This procedure lasted up to the anthesis of the last flower of the thyrses.
To identify and quantify the superposition of female and male flowers within thyrses, synflorescences and individuals, as well as to study the patterns of flowering at plant and population levels, we selected 31 plants that were labelled and analysed once a week, from 27 March to 20 June 2009, and from 7 April to 4 June 2010, comprising 12 observations each year. Monitoring the same plants for two consecutive flowering periods allowed us to verify annual changes in the dynamics of alternating male and female flowers within and between plants. Six thyrses and six synflorescences from each of the 31 plants were randomly chosen every week; they were analysed with respect to the total number of male and female flowers in anthesis. At the end of the flowering periods, 1945 thyrses (n = 909 in 2009 and n = 1036 in 2010) and 1851 synflorescences (n = 842 in 2009 and n = 1009 in 2010) were sampled. To examine the difference between the number of male and female thyrses/synflorescences produced per plant, a Chi-square (χ 2) test was carried out after the data were checked for normality. In both cases, significant values of p < 0.05 were considered. To test differences between the opening rhythm of male and female flowers, we compared the average number of flowers in anthesis per day in thyrses/synflorescences in male and female phases, with the Student’s t test. The analyses were performed using the Statistic 8.0 Program (StatSoft Inc 2007).
The activity (presence and absence) and intensity of female and male flowering were evaluated during the entire period. To estimate the flowering intensity (each plant, once a week), was categorized from 0 to 3, as follows: 0—absence of event; 1—event with magnitude from 1 to 33 %; 2—from 34 to 66 %; and 3—from 66 to 100 % (modified from Fournier 1974, on the number of categories) and the results are shown in the phenological graphic (Fig. 6).
Two voucher specimens, G.V. Somner and H. Lima (1320, 1467), were deposited in the herbarium RBR, located in the Departamento de Botânica da Universidade Federal Rural do Rio de Janeiro.
Results
Floral biology
Flowers are seemingly bisexual but functionally unisexual, zygomorphic, white, nectariferous (Figs. 2a–c, n, 3a, b) and scented. Buds start to open between 8:20 p.m. and 10:00 p.m. The anterior emarginate sepal is the first to open, followed by the others. At this stage, a small aperture appears at the bud apex, a feature that is concurrent with the onset of nectar secretion. The four petal appendages are connivent by the villose margin and form a floral pseudo-tube, where nectar accumulates (Figs. 2c, n, 3c). The yellowish fleshy appendage crests function as a nectar guide (Fig. 3a, b, e, f). Male flowers are larger (5.3 ± 0.5 mm) than female ones (3.3 ± 0.4 mm; t = 15.1, p < 0.01). In both, reproductive structures are dislodged to the anterior part of the receptacle (Fig. 3a, b) and curved towards the appendage crests (Fig. 2a–c, n). Anther dehiscence occurs in half-opened buds, between 1:40 a.m. and 3:40 a.m., and the released pollen grains are 95 % viable (n = 300 pollen grains in five plants). At about 4:30 a.m., the flowers begin to release a weak, sweet odour. The base of the petals, filaments and the emarginate sepals of male and female flowers were tinged by neutral red, suggesting the presence of osmophores. Opening of female and male flowers is completed between 4:30 a.m. and 6:00 a.m. At this time, many insects already visit the flowers, however, only at 6:30 a.m. the female flowers expose the three stigmatic areas, which change from greenish to whitish and become humid when receptive. The staminodes of female flowers resemble the stamens, but anthers are indehiscent with 100 % non-viable pollen grains (n = 300 pollen grains in five plants).
The flowers last for 2 days and, after this period, the corolla closes, the floral structures gradually change from white to brown, and the nectar lobes shrink. The petals and other floral structures remain until the initial development of the fruit, and the sepals are persistent.
Floral visitors
The flowers are visited by a great variety of insects (Table 1). Visits begin when the sun rises at 5:45 a.m. (in the summer) and are intense until 2:00 p.m., with a peak around 10:00 a.m. (Fig. 4). Although no data was recorded after 2:00 p.m., insects were observed to forage as late as 4:00 p.m. The majority of visitors use the nectar as a food reward, although the beetle Astylus lineatus and the bee Trigona spinipes (author names appear in Table 1) also collect pollen grains directly from the anthers of male flowers. Bees are the most frequent visitors of both male and female flowers (82.4 %, of those 60.2 % by Apis mellifera and 22.2 % by native bees; n = 108 observed visits), and were recorded in all plants observed (Fig. 3d, e). In general, they hold the flower or the thyrse with the first pair of legs, curve the front part of the body towards the floral pseudo-tube and introduce the tongue into the pseudo-tube to suck nectar. This behaviour leads the bees to touch the anthers of the male flowers and the stigmas of the female flowers with the front part of the head. Bees are considered to be the main pollinators of the species. Despite the great number of Lepidoptera species observed as floral visitors, none of them are frequent, except Rekoa palegon. Lepidoptera did not always result in touching the anthers or the stigmas.
Reproductive phenology
Flowering is subannual, with two blooming episodes, preceded by intense leaf production and separated by an interval of 4 months. The first episode begins in March and lasts until the end of June, that is, partly included in the hot rainy season (October–March) and partly in the cold dry season (April–September). This episode is longer, with peak flowering between the end of April and the first fortnight of May, when almost all individuals of the population flower intensely. In the second blooming episode, most plants either do not flower or bloom inconspicuously. This episode begins in November and lasts until the end of December, within the hot rainy season, with peak flowering from mid-November to early December.
The mature fruit is a reddish 3-winged septifragal capsule, exposing 1–3 black seeds, 2/3 covered by a white and fleshy sarcotesta. The fruits ripen from 2.5 to 3.5 months after pollination. The seeds are released from early July to the first week of October and from February to mid-March.
Sequence of male and female flowers in thyrses
The experiment of bagging thyrses showed five types of thyrses, taking into consideration the sex of the first flower and the number of alternating developmental cycles of male and female flowers. Thyrses with only male flowers were rare and those with only females were not seen (though data from sex expression in the plants and in the population, described later, showed one female plant). In general, thyrses alternated the sequence in which male and female flowers were being produced once, twice or three times. Thyrses with the sequence ‘male–female–male’ flowers were the most common and those starting with the production of female flowers were rare (Table 2). In the thyrses, male flowers opened in small daily quantities, but within longer periods than the female flowers, which all opened in the course of a few days (Fig. 5).
Relative frequency of male (grey columns) and female (black columns) flowers observed from a sample of six thyrses in five plants of Paullinia weinmanniifolia at the Maricá Environmental Protection Area, Rio de Janeiro State, Brazil. Male and female flowers were found in the same interval of time (3–9 days) but they were at different stages—male flowers in anthesis and female flowers in senescent conditions, or vice versa
Superposition of female and male flowers in thyrses and synflorescences
Thyrses and synflorescences in the male phase were much more frequent than those with flowers in the female phase (thyrses: χ 2 = 1011.7, p < 0.0001; synflorescences: χ 2 = 1000.1; p < 0.0001). However, once again, the average number of male flowers in anthesis per day was lower than that of the female flowers (thyrses: t = −8.94, p < 0.0001; synflorescences: t = −6.09, p < 0.0001), due to the high concentration of the latter in a short period of activity (Table 3), confirming the results shown in Fig. 5. Overlap of male and female flowers on any day was not recorded in thyrses and synflorescences (Table 3; Fig. 3e, f).
Sex expression in the plants and in the population
Plants display a complex flowering pattern (Fig. 6). The majority flowered in a duodichogamous way. Six plants in 2009 (7, 11, 15, 25, 27, 30) and nine in 2010 (1, 4, 7, 9, 14, 17, 26, 27, 31) produced synchronized cycles of flowers in the sequence ‘male → female → male’. More cycles also occurred (plants 14, 17, 29 in 2009 and 11, 15, 21, 29 in 2010). Rarely (plants 26 in 2009 and 22 in 2010), we found sequences in an opposite way, i.e. ‘female → male → female’ and, as in the first case, we also observed more cycles (plants 1, 4, 23, 24 in 2009 and 13, 19, 20, 23 in 2010). Some plants flowered dichogamously, including protandrous (‘male → female’: plants 19, 28 in 2009 and plants 8, 30 in 2010) and protogynous (‘female → male’: plants 13, 31 in 2009 and 6, 18 in 2010). We also observed plants that produced only male flowers (plants 2, 6, 8, 16, 18, 20 in 2009 and plants 2, 5, 10, 16 in 2010) or only female flowers (plant 21 in 2009).
Staggering of male (continuous lines) and female (dashed lines) phases in 31 plants of Paullinia weinmanniifolia during 12 weeks of flowering in 2009 and the same plants in 2010. The degree of line width varies with the intensity of flowering, increasing from 1 (lower intensity) to 3 (higher intensity), according to Fournier (1974, modified). The overlap of dashed and continuous lines means superposition of sexes at the plant level
Among 31 plants studied in two consecutive years, we recorded overlaps of male and female flowers, only at the plant level, ten times in 2009 and seven times in 2010. Considering the population level, there are always more plants in the male phase than in the female phase, although both are always present (Fig. 6). The experiments conducted in 2009/2010 (Fig. 6) showed that many of the plants studied had a labile sex expression: (1) plants that began to bloom with male flowers in 2009, began to bloom with female flowers in the following year, or vice versa (plants 1, 4, 6, 18, 19, 20, 21, 22, 24, 26, 31); (2) plants that behaved as males in 2009 produced male and female flowers in 2010 (plants 6, 8, 18, 20). However, two of them (plants 2, 16) maintained the male sexual expression in both years; (3) one plant (plant 21) that produced only female flowers in 2009 had male and female flowers in 2010.
Discussion
This is the first report of labile sex expression for the genus Paullinia, as well as for the tribe Paullinieae. Most individuals showed a pattern of flowering in which there are two cycles of male with an intervening cycle of female flowers, suggesting that P. weinmanniifolia is a duodichogamous species (sensu Lloyd and Webb 1986). This temporal sexual system is known for very few species (Luo et al. 2007), most of them in shrubs or trees of Sapindaceae such as Acer (references see Renner et al. 2007; Shang et al. 2012), Allophylus L. (Aluri et al. 1998), Cupania L. (Bawa 1977), Dipteronia Oliv., Hippocastanum Mill., Deinbollia Schmach. and Thonn., Koelreuteria Laxm. (De Jong 1976), Pappea Sond. and Harv. (Robbertse et al. 2011), Sapindus L. (Subba Reddi et al. 1983) and Talisia L. (Acevedo-Rodríguez 1993). Although the majority of P. weinmanniifolia plants studied presented the typical duodichogamous pattern of flowering, we found many variations in the individual sex expression. Based on these variations, we think that it is more prudent to say that the sexual system of P. weinmanniifolia is predominantly characterized by an alternation of male and female flowers, which does not always follow the same pattern. In 2009, the occurrence in the population of plants starting to bloom with male flowers and plants starting to bloom with female ones led us to suspect the occurrence of heterodichogamy (Renner 2001; Sato 2002; Renner et al. 2007) or some kind of sexual polymorphism similar to that described by Shang et al. (2012) for Acer pictum Thunb. subsp. mono (Maxim.) H. Ohashi, whose populations included three sex phenotypes (duodichogamous 69.1 %, protandrous 19.6 %, protogynous 11.3 %). Moreover, in the same year, the observation of plants producing just one type of flower, especially male ones, could have indicated the presence of andromonoecy. However, in most P. weinmanniifolia plants studied, the individual sexual expression showed plasticity between 2009 and 2010 flowering, suggesting that environmental features rather than genetic are responsible for the changes observed. Golenberg and West (2013) pointed out that the two sex determination systems—‘environmental sex determination’ and ‘genetic sex determination’—are genetic, since environmental factors may regulate the production of hormones involved in the differential expression of genes related to floral development. These interactions may generate effects on the sexual plasticity of some species. We concluded that P. weinmanniifolia has a somewhat labile sex expression, a phenomenon that was also found in Acer (Korpelainen 1998; Renner et al. 2007; Shang et al. 2012). Because of the variation in sex expression, more years of field work are necessary to understand the lability in this species and the correlations with environmental parameters.
Our results about the superposition of male and female flowers in P. weinmanniifolia showed that although thyrses have buds of the two sexes, female and male flowers in anthesis were never found simultaneously in the same thyrse or in the same synflorescence. Nevertheless, the two flower morphs overlapped, although rarely, in different branches of the same individual. Therefore, the sexual synchrony within thyrses, within synflorescences, and within plants is evident. This pattern of flowering maximizes the level of outcrossing, since the temporal separation of male and female flowers on the same plant is precise enough for the species to be regarded as obligatorily xenogamous. The synchrony mentioned above was also suggested for other species of Paullinieae as P. cupana Kunth (Moreira Filho et al. 1975), Urvillea ulmacea Kunth (Zapata and Arroyo 1978) and Allophylus serratus (Aluri et al. 1998), but in the self-compatible Cardiospermum halicacabum, Rama Das et al. (1997) did not find staggering of sexual phases.
In P. weinmanniifolia, there are more male flowers, which are produced in longer periods than the female ones; this has also been recorded for P. rugosa (Benth. ex Radlk.) and P. cupana (Escobar et al. 1984; Paula 1989). This feature seems to be typical for the genus Paullinia since analysis of herbarium material always shows synflorescences with only female flowers or with only male flowers, the latter being much more frequent (G.V. Somner personal observation).
Like many other Sapindaceae (Radlkofer 1931–1934; Ferrucci 1991; Rama Das et al. 1997), P. weinmanniifolia has female flowers with indehiscent staminodes very similar to the stamens of male flowers, including the production of pollen grains, which are 100 % sterile. However, viable pollen grains in non-dehiscing anthers of female flowers have been reported in Acer rubrum L. (Cane 1993), Cupania guatemalensis (Bawa 1977), C. emarginata Cambess. (Lima personal comm.) and Tina striata Radlk. (Vary et al. 2011). In the latter, the authors found loss of function in the pollen grains from female flowers based on the reduced ability to germinate and potential to fertilize ovules. It is noteworthy that Appanah (1982) recorded androdioecy in Xerospermum intermedium Radlk., where the monoclinous flowers have indehiscent anthers during the two first days of anthesis, but on the third day, the anthers open slightly, liberating viable pollen grains on the stigmas, promoting self-pollination. Palynological studies including analysis of the viability of pollen grains from female flowers of Sapindaceae species may contribute to elucidate the evolution of sexual systems in the group.
Bees are the main pollinators of P. weinmanniifolia. This is also observed in other species of Sapindaceae such as Allophylus serratus (Aluri et al. 1998), Cupania guatemalensis (Bawa 1977), Paullinia cupana (Escobar et al. 1984), P. rugosa (Paula 1989) and Xerospermum intermedium (Appanah 1982). In these species, nectar is the main floral resource, but pollen apparently in Cupania guatemalensis (Bawa 1977) and Talisia striata (Vary et al. 2011).
This study broadens the knowledge of sexual strategies in the family Sapindaceae, since it presents the first results on the alternation of male and female flowers, in a highly synchronous manner, for one species of Paullinieae. All the Sapindaceae liana species are monoecious, although the distribution of dichogamy, heterodichogamy, duodichogamy and labile sex expression in the group is still poorly known. The latest phylogenetic studies in Sapindaceae showed that the genera of Paullinieae (sensu Radlkofer 1931) are nested within the Thouinieae, therefore calling for the merging of the two tribes as Paullinieae–Thouinieae (Acevedo-Rodríguez 1993; Harrington et al. 2005; Buerki et al. 2009), recently treated as Paullinieae by Acevedo-Rodríguez et al. (2010). This expanded tribe includes monoecious climbers and monoecious and dioecious arboreal genera, as Allophylus, indicating the potential to evolve separate sexes. Phylogenetic analyses in Acer allowed inferences about the evolution of sexual systems, suggesting transitions from monoecy with duodichogamous flowering to dioecy (Renner et al. 2007). Our results showed that individuals of P. weinmanniifolia seem to constantly adjust their sex allocation, and that duodichogamy can prevent self-fertilization by geitonogamy, as there are very few overlaps between male and female flowering in the same plant. These traits seem to guarantee the production of vigorous seeds through outcrossing, hindering the evolution of dioecy by the establishment of male-sterile mutants in the population, or by disruptive selection on female and male sex allocation gradually increasing gender specialization, as defined in the models for the evolution of dioecy (Charlesworth and Charlesworth 1978; Barrett 2002). On the other hand, all dioecious Sapindaceae are shrubs or trees, and according to Vamosi et al. (2003), dioecy is associated with the woody growth form. Future studies on the sexual system of Sapindaceae lianas may clarify whether the precise temporal separation between male and female phases, combined with some flexibility in sexual allocation, were advantageous outputs for Sapindaceae climbers in achieving outcrossing rates as high as in dioecious species. Moreover, a phylogeny for the tribe is needed to estimate the diversity of the sex expression in an evolutionary approach.
References
Acevedo-Rodríguez P (1993) Systematics of Serjania (Sapindaceae). Part I: a revision of Serjania sect. Platycoccus. Mem New York Bot Gard 67:1–93
Acevedo-Rodríguez P, van Welzen PC, Adema F, van der Ham RWJM (2010) Sapindaceae. In: Kubitzki K (ed) Flowering plants, Eudicots: Sapindales, Cucurbitales, Myrtaceae. The families and genera of vascular plants, vol 10. Springer, Berlin, pp 357–407
Aluri JSR, Subba Reddi C, Rama Das K (1998) Temporal dioecism and pollination by wasps and bees in Allophylus serratus (Roxb.) Radlk. (Sapindaceae). Pl Spec Biol 13:1–5
Appanah S (1982) Pollination of androdioecious Xerospermum intermedium Radlk. (Sapindaceae) in a rain forest. Biol J Linn Soc 18:11–34
Barrett SCH (2002) The evolution of plant sexual diversity. Nat Rev Genet 3:274–284
Bawa KS (1977) The reproductive biology of Cupania guatemalensis Radlk. (Sapindaceae). Evolution 31:52–63
Buerki S, Forest F, Acevedo-Rodríguez P, Callmander MW, Nylander JAA, Harrington M, Sanmartín I, Küpfer P, Alvarez N (2009) Plastid and nuclear DNA markers reveal intricate relationships at subfamilial and tribal levels in the soapberry family (Sapindaceae). Molec Phylogen Evol 51:238–258
Cane JH (1993) Reproductive role of sterile pollen in cryptically dioecious species of flowering plants. Curr Sci 65:223–225
Charlesworth D, Charlesworth B (1978) A model for the evolution of dioecy and gynodioecy. Amer Naturalist 112:975–997
Croat TB (1976) Sapindaceae. In: Flora of Panama. Ann Missouri Bot Gard 63:509–522
De Jong PC (1976) Flowering and sex expression in Acer L. A biosystematic study. Meded Landbouwhogeschool Wageningen 76:1–201
Endress PK, Matthews ML (2006) Elaborate petals and staminodes in eudicots: diversity, function, and evolution. Org Divers Evol 6:257–293
Escobar JR, Correa MPF, Aguilera FJP (1984) Estruturas florais, floração e técnicas para a polinização controlada do guaranazeiro. Pesq Agropec Brasil 19:615–622
Ferrucci MS (1991) Sapindaceae. In: Spichiger R, Ramella L (eds) Flora del Paraguay. Conservatoire et Jardin Botaniques de la Ville de Genève and Missouri Botanical Garden, pp 1–144
Fournier LA (1974) Un método cuantitativo para la medición de características fenológicas en árboles. Turrialba 24:422–423
Gadek PA, Fernando ES, Quinn CJ, Hoot SB, Terrazas T, Sheahan MC, Chase MW (1996) Sapindales: molecular delimitations and infraordinal groups. Amer J Bot 83:802–811
Golenberg EM, West NH (2013) Hormonal interactions and gene regulation can link monoecy and environmental plasticity to the evolution of dioecy in plants. Amer J Bot 100:1–16
Harrington MG, Edwards KJ, Johnson SA, Chase MW, Gadek PA (2005) Phylogenetic inference in Sapindaceae sensu lato using plastid matK and rbcL DNA sequences. Syst Bot 30:366–382
Johansen DA (1940) Plant microtechnique. McGraw-Hill Book Company, New York
Judd WS, Campbell CS, Kellogg EA, Stevens PF, Donoghue MJ (2009) Sistemática Vegetal: um enfoque filogenético, 3rd edn. Artmed, Porto Alegre, pp 438–440
Kearns CA, Inouye D (1993) Techniques for pollinations biologists. University Press of Colorado, Boulder
Korpelainen H (1998) Labile sex expression in plants. Biol Rev Cambridge Philos Soc 73:157–180
Lloyd DG, Webb CJ (1986) The avoidance of interference between the presentation of pollen and stigmas in Angiosperms I. Dichogamy. New Zealand J Bot 24:135–162
Luo S, Zhang D, Renner S (2007) Duodichogamy and androdioecy in the Chinese Phyllanthaceae Bridelia tomentosa. Amer J Bot 94:260–265
Menezes LFT, Araújo DSD (2005) Formações vegetais da restinga da Marambaia, Rio de Janeiro. In: Menezes LFT, Peixoto AL, Araujo DSD (eds) História Natural da Marambaia. EDUR, Seropédica, pp 67–120
Moreira Filho A, Ribeiro OC, Ferreira MA, Martins GA (1975) Polinização e polinizadores de guaraná. Inf Téc ACAR-AM 3:4–7
Ormond WT, Pinheiro MCB, Lima HA, Correia MCR, Castro AC (1991) Sexualidade das plantas da restinga de Maricá, RJ. Bol Mus Nac Rio de Janeiro Bot 87:1–24
Paula NMC (1989) Aspectos da biologia reprodutiva de Paullinia rugosa Benth. ex Radlk. (Sapindaceae). Dissertation. Instituto Nacional de Pesquisa da Amazônia (INPA) e Fundação Universidade do Amazonas
Radford AE, Dickison WC, Massey JR, Bell CR (1974) Vascular plant systematics. Harper and Row Publishers, New York
Radlkofer LAT (1931–1934) Sapindaceae. In: Engler A (ed) Das Pflanzenreich IV, 165 (Heft 98 a–h). Verlag von Wilhelm Engelmann, Leipzig, pp 1–1539
Rama Das K, Ruddi CS, Aluri RJS, Atluri JB (1997) Sexual system and pollination ecology of Cardiospermum halicacabum L. (Sapindaceae). J Bombay Nat Hist Soc 94:333–341
Renner SS (2001) How common is heterodichogamy? Trends Ecol Evol 16:595–597
Renner SS, Beenken L, Grimm GW, Kocyan A, Ricklefs RE (2007) The evolution of dioecy, heterodichogamy, and labile sex expression in Acer. Evolution 61–11:2701–2719
Robbertse PJ, Du Toit ES, Cloete MO (2011) Gender expression and inflorescence structure of Pappea capensis Eckl. and Zeyh. (Sapindaceae). S African J Bot 77:425–429
Sato T (2002) Phenology of sex expression and gender variation in a heterodichogamous maple, Acer japonicum. Ecology 83:1226–1238
Shang H, Luo Y-B, Bai W-N (2012) Influence of asymmetrical mating patterns and male reproductive success on the maintenance of sexual polymorphism in Acer pictum subsp. mono (Aceraceae). Molec Ecol 21:3869–3878
StatSoft Inc (2007) Statistica (data analysis software system) version 8.0. http://www.statsoft.com
Subba Reddi C, Reddi EUB, Reddi NS, Reddi PS (1983) Reproductive ecology of Sapindus emarginatus Vahl (Sapindaceae). Proc Indian Acad Sci Pl Sci 49:57–72
Vamosi JC, Otto SP, Barrett SCH (2003) Phylogenetic analysis of the ecological correlates of dioecy. J Evol Biol 16:1006–1018
Vary LB, Sakai AK, Weller SG (2011) Morphological and functional sex expression in the Malagasy endemic Tina striata (Sapindaceae). Amer J Bot 98(6):1040–1048
Verdú M, Gleiser G (2005) Adaptative evolution of reproductive and vegetative traits driven by breeding systems. New Phytol 169:409–417
Vogel S (1990) The role of scent glands in pollination: On the structure and function of osmophores. Amerind Publishing Co., Pvt. Ltd, New Delhi, New Delhi
Zapata TR, Arroyo MTK (1978) Plant reproductive ecology of a secondary deciduous tropical forest in Venezuela. Biotropica 10:221–230
Acknowledgments
We would like to thank the entomologists Dr. Antonio José Mayhé Nunes, from the Universidade Federal Rural do Rio de Janeiro (UFRRJ), Dra. Cátia Mello Patiu, Dra. Márcia Souto Couri, and biologist Alexandre Soares, from Museu Nacional, Universidade Federal do Rio de Janeiro (UFRJ), for identification of the insects, Mrs. Glória Gonçalves, for the illustrations, Dr. Ivo Abraão Araújo da Silva, for support on statistical aspects, and Dra. Cristine Rodrigues Benevides, for criticisms and suggestions.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
None.
Additional information
Handling editor: Peter K. Endress.
Rights and permissions
About this article
Cite this article
de Lima, H.A., Somner, G.V. & Giulietti, A.M. Duodichogamy and sex lability in Sapindaceae: the case of Paullinia weinmanniifolia . Plant Syst Evol 302, 109–120 (2016). https://doi.org/10.1007/s00606-015-1247-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00606-015-1247-5