Abstract
Taxonomic features of African species of Panicum sections Clavelligerae and Pectinatae are reviewed and compared with Dichanthelium and other taxa in tribe Paniceae. The new subtribe Dichantheliinae is proposed on the basis of molecular and morphological characters: it includes non-Kranz species, growing at forests edges or in mountain grasslands with membranous-ciliate ligules, lax and open inflorescences, ellipsoid to oblongoid spikelets, and an indurate upper anthecium. Within the Dichantheliinae, the new genus Adenochloa is also established on the basis of chloroplast ndhF sequences and morphological characters, i.e., plants with clavellate multicellular, and glandular hairs present on blades, main axis, branches, and pedicels of inflorescences; ellipsoid to oblongoid, glabrous spikelets, with a lower glume 1/3 to more than 1/2 the length of the spikelet; a 7–13-nerved upper glume and a 5–9-nerved lower lemma; the upper anthecium is indurate, pilose or glabrous. Adenochloa includes 14 species from Africa and Madagascar. The new combinations: Adenochloa adenophylla, A. adenophora, A. bullockii, A. claytonii, A. ecklonii, A. flacciflora, A. habrothrix, A. hymeniochila, A. lukwangulense, A. nigromarginata, A. pectinella, A. pole-evansii, A. sadinii, and A. squarrosa are proposed and the new genus is compared with other genera of the tribe Paniceae. Also, lectotypes are designated for Brachiaria sadinii, Panicum adenophorum, P. adenophyllum, P. ecklonii, P. katentaniense, P. kisantuense, P. hymeniochilum, P. hymeniochilum var. glandulosum, P. snowdenii, and P. scandens; Panicum glanduliferum and P. omega are treated as new synonyms of Adenochloa hymeniochila and A. pectinella, respectively. Finally, new illustrations are provided for 6 species of the new genus.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Sections Clavelligerae and Pectinatae, of Panicum L., were established by Stapf (1920), in his treatment of grasses of Tropical Africa; the former was based on the presence of glandular hairs on the inflorescences, culms and blades, and the latter by having glumes pectinately toothed. Stapf (1920) included four species in Clavelligerae and two in Pectinatae. Renvoize (1968) reviewed both sections, mentioning that they share an oblong shape of the spikelet, and the presence of clavellate tipped hairs on the axis and branches of the inflorescence. These clavate glandular hairs were described (Kabuye and Wood 1968) as multicellular hairs, present in culms, sheaths, blades, and panicle axis and branches of species of Clavelligerae and Pectinatae; Kabuye and Wood (1968) also distinguished these hairs from those present in P. deustum Thunb., which in the latter species are unicellular and with swollen tips. Renvoize (1968) arranged four species in sect. Pectinatae, P. ecklonii Nees, P. lukwangulense Pilg., P. pectinatum Rendle, and P. pectinellum Stapf, and 10 species in Clavelligerae: P. adenophorum K.Schum., P. claytonii Renv., P. deustum Thunb., P. flacciflorum Stapf, P. habrothrix Renv., P. hymeniochilum Nees, P. nigromarginatum Robyns, P. pole-evansii C.E.Hubb., P. sadinii (Vanderyst) Renv., and P. snowdenii C.E.Hubb. Later, Renvoize (1980) described P. omega in sect. Pectinatae, and P. bullockii (Renvoize 1989b) in sect. Clavelligerae. Recent floristic treatments of the flora of Africa recognized 11 and 14 species in section Clavelligerae (Clayton and Renvoize 1982), and section Pectinatae (Renvoize 1989a). It should be pointed out that P. deustum Thunb., placed by Renvoize (1968) in sect. Clavelligerae is an “incertae sedis” species of subtribe Melinidinae (Salariato et al. 2010). Also, Panicum glanduliferum K.Schum., a species from Madagascar (Bosser 1969) was included among the analyzed species of sect. Clavelligerae. Based on a Panicoid ndhF sequence analysis, Aliscioni et al. (2003) and Morrone et al. (2012) discussed the systematic position of two species of Clavelligerae which were included in “clade A”, together with five species of the genus Dichanthelium.
Polyphyly of the genus Panicum has been confirmed in several morphological and phylogenetic studies of Panicoideae based on chloroplast (ndhF, trnL-F, rpoC 2) and ribosomal nuclear (ETS) sequence data (Gómez-Martínez and Culham 2000; Zuloaga et al. 2000; Duvall et al. 2001, 2003; Giussani et al. 2001; Aliscioni et al. 2003). Aliscioni et al. (2003) restricted Panicum to those taxa usually placed in Panicum subg. Panicum and characterized it as including caespitose plants, with ciliate or membranous-ciliate ligules, spikelets arranged in an open and lax inflorescence, with the upper glume and lower lemma (5–)7–13 nerved, the upper anthecium indurate, and the upper palea with simple or compound papillae toward the apex. Based on four plastid markers, Christin et al. (2009) and Zimmermann et al. (2013) also indicated, that Panicum should be restricted to a set of species all using the C4 NAD-me photosynthesis subtype. Aliscioni et al. (2003) transferred several species to other panicoid genera [such as Dichanthelium (Hitchc. & Chase) Gould, Hymenachne P.Beauv., and Steinchisma Raf.], and recognized Phanopyrum (Raf.) Nash as an independent genus. Aliscioni et al. (2003) also suggested that all “incertae sedis” species of Panicum should be segregated from the genus and transferred to new taxa or to other extant genera. As a result, several species were transferred recently to other panicoid genera, i.e., Aakia (Grande Allende 2014), Apochloa Zuloaga & Morrone (Sede et al. 2008), Canastra Morrone, Zuloaga, Davidse & Filg. (Zuloaga et al. 2006), Coleataenia Griseb. (Soreng 2010), Cyphonanthus Zuloaga & Morrone (Morrone et al. 2007), Hopia Zuloaga & Morrone (Zuloaga et al. 2007), Ocellochloa Zuloaga & Morrone (Sede et al. 2009), Parodiophyllochloa Zuloaga & Morrone (Morrone et al. 2008), Renvoizea Zuloaga & Morrone (Sede et al. 2008), Stephostachys Zuloaga & Morrone (Zuloaga et al. 2010), Trichanthecium Zuloaga & Morrone (Zuloaga et al. 2011), and Zuloagaea Bess (Bess et al. 2006).
The aims of this study are: (a) to analyze, with additional molecular and morphological data, clade A of Morrone et al. (2012) to define its taxonomic position, and (b) to evaluate the systematic positions of sects. Clavelligerae and Pectinatae using the chloroplast (ndhF) DNA phylogeny of Panicoideae.
Materials and methods
Morphological data
Morphological characters were recorded from herbarium specimens from BAA, BR, G, K, MA, MO, P, SI, and US (acronyms after Thiers 2013).
Molecular data and phylogenetic analyses
A total of 20 new ndhF sequences belonging to Panicum sects. Clavelligerae and Pectinatae and to genus Dichanthelium were obtained from herbarium specimens for the present work (GenBank accession numbers: KP173693-KP173711). The sequences obtained were added to the 123-ndhF Panicoid matrix of Aliscioni et al. (2003). Details of the 143 taxa analyzed and GenBank accession numbers are available in "Appendix".
DNA sequencing. DNA was isolated, from herbarium material, using the DNeasy Plant Mini Kit (Qiagen, Hilden, Germany) following the manufacturer’s recommendations. The plastid marker, ndhF, was amplified by polymerase chain reaction (PCR). The complete ndhF gene (ca. 2,100 bp) was amplified using primers specified by Olmstead and Sweere (1994) and Aliscioni et al. (2003). Four fragments were amplified (5F-536R, 536F-972R, 972F-1666R and 1666F-3R).
PCR reactions were performed in 25 µL final volumes with 50–100 ng of template DNA, 0.2 µM of each primer, 25 µM dNTP, 5 mM MgCl2 1× buffer and 0.3 units of Taq polymerase provided by Invitrogen Life Technologies (Brazil). The PCR reactions were carried out using the following parameters: 1 cycle of 94 °C for 5 min, 39 cycles of 94 °C for 30 s, 48 °C for 1 min, and 72 °C for 1 min 30 s, and a final extension cycle of 72 °C for 10 min. PCR products were run out on a 1 % TBE agarose gel stained with SYBR Safe DNA gel stain (Invitrogen) and visualized in a blue light transilluminator. Automated sequencing was performed by Macrogen, Inc. (Seoul, South Korea). Forward and reverse strands were sequenced of each template to reach the complete sequence of each voucher. Alignment was manually performed, using BioEdit ver. 5.0.9 (Hall 1999). The alignment is available at Treebase under study accession S15539.
Phylogenetic analyses
A maximum parsimony (MP) analysis was performed using TNT ver. 1.1 (Goloboff et al. 2008). All characters were equally weighted, and gaps were scored as missing data. Prior to heuristic searches, all uninformative characters were deactivated. The searches involved 1000 replicates, each of which generated a Wagner tree using a random addition sequence of taxa from the data matrix, swapping the initial tree with TBR (tree bisection and reconnection) and retaining a maximum of 10 trees in each replicate. Subsequently, all optimal trees were swapped using TBR, holding a maximum of 10,000 trees. Branches with ambiguous length of 0 or 1 were collapsed, according to collapsing rules. A strict consensus tree was generated from the most parsimonious trees. Branch supports were estimated with Bootstrap (Felsenstein 1985) using a total of 10,000 replicates. Each replicate was analyzed using 10 Wagner trees as a starting point followed by TBR branch swapping, saving only one tree per replicate. Bootstrap values (BS) over 80 % are reported.
A Bayesian phylogenetic analysis was also performed. The general time reversible substitution model (GTR+I+G) was chosen using the Akaike Information Criterion (AIC) as implemented in jModeltest 2.1.1 (Darriba et al. 2012). Four Markov chains were run simultaneously in two independent runs for 5 million generations in MrBayes v.3.1.2 (Huelsenbeck and Ronquist 2001). Trees were sampled every 1,000 generations, discarding the first 25 % of the samples as burn-in. Convergence diagnostics for log likelihood values were assessed visually using Tracer v.1.5.0 software (Rambaut and Drummond 2007); to calculate the Bayesian posterior probabilities (PP), trees prior to stability of the average standard deviation of split frequencies were excluded, and the remaining trees were used to generate a 50 % majority rule consensus tree.
Results
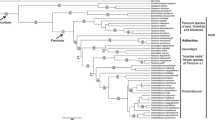
The analyzed matrix consisted of 143 taxa and 2074 characters, of which 445 were parsimony informative. The MP analysis resulted in more than 10,000 most parsimonious trees of 1,600 steps. The strict consensus tree is shown in Fig. 1. Parsimony and Bayesian consensus trees were fully congruent, showing the same strongly supported clades. Posterior probabilities of the Bayesian analysis are indicated in the cladogram of Fig. 1.
Strict consensus tree obtained in the parsimony analysis of the ndhF Panicoid matrix including the new sequences belonging to sections Clavelligerae and Pectinatae and to Dichanthelium. The new sequences are indicated with asterisk. Bootstrap values are shown above branches. Posterior probabilities ≥0.8 obtained in the Bayesian analysis are indicated below branches. Bars indicate tribes and subtribes according to Morrone et al. (2012)
The consensus tree shows that clade A of Morrone et al. (2012) is supported by nine synapomorphic positions, a Bootstrap value (BS) = 84 %, and posterior probabilities (PP) = 1. Within this clade, all analyzed species of Clavelligerae and Pectinatae grouped together with BS = 87 % and PP = 0.86. Three molecular synapomorphies support this clade at positions 74, 102 and 209 of the ndhF Panicoid matrix, respectively. The Clavelligerae–Pectinatae clade appears as the sister group of Dichanthelium, which was monophyletic (BS = 77 %, PP = 1).
Within the Clavelligerae–Pectinatae clade, P. peteri appears as the sister taxon of the remaining species, which are strongly supported as a monophyletic group with 99 % of BS and 0.97 of PP. The two analyzed species, previously assigned to sect. Pectinatae, form a well-supported clade (BS = 100 %, PP = 1) that is the sister group of a polytomy (BS = 94 %, PP = 1). Three subclades are found within this polytomy: P. sadinii–P. bullocki (BS = 100 %, PP = 1), P. nigromarginatum–P. pole-evansii–P. hymeniochilum (BS = 93 %, PP = 1), and P. habrothix–P. claytonii–P. adenophorum (BS = 100 %, PP = 1).
As previously mentioned, the sister group of the Clavelligerae–Pectinatae clade is the genus Dichanthelium. Dichanthelium cumbucana appears as the sister taxon of the remaining species of that genus, while the remaining species are supported by a BS of 82 % and PP of 1.
Discussion
Aliscioni et al. (2003) treated Dichanthelium (120 species approx.) as an independent, monophyletic genus, with sect. Clavelligerae as its sister taxon. These authors mentioned that section Clavelligerae should be segregated from Panicum s.str., its final position pending the study of more species from this section. Later, Morrone et al. (2012) stressed that Dichanthelium and species of Clavelligerae, that grouped in a well-supported clade A, still have an uncertain position within the Paniceae, due to the limited number of taxa examined. Our study allows us to conclude, after the analysis of 12 (of 14) species of Clavelligerae/Pectinatae, and 14 species of Dichanthelium, that this clade is well supported and morphologically coherent; all taxa share membranous-ciliate ligules, lax and open inflorescences, ellipsoid to oblongoid spikelets, the lower glume present, 1–7-nerved, a (5–)7–13-nerved upper glume and lower lemma, the upper anthecium indurate, with simple papillae usually present and regularly arranged, with macrohairs present or absent. Also, species in this clade are non-Kranz (Brown 1977; Zuloaga et al. 1993; Osborne et al. 2014). Considering molecular and morphological characters, we are here describing a new subtribe within the Paniceae to contain the members of these two clades and allied species.
Species of Clavelligerae/Pectinatae differ from Dichanthelium by the presence of pedicellate multicellular glandular hairs on the culms, leaves, and inflorescences. In addition, foliar dimorphism and cleistogamy are absent (vs. pedicellate glandular hairs absent, and foliar dimorphism and cleistogamy usually present in species of Dichanthelium).
Panicum s.str. differs from species of Clavelligerae/Pectinatae by including taxa without clavellate tipped hairs, all over the plants, the spikelest without pectinate glumes and lower lemma, and upper anthecium with simple or compound papillae at the top of the palea and lemma; also, all species of Panicum s.str. are C4, of the NAD-me subtype.
Based on the discussion above, and because species of Clavelligerae/Pectinatae cannot be included in any genus of tribe Paniceae or subtribe Panicinae (Morrone et al. 2012), we describe this taxon as a new genus.
The phylogenetic analysis has also shown several relationships within the boundaries of this taxon (Fig. 1), which agree with morphological similarities between the species, as follows:
Panicum peteri, which appears in the molecular analysis as sister to all remaining species, differs from these by its inflorescences that are partially included in the upper blades, leaves ovate lanceolate, lower glume 7–9-nerved, lower palea developed, and the upper anthecium crested and glabrous.
All species previously classified in sect. Pectinatae, P. lukwangulense, P. pectinellum, and P. ecklonii share the presence of pectinate glumes, with the upper glume shorter than the upper anthecium, lower palea and lower flower absent, and the upper anthecium pilose. Panicum flacciflorum is distinguished by the presence of a stipitate upper anthecium; this is an annual species with spikelets having the lower palea developed. Panicum nigromarginatum, P. hymeniochilum, and P. pole-evansii are annual plants, freely branching at the lower nodes, with the upper anthecium glabrous, papillose, and crested at the apex. Panicum sadinii and P. bullockii are perennials, conspicuously branching at the lower nodes, the pedicels with long macrohairs, and spikelets with the upper anthecium glabrous, smooth, and crested. Finally, Panicum adenophorum, P. claytonii and P. habrothrix share the presence of lower palea and lower flower developed, the upper anthecium papillose and pilose toward the apex; both P. adenophorum (a perennial plant) and P. habrothrix (annual species) have long whitish macrohairs on pedicles, these being absent in P. claytonii.
Taxonomic treatment
Dichantheliinae Zuloaga, subtribe nov.—TYPE: Dichanthelium (Hitchc. & Chase) Gould
Annuals or perennials, cespitose or with culms decumbent and rooting at the lower nodes, with or without foliar dimorphism; ligules membranous-ciliate; blades filiform to ovate lanceolate, with or without glandular hairs. Inflorescence lax and open, spikelets ellipsoid to oblongoid, pilose or glabrous; lower glume present, 0–9-nerved; upper glume and lower lemma (5–)7–13-nerved; lower palea present or absent, lower flower male or absent; upper anthecium indurate; caryopsis with a punctiform to oblong hilum. C3 photosynthetic pathway; basic chromosome number x = 9.
Subtribe with 2 genera, Adenochloa and Dichanthelium, which are found in Africa and America, respectively.
Adenochloa Zuloaga, gen. nov.—TYPE SPECIES: Adenochloa hymeniochila (Nees) Zuloaga = Panicum hymeniochilum Nees, Fl. Afr. Austral. III: 46. 1841.
= Polyneura Peter, Repert. Spec. Nov. Regni Veg. Beih. 40 (1, Anhang): 53. 1929, nom. illeg. (Art. 53), non (J. Agardh) Kykin.
Annuals or perennials, with or without conspicuous rhizomes, culms scrambling and slender, decumbent and rooting at the lower nodes to ascending or erect, simple or branching; internodes hollow, terete; nodes pilose or glabrous; sheaths striate, with or without clavellate glandular hairs; ligules membranous-ciliate; blades linear lanceolate to ovate lanceolate, flat, cordate and amplexicaulous to subcordate or rounded at the base, the apex acuminate, with or without clavellate glandular hairs, otherwise pilose to glabrous. Inflorescence an open panicle, subexserted to exserted from the uppermost leaves, sparsely to densely clavellate hairy, glandular hairs occasionally absent; main axis terete, first-order branches divergent; pedicels claviform, with or without clavellate hairs and with or without long macrohairs, some of them exceeding the length of the spikelet; sessile glands present or absent. Spikelets oblongoid to ellipsoid, glabrous, upper glume and lower lemma subequal or the upper glume shorter than the upper anthecium, membranous; lower glume 1/3 to 3/4 the length of the spikelet, 0–9-nerved, pectinate or not; upper glume 7–13-nerved, pectinate or not; lower lemma glumiform, 5–11-nerved, pectinate or not; lower palea as long as the upper anthecium, hyaline and with short hairs at the margins, to reduced or absent; lower flower male or absent. Upper anthecium narrowly ellipsoid, indurate, with macrohairs toward the apex of the lemma or glabrous, with or without simple papillae distributed all over the lemma and palea, the lemma crested or not at the apex. Caryopsis ellipsoid; hilum oblong, embryo less than half the length of the caryopsis.
Etymology—the name of the new genus makes reference to the presence of glandular hairs on vegetative and/or reproductive portions of the plants.
Distribution and habitat—an African genus, with one species growing also in Madagascar.
Observation—genus with 14 species.
Key to the species
1. Glumes and lower lemma pectinate
2
1′. Glumes and lower lemma entire, not pectinate
4
2. Plants 0.7–1.6 m tall; blades 20–50 × 1–2 cm; inflorescences 13–40 cm long
Adenochloa lukwangulense
2′. Plants 0.15–0.8(–0.9) m tall; blades 4–14– (–18) × 0.1–0.7 cm; inflorescences 5–15(–17) cm long
3
3. Spikelets 2.5–3.4 mm long
Adenophora ecklonii
3′. Spikelets 1.6–2 mm long
Adenophora pectinella
4. Upper anthecium glabrous, the upper lemma crested at the apex, usually greenish
5
4′. Upper anthecium conspicuously pilose at the apex, the upper lemma not crested, pale
11
5. Spikelets 1.2–1.8 mm long; upper glume shorter than the upper anthecium, bracts and upper anthecium with black spots at the apex
Adenochloa nigromarginata
5′. Spikelets 2–4.5 mm long; upper glume as long or longer than the upper anthecium, bracts and upper anthecium without black spots at the apex
6
6. Blades symmetric, linear lanceolate to lanceolate, up to 1.2 cm wide; culms herbaceous, simple or branching, plants annual; lower glume 0–3-nerved, upper glume 7–9-nerved
7
6′. Blades asymmetric, lanceolate to ovate lanceolate, up to 2 cm wide; culms rigid, freely branching, plants perennial; lower glume 5–9-nerved, upper glume 9–13-nerved
8
7. Lower glume 1/2 to 2/3 the length of the spikelet; upper anthecium smooth; lower palea reduced, lower flower absent
Adenochloa hymeniochila
7′. Lower glume 1/3 to 1/2 the length of the spikelet; upper anthecium with simple papillae all over its surface; lower palea developed, lower flower present
Adenochloa pole-evansii
8. Inflorescence with sessile glands on the main axis and branches, with pedicellate glandular hairs only on the main axis
Adenochloa adenophylla
8′. Inflorescence without sessile glands on the main axis and branches, with pedicellate glandular hairs all over the inflorescence, including the pedicels
9
9. Spikelets 4.3–5 mm long; lower glume less than 1/2 the length of the spikelet, 7–9-nerved
Adenochloa squarrosa
9′. Spikelets 2.5–3.5 mm long; lower glume 1/2 to 3/4 the length of the spikelet, 3–5-nerved
10
10. Plants 20–40 cm tall; blades 3.5–4.5 × 0.6–0.8 cm
Adenochloa bullockii
10′. Plants 90–140 cm tall; blades 5–14 × 1–2 cm
Adenochloa sadinii
11. Pedicels with whitish macrohairs longer than the spikelet
12
11′. Pedicels without long whitish macrohairs
13
12. Plants annual, 18–20 cm tall, spikelets 2–2.2 mm long
Adenochloa habrothrix
12′. Plants perennial, 45–90 cm tall, spikelets 2.5–3(–3.8) mm long
Adenochloa adenophora
13. Plants perennial, strongly rhizomatous; spikelets 3.5–5.5 mm long; upper anthecium non-stipitate
Adenochloa claytonii
13′. Plants annual; spikelets 3–4 mm long; upper anthecium stipitate
denochloa flacciflora
1. Adenochloa adenophora (K.Schum.) Zuloaga, comb. nov.
≡ Panicum adenophorum K.Schum., Pflanzenw. Ost-Afrikas C: 103. 1895.—TYPE:Uganda. Ankole District: Seengebiet (Ruhanga), 26 Apr 1891, F. Stuhlmann 2143 (lectotype designated here, B barcode B100167793!; isolectotypes, US barcodes US1063855!, US1715360!) Fig. 2.
Adenochloa adenophora (K.Schum.) Zuloaga. a Habit; b ligular region; c portion of inflorescence; d spikelet, ventral view; e spikelet, dorsal view; f lower palea, ventral view; g upper anthecium, dorsal view; h upper palea, ventral view; i caryopsis, scutelar view with stamens and stigmas; j caryopsis, hilar view [a–j from Namaganda 921 (K)]
Distribution and habitat—this species grows in grasslands, on sandy soils, or in forest margins of Burundi, Democratic Republic of the Congo, Malawi, Tanzania, Uganda, and Zambia, between 1,800 and 2,300 m elevation.
Adenochloa adenophora is morphologically similar to A. habrothrix, the latter differing by being an annual species with inflorescence 3–4 cm long, and spikelets with the upper glume and lower lemma 7 nerved.
2. Adenochloa adenophylla (Pilg.) Zuloaga, comb. nov.
≡ Panicum adenophyllum Pilg., Notizbl. Bot. Gart. Berlin-Dahlem 13: 260. 1936.—TYPE: Tanzania. Lindi District: Bezirk Lindi, ca. 70 km NW von Lindi, Muera plateau, 24 Feb 1935, H.J. Schlieben 6064 (lectotype designated here, B barcode B100167792!; isolectotypes, B barcode B100167792!, BM barcode BM000923097!, BR barcode BR0000008760807!, LISC barcode LISC003459!, M barcode M0103871!, MA barcode MA175812!, S barcode SS-G-4477!).
Distribution and habitat—Tanzania, a scrambling perennial growing in forest margins ca. 700 m elevation.
Clayton and Renvoize (1982) considered this species a synonym of A. adenophora, without any further explanation. Nevertheless, A. adenophylla can be distinguished from A. adenophora by having sessile glands all over the inflorescence, clavellate hairs only present on the main axis, pedicels without glands or macrohairs, and upper anthecium glabrous (vs. sessile glands absent and conspicuous clavellate hairs on the main axis, branches of the inflorescence and pedicels, pedicels with clavellate hairs and long macrohairs, and upper anthecium pilose toward the apex in A. adenophora).
Several syntypes of the specimen Schlieben 6064 were examined, of which the collection B barcode B100167792 was selected as lectotype of the species.
3. Adenochloa bullockii (Renvoize) Zuloaga, comb. nov.
≡ Panicum bullockii Renvoize, Kew Bull. 44: 543. 1989.—TYPE: Zambia. Kasama, Chisimbia Falls, 25 km WNW of Kasama, 28 Feb 1970, in sandy soils pockets among rocks, R. B. Drummond and G. Williamson 10092 (holotype, SRGH barcode SRGH109633-0!; isotype, K!).
Distribution and habitat—only known from Zambia, where it grows in mountain slopes on sandy soils between 1,700 and 2,200 m elevation.
This species is morphologically similar to A. sadinii, from which it differs by including plants strongly rhizomatous and 20–40 cm tall (vs. plants not strongly rhizomatous, scrambling on the vegetation and up to 1.50 m tall).
4. Adenochloa claytonii (Renvoize) Zuloaga, comb. nov.
≡ Panicum claytonii Renvoize, Kew Bull. 22: 484, f. 1-12. 1968.—TYPE: Zambia. Nyika Plateau, Lake Laulime, grassland, 2150 m, 24 Oct 1958, N.K.B. Robson 332 (holotype, K barcode K000282438!; isotypes, BR barcode BR0000008760692!, EA barcode EA000000476!, LISC barcode LISC003461!, SRGH barcode SRGH0106318-0!, WAG barcode WAG0001527!).
Distribution and habitat—this species grows in the Democratic Republic of the Congo, Malawi, Tanzania, and Zambia, where it is found climbing on shrubs along forest margins, growing in reddish laterite soils between 1800 and 2400 m elevation.
Adenophora claytonii is a species with conspicuous rhizomes and erect culms, usually unbranched. This species is morphologically similar to A. adenophora, differing by the absence of long whitish macrohairs on pedicels, spikelets 3.5–5.5 mm long, lower glume 7–9-nerved, and upper glume and lower lemma 9–11-nerved (vs. pedicels with long whitish macrohairs, spikelets 2.5–3 mm long, lower glume 3-nerved, and upper glume and lower lemma 7–9-nerved in A. adenophora).
5. Adenochloa ecklonii (Nees) Zuloaga, comb. nov.
≡ Panicum ecklonii Nees, Fl. Afr. Austral. Ill.: 43. 1841.—TYPE: South Africa. Katberg, J.F. Drège s.n. (lectotype designated here, P barcode P00442154!; isolectotype, LE-TRIN.689.2) Fig. 3.
= Panicum pectinatum Rendle, Trans. Linn. Soc. London, Bot. 4: 54, tab. 10, Fig. 1–6. 1894.—TYPE: Malawi. Mt. Milanji, A. White 10 (holotype, BM barcode BM000923138!; isotype, K barcode K000255542!, US barcode US1445182!, fragment).
= Panicum catangense Chiov., Ann. Bot. (Rome) 13: 44. 1914.—TYPE: Democratic Republic of the Congo: “Catanga, plateau bianos a Katantania”, Nov 1912, E.A.R. Bovone 82 (holotype, TO!).
= Panicum katentaniense Robyns, Mém. Inst. Roy. Colon. Belge, Sect. Sci. Nat. (8vo) 1(6): 57, tab. 5, A-F. 1932.—TYPE: Democratic Republic of the Congo. Katanga: Plate des Bianos, Katentania, Nov 1912, H.A. Homblé 706 (lectotype designated here, BR barcode BR0000008760814!; isolectotypes, BR barcode BR0000008761217, K, US barcode US1538736!).
Distribution and habitat—this species is present in Sierra Leone and from Cameroon, Democratic Republic of the Congo, Mozambique, Malawi and Zambia to South Africa; it grows in montane grasslands, on rocky places, between 1,100 and 2,600 m.
Adenochloa ecklonii is morphologically similar to A. lukwangulense, both species with pectinate glumes and lower lemma, the upper glume shorter than the spikelet, with the upper anthecium pilose at the apex. It differs from the latter by being smaller plants, 15–75 cm tall, with inflorescences 6–15 cm long; the presence of burned basal leaves indicates that this species is frequent in burned areas. Plants are characterized by being robust, cespitose perennials, densely hispid along blades and culms, with glandular hairs all over the terminal inflorescence; the spikelets are nearly 4 mm long, with the lower glume 1/2 or less the length of the spikelet, the upper glume usually not covering the upper anthecium, the latter with macrohairs toward the distal portion.
Adenochloa ecklonii differs from A. pectinella by including plants with simple culms, blades mostly basal, blades linear to linear lanceolate, 0.2–0.5 cm wide, spikelets 2.5–3.5 mm long (vs. plants with branching culms, blades arranged along the culms, lanceolate, 0.4–0.8 cm wide, and spikelets 1.8–2 mm long in the latter species).
When describing P. ecklonii, Nees (1841) cited two syntypes, Ecklon s.n. and Drége s.n. The specimen Drége from P is selected as lectotype of the species since it agrees with the protologue and is a well preserved specimen. Also, the specimen Homblé 706 from BR (barcode BR8760814) is selected as lectotype of Panicum katentaniense Robyns.
6. Adenochloa flacciflora (Stapf) Zuloaga, comb. nov.
≡ Panicum flacciflorum Stapf, Fl. Trop. Afr. 9: 720. 1920.—TYPE: Tanzania. Kahama-Tabora District: Miniga, 3800 ft, J.A. Grant s.n. (holotype, K 000255546!; isotype, US barcode US1445207) Fig. 4.
Adenochloa flacciflora (Stapf) Zuloaga. a Habit; b ligular region; c, portion of the inflorescence; d, spikelet, dorsal view; e spikelet, ventral view; f lower palea; g upper anthecium, dorsal view; h upper palea with caryopsis; i caryopsis, embryo view; j caryopsis, hilum view [a–j from Bidgood and Darbyshire 5501 (K)]
= Panicum microcephalum Peter, Repert. Spec. Nov. Regni Veg. Beih. 40 (1, Anhang): 44, tab. 25, Fig. 2. 1929.—TYPE: Burundi. Deutsch Ost. Afr. Avinsa: westl. von Lugufu, G.A. Peter 36484, 36546 (syntypes, B?, not located).
= Panicum microcephalum fo. nanum Peter, Repert. Spec. Nov. Regni Veg. Beih. 40 (1, Anhang): 44. 1929.—TYPE: Burundi, without locality, G.A. Peter 38174 (holotype, B?, not located).
Distribution and habitat—growing in open and sandy soils in Burundi, Tanzania, Zambia, and Uganda, between 1,000 and 1,500 m elevation.
Adenochloa flacciflora is characterized by being an annual, cespitose plant, with open and lax terminal inflorescences, the spikelets 3–4 mm long, and the upper anthecium with a manifest, thick stipe below, being the anthecium papillose, with simple papillae regularly distributed, and macrohairs toward the apex of lemma and palea.
7. Adenochloa habrothrix (Renvoize) Zuloaga, comb. nov.
≡ Panicum habrothrix Renvoize, Kew Bull. 22: 486, Fig. 2. 1968.—TYPE: Zambia. Mwinilunga District: just S of Matonchi Farm, in Brachystegia woodland, 24 Jan 1938, E.W.B.H. Milne-Redhead 4305 (holotype, K barcode K000282440!; isotypes, BR barcode BR0000008761378!, LISC barcode LISC003462!, PRE barcode PRE0747957-0!).
Distribution and habitat—Burundi and Zambia, where it is found in forest shade, at elevations ca. 1,000 m.
Adenochloa habrothrix is morphologically similar to A. adenophora and A. claytonii; it differs from the former by its annual habit, plants 20–40 cm tall, spikelets 2–2.2 mm long, and upper anthecium glabrous (vs. perennial, 30–100 cm tall, and spikelets 2.5–3(–3.6) mm long, and upper anthecium pilose in A. adenophora); it is distinguished from A. claytonii by its annual habit, the presence of long whitish macrohairs on pedicels, spikelets 2–2.2 mm long (vs. a perennial habit, absence of macrohairs on pedicels, and spikelets 3.5–5.5 mm long in the A. claytonii).
8. Adenochloa hymeniochila (Nees) Zuloaga, comb. nov.
≡ Panicum hymeniochilum Nees, Fl. Afr. Austral. Ill.: 46. 1841.—TYPE: South Africa: Natal, “in graminosis inter Omsamculo et Omcomas alt 500´”, J.F. Drège 4247 (lectotype designated here, P barcode P00444247!; isolectotype, K barcode K000282468!) Fig. 5.
= Panicum hymeniochilum var. glandulosum Nees, Fl. Afr. Austral. Ill.: 47. 1841.—TYPE: South Africa. Natal: near the Umlazi river, 25 Mar 1832, J.F. Drège s.n. (—TYPE: P barcode P00442175!; isolectotypes, HAL barcode HAL0063367!, K barcode K000282469).
= Panicum glanduliferum K.Schum., Abh. Naturwill. Vereine Bremen 9: 401. 1887.—TYPE: Madagascar: “Im Sümpfen ohne genauere Standortsangabe”, 8 Nov 1877, C. Rutenberg s.n. (not located).
= Panicum filiculme Hack. ex Schinz, Bull. Herb. Boissier 3(8): 377. 1895.—TYPE: South Africa. Natal: inter Pinetown et Umbilo, A. Rehmann 8049 (holotype, Z; isotypes, K barcode K000255480!, US-80764!).
= Panicum schlechteri Hack., Bull. Herb. Boissier 7(1): 24. 1899.—TYPE: South Africa. Natal: «in humidis prope Hilton Road (Terra Capens, orientalis)», 17 Feb 1895, F.R.R. Schlechter 6759 (holotype, Z; isotype, BOL barcode BOL139355!).
= Panicum snowdenii C.E.Hubb., Bull. Misc. Inform. Kew 1928(4): 132–133. 1928.—TYPE: Uganda. Mt. Elgon, Butandiga, 2100-2400 m, in shade of and near bushes, following cultivation, 19 Aug 1927, J.D. Snowden 1188 (lectotype designated here, K barcode K000255553!; isolectotypes, BM barcode BM000923145!, BR barcode BR0000008768186!, K barcode K000255554!, MO barcode MO1660941!, PRE barcode PRE0033229-0!, SRGH barcode SRGH0107002-0, US barcodes US140001!, US00731198!).
= Sacciolepis semienensis Chiov., Pl. Nov. Minus Not. Aethiopia: 25. 1928.—TYPE: Ethiopia. Semien, E. Chiovenda 937 (holotype, FT barcode FT000239!).
– (“Panicum semienense Chiov.”, Pl. Nov. Minus Not. Aethiopia: 25. 1928, pro syn. nom. nud.)
= Panicum kisantuense Vanderyst ex Robyns, Mém. Inst. Roy. Colon. Belge, Sect. Sci. Nat. (8vo) 1(6): 25, tab. 1, G-M. 1932.—TYPE: Zaire. Kisantu, 13 Apr 1916, H. Vanderyst 6010 (lectotype, BR barcode BR000000876513!, designated by Renzoize, Kew Bull. 22: 488. 1968; isolectotype, BR barcode BR000000876217!).
= Panicum djalonense A.Chev., Rev. Bot. Appl. Agric. Trop. 14: 27. 1934.—TYPE: French Guinea. Lit de la rivière Mafin à Dalaba, 1350 m, 16 Apr 1907, O. Caille 18139 (holotype, P!; isotypes, BR barcode BR0000008769176!, K barcode K000257019!).
Distribution and habitat—A widespread species which inhabits humid, shady areas, at river margins in Botswana, Burundi, Cote d`Ivoire, Ethiopia, Guinée, Malawi, Mozambique, Zambia, and Zimbabwe; 600–2,800 m elevation. This species is also found in Madagascar.
Adenochloa hymeniochila is included, together with A. nigromarginata and A. pole-evansii, in a group of aquatic annual species, with culms conspicuously branching at the lower nodes, blades lanceolate, lower glume 1/3 to 1/2 the length of the spikelet, nerveless to 1-nerved, and upper anthecium glabrous, papillose. Adenochloa pole-evansii differs from A. hymeniochila and A. nigromarginata by having a developed lower palea enclosing a lower flower (vs. lower palea reduced and lower flower absent); also, A. nigromarginata has 1.2–1.8 mm long spikelets, black at the upper portion, upper glume shorter than the upper anthecium, and upper anthecium black towards the apex (vs. spikelets 2–3 mm long, upper glume as long as or longer than the upper anthecium, and upper anthecium pale in A. hymeniochila).
The type specimen of P. glanduliferum is not preserved at B; nevertheless, the analysis of the original description, and additional material of the species preserved at P from Madagascar, allowed us to conclude that this species is a synonym of A. hymeniochila.
9. Adenochloa lukwangulense (Pilg.) Zuloaga, comb. nov.
≡ Panicum lukwangulense Pilg., Notizbl. Bot. Gart. Berlin-Dahlem 12: 380. 1935.—TYPE: Tanzania. Uluguru Mts., Lukwangule Plateau, 20 Feb 1933, H.J. Schlieben 3520 (holotype, B; isotypes, BM barcode BM000923146!, BR barcode BR0000008769183!, LISC barcode LISC003465!, M barcode M0103983!, MA barcodes MA175716!, 175716-2!, MO barcode MO1710404!, P barcode P00442181!, PRE barcode PRE0033219-0!).
Distribution and habitat—Malawi and Tanzania, where it is found in montane grasslands, between 2,300 and 2,900 m elevation.
This species is characterized by being a robust, cespitose perennial with culms up to 160 cm tall, densely hispid along blades and culms, with glandular hairs all over the terminal inflorescence, the panicles 10–40 cm long; the spikelets are 2.6–3.7 mm long, with the lower glume 1/2 or less the length of the spikelet, the upper glume usually not covering the upper anthecium, lower palea and lower flower absent, and upper anthecium with macrohairs toward the distal portion.
10. Adenochloa nigromarginata (Robyns) Zuloaga, comb. nov.
≡ Panicum nigromarginatum Robyns, Mém. Inst. Roy. Colon. Belge, Sect. Sci. Nat. (8vo) 1(6): 24, tab. 1, A-F. 1932.—TYPE: Democratic Republic of the Congo. Shaba: Welgelegen, 2 May 1912, J.C.C. Bequaert 385 (holotype, BR barcode BR0000008769381!; isotypes, K barcode K000256717!, K barcode K000256718!, US barcode US1538733!, fragment).
Distribution and habitat—it grows in swampy places, in forest margins, of the Democratic Republic of the Congo, Uganda, and Zambia, ca. 1400 m.
Adenochloa nigromarginata differs from A. hymeniochila and A. pole-evansii by its spikelets 1.2–1.8 mm long, dark, and also by having a dark upper anthecium (see relationships of this species under A. hymeniochila).
11. Adenochloa pectinella (Stapf) Zuloaga, comb. nov.
≡ Panicum pectinellum Stapf, Fl. Trop. Afr. 9: 720. 1920.—TYPE: Democratic Republic of the Congo. Katanga: near Elisabethville, on dry wooded ground, Feb 1912, H. Homblé 54 (holotype, K!; isotypes, BR barcodes BR0000008769053!, BR0000008768728!) Fig. 6.
= Panicum omega Renvoize, Kew Bull. 34(3): 551. 1979[1980].—TYPE: Tanzania. Mbeya District: World's End View, 25 Dec 1969, R. Wingfield 512 (holotype, K barcode K000282426!; isotype, EA barcode EA000000479!).
Distribution and habitat—Angola, Democratic Republic of the Congo, Malawi, and Zambia, growing in woodlands or rocky grassland; 1100–2000 m.
When describing P. omega, Renvoize (1980) differentiated the species from P. pectinellum by its compact base, basal blades, and absence of glandular hairs. However, we consider that the single collection of P. omega represents a depauperate specimen of P. pectinellum, and therefore, we include this species in the synonymy of the latter. See relationships of A. pectinella under A. ecklonii.
12. Adenochloa pole-evansii (C.E.Hubb.) Zuloaga, comb. nov.
≡ Panicum pole-evansii C.E.Hubb., Bull. Misc. Inform. Kew 1934: 113. 1934.—TYPE: Zambia. At river 9 miles south of Lake Tanganyka, creeping over rocks, July 1930, I.B. Pole Evans 3039 (holotype, K).
Distribution and habitat—Democratic Republic of the Congo, Tanzania, and Zambia, in margins of streams and swamps, in areas partially shaded; 1,200–1,500 m.
Adenochloa pole-evansii is morphologically related to A. hymeniochila, differing from the latter species by its reduced lower palea, without enclosing a lower flower (vs. lower palea and lower flower developed in A. hymeniochila), spikelets ellipsoid (vs. spikelets lanceolate), and, as already pointed out by Clayton and Renvoize (1982), by the spikelets not appressed on the branches (vs. spikelets appressed on the branches in the latter species).
13. Adenochloa sadinii (Vanderyst) Zuloaga, comb. nov.
≡ Brachiaria sadinii Vanderyst, Bull. Agric. Congo Belge, 10: 244. 1919.
≡ Panicum sadinii (Vanderyst) Renvoize, Kew Bull. 22: 485. 1968.—TYPE: Democratic Republic of the Congo. Kitiaka, Apr 1917, H. Vanderyst 6310 (lectotype designated here, BR barcode BR0000008766113!).
= Panicum acuminatifolium Robyns, Mém. Inst. Roy. Colon. Belge, Sect. Sci. Nat. (8vo) 1(6): 27, tab. 2 A-F. 1932.—TYPE: Democratic Republic of the Congo. District forestier Central Banalia, 8 Dec 1913, J.C. Bequaert 1402 (holotype, BR barcode BR0000008768759!; isotypes, K barcode K000256719!, US barcode US1538738!).
= Panicum lineatum Trin., Sp. Gram. 2(20): tab. 233. 1829, nom. illeg. (Art. 53), non Schumach., 1828.—TYPE: Sierra Leone. Without locality, J. Lindley s.n. (isotype, K barcode K000257018!).
= Panicum scandens Mez, Bot. Jahrb. Syst. 57: 190. 1921, nom. illeg. (Art. 53), non Trin., 1826.—TYPE: Liberia. Sinoe, 24 Nov 1908, M. Dinklage 2326 (lectotype designated here, B barcode B10-016891!; isolectotypes, B barcodes B10-016890!, B10-016892!).
Distribution and habitat—a species from western Africa, where it is found in Angola, the Democratic Republic of the Congo, and Zambia; it is frequent in margins of forests.
Renvoize (1968) cited the following specimens as syntypes of Brachiaria sadinii: Vanderyst 5325, 5341, and 6313, all from BR. However, the original description by Vanderyst cited the syntypes: “AR, Kitiaka, avril 1917; Kindundu (sans numéro); région d´Idiofa, 8642; Haut-Kwilu, 3356, janvier 1914. The specimen Vanderyst 6310, kept at BR, agrees with the protologue and is therefore designated as lectotype of the species.
14. Adenochloa squarrosa (Peter) Zuloaga, comb. nov.
≡ Polyneura squarrosa Peter, Repert. Spec. Nov. Regni Veg. Beih. 40 (1, Anhang): 53–54, tab. 30, Fig. 1, 1929.
≡ Panicum peteri Pilg., Nat. Pflanzenfam. 14e: 20. 1940.
≡ Brachiaria squarrosa (Peter) Clayton, Kew Bull. 34(3): 559. 1979[1980].—TYPE: Tanzania. Uzuramo District: Pugu, 28 Oct 1925, G.A. Peter 31511 (lectotype, B barcode B10-0168856!; isolectotypes, K barcode K000255545!, US barcode US00141251!, fragment) Fig. 7.
Distribution and habitat—Eastern Africa, in Mozambique, Tanzania, and Zimbabwe, where it is found in forest edges in humid habitats; 100–700 m.
This species is characterized by its rigid culms profusely branching from the lower nodes, blades ovate lanceolate, flat and asymmetric at the base, the leaves with many glandular hairs; the inflorescences are partially exserted on the culms, and the spikelets have a lower glume 1/2 the length of the spikelet, 7–9-nerved, the upper glume and lower lemma are 9–13-nerved.
References
Aliscioni SS, Giussani LM, Zuloaga FO, Kellogg EA (2003) A molecular phylogeny of Panicum (Poaceae: Paniceae): tests of monophyly and phylogenetic placement within the Panicoideae. Amer J Bot 90:796–821. doi:10.3732/ajb.90.5.796
Bess E, Doust ANL, Davidse G, Kellogg EA (2006) Zuloagaea, a new genus of neotropical grass within the “Bristle Clade” (Poaceae: Paniceae). Syst Bot 31:656–670
Bosser J (1969) Graminées des paturages et des cultures a Madagascar. Mem ORSTOM 35:1440
Brown WV (1977) The Kranz syndrome and its subtypes in grass systematics. Mem Torrey Bot Club 23:1–97
Christin PA, Salamin N, Kellogg EA, Vicentini A, Besnard G (2009) Integrating phylogeny into studies of C4 variation in the grasses. Pl Physiol 149:82–87
Clayton WD, Renvoize SA (1982) Gramineae (Part 3). In: Polhill RM (ed.), Fl Trop E Africa, Gram (Part 3), pp 461–499
Darriba D, Taboada GL, Doallo R, Posada D (2012) jModelTest 2: more models, new heuristics and parallel computing. Nature Meth 9:772. doi:10.1038/nmeth.2109
Duvall MR, Noll JD, Minn AH (2001) Phylogenetics of Paniceae (Poaceae). Amer J Bot 88:1988–1992
Duvall MR, Saar DE, Grayburn WS, Holbrook GP (2003) Complex transitions between C3 and C4 photosynthesis during the evolution of paniceae: a phylogenetic case study emphasizing the position of Steinchisma hians (Poaceae), a C3–C4 intermediate. Int J Pl Sci 164:949–958
Felsenstein J (1985) Confidence limits on phylogenies: an approach using the bootstrap. Evolution 39:783–791. doi:10.2307/2408678
Giussani LM, Cota-Sanchez JH, Zuloaga FO, Kellogg EA (2001) A molecular phylogeny of the grass subfamily Panicoideae (Poaceae) shows multiple origins of C4 photosynthesis. Amer J Bot 88:1993–2012
Goloboff PA, Farris JS, Nixon KC (2008) TNT, a free program for phylogenetic analysis. Cladistics 24:774–786
Gómez-Martínez R, Culham A (2000) Phylogeny of the subfamily Panicoideae with emphasis on the tribe Paniceae: evidence from the trnL-F cpDNA region. In: Jacobs SWL, Everett JE (eds) Grasses: systematics and evolution. Commonwealth Scientific and Industrial Research Organization (CSIRO) Publishing, Victoria, Collingwood, pp 136–140
Grande Allende JR (2014) Novitates Agrostologicae. IV. Additional segregates from Panicum incertae sedis. Phytoneuron 22:1–6
Hall TA (1999) BioEdit: a user-friendly biological sequence alignment editor and analysis program for Windows 95/98/NT. Nucleic Acids Symp Series 41:95–98
Huelsenbeck JP, Ronquist F (2001) MRBAYES: Bayesian inference of phylogenetic trees. Bioinformatics 17:754–755
Kabuye CHS, Wood D (1968) A first record of multicellular glandular hairs in the Gramineae. Bot J Linn Soc 62:69–70
Morrone O, Scataglini MA, Zuloaga FO (2007) Cyphonanthus, a new genus segregated from Panicum (Poaceae: Panicoideae: Paniceae) based on morphological, anatomical and molecular data. Taxon 56:521–532
Morrone O, Denham SS, Aliscioni SS, Zuloaga FO (2008) Parodiophyllochloa, a new genus segregated from Panicum (Paniceae, Poaceae) based on morphological and molecular data. Syst Bot 33:66–76. doi:10.1600/036364408783887393
Morrone O, Aagesen L, Scataglini MA, Salariato DL, Denham SS, Chemisquy MA, Sede SM, Giussani LM, Kellogg EA, Zuloaga FO (2012) Phylogeny of the Paniceae (Poaceae: Panicoideae): integrating plastid DNA sequences and morphology into a new classification. Cladistics 28:333–356. doi:10.1111/j.1096-0031.2011.00384.x
Nees von Esenbeck CGN (1841) Fl Afr Austral Ill. I. Gramineae: 1–490. Glogau
Olmstead RG, Sweere JA (1994) Combining data in phylogenetic systematics: an empirical approach using three molecular data sets in the solanaceae. Syst Biol 43:467–481. doi:10.1093/sysbio/43.4.467
Osborne CP, Salomaa A, Kluyver TA, Visser V, Kellogg EA, Morrone O, Vorontsova MS, Clayton WD, Simpson DA (2014) A global database of C4 photosynthesis in grasses. New Phytol. doi:10.1111/nph.12942
Rambaut A, Drummond A (2007) Tracer, version 1.4. Computer program and documentation distributed by the author, website http://tree.bio.ed.ac.uk/software/tracer/
Renvoize SA (1968) Studies in the Gramineae: XVIII. Kew Bull 22:481–488
Renvoize SA (1979 [1980]) New species of Panicum (Poaceae) from tropical Africa. Kew Bull 34:551–555
Renvoize SA (1989a) Panicum. In Launert E, Pope GV (eds) Flora Zambesiaca. pp 9–40
Renvoize SA (1989b) New species of Panicum (Poaceae) from southern tropical Africa. Kew Bull 44:543–546
Salariato DL, Zuloaga FO, Giussani LM, Morrone O (2010) Molecular phylogeny of the subtribe Melinidinae (Poaceae:Panicoideae:Paniceae) and evolutionary trends in the homogenization of inflorescences. Molec Phylogen Evol 56:355–369
Sede SM, Morrone O, Giussani LM, Zuloaga FO (2008) Phylogenetic studies in the Paniceae (Poaceae): a realignment of section Lorea of Panicum. Syst Bot 33:284–300. doi:10.1600/036364408784571626
Sede SM, Zuloaga FO, Morrone O (2009) Phylogenetic studies in the Paniceae (Poaceae-Panicoideae): Ocellochloa, a new genus from the New World. Syst Bot 34:684–692. doi:10.1600/036364409790139655
Soreng R (2010) Coleataenia Griseb. (1879): the correct name for Sorengia Zuloaga and Morrone (2010) (Poaceae: Paniceae). J Bot Res Inst Texas 4:691–692
Stapf O (1920) Panicum. In: Prain D (ed.) Flora of Tropical Africa, vol IX. L. Reeve & Co. Ltd, London, pp. 638–738
Thiers B ([continuously updated]) Index Herbariorum: A global directory of public herbaria and associated staff. New York Botanical Garden’s Virtual Herbarium. http://sweetgum.nybg.org/ih/. Accessed 2013
Zimmermann T, Bocksberger G, Brüggemann W, Berberich T (2013) Phylogenetic relationship and molecular taxonomy of African grasses of the genus Panicum inferred from four chloroplast DNA-barcodes and nuclear gene sequences. J Pl Res 126:363–371
Zuloaga FO, Morrone O, Ellis RP (1993) A revision of Panicum subg. Dichanthelium (Poaceae: Panicoideae: Paniceae) in Mesoamerica, the West Indies, and South America. Ann Missouri Bot Gard 80:119–190
Zuloaga FO, Morrone O, Giussani LM (2000) A cladistic analysis of the Paniceae: a preliminary approach. In: Jacobs SWL, Everett JE (eds) Grasses: Systematics and Evolution. Commonwealth Scientific and Industrial Research Organization (CSIRO) Publishing, Victoria, Collingwood, pp 123–135
Zuloaga FO, Giussani LM, Morrone O (2006) On the taxonomic position of Panicum aristellum (Poaceae: Panicoideae: Paniceae). Syst Bot 31:497–505. doi:10.1043/05-56.1
Zuloaga FO, Giussani LM, Morrone O (2007) Hopia, a new genus segregated from Panicum (Poaceae: Panicoideae: Paniceae). Taxon 56:145–156
Zuloaga FO, Scataglini MA, Morrone O (2010) A phylogenetic evaluation of Panicum sects. Agrostoidea, Megista, Prionitia and Tenera (Panicoideae, Poaceae): two new genera. Stephostachys Sorengia. Taxon 59:1535–1546
Zuloaga FO, Morrone O, Scataglini MA (2011) Monograph of Trichanthecium, a new genus segregated from Panicum (Poaceae, Paniceae) based on morphological and molecular data. Syst Bot Monogr 94:1–101
Acknowledgments
Funding of this research was provided by CONICET, grant 11220100100207. We like to express our deep gratitude to Francisco Rojas and Marcelo Moreno for the illustrations included in this contribution. We especially appreciate the help of the Editor-in-Chief Karol Marhold, Rob Soreng, and two additional anonymous reviewers who provided useful suggestions to improve an early version of this paper.
Author information
Authors and Affiliations
Corresponding author
Additional information
Handling editor: Karol Marhold.
Appendix: List of taxa used in the molecular analysis and GenBank accession numbers. For new sequences (*) voucher information is given
Appendix: List of taxa used in the molecular analysis and GenBank accession numbers. For new sequences (*) voucher information is given
Tribe Andropogoneae. Andropogon gerardii Vitman, AF117391; Bothriochloa bladhii (Retz.) S.T.Blake, AF117395; Chionachne koenigii (Spreng.) Thwaites, AF117397; Cymbopogon flexuosus (Nees ex Steud.) Will. Watson, AF117404; Phacelurus digitatus (Sibth. & Sm.) Griseb., AF117418. Sorghum bicolor (L.) Moench, U21981; Zea mays L., U21985. Tribe Arundinelleae. Arundinella hirta (Thunb.) Tanaka, AF117393. Tribe Chasmanthieae. Chasmanthium laxum (L.) H.O.Yates, U27296. Tribe Paniceae. Acroceras zizanioides (Kunth) Dandy, AY029618; Cenchrus ciliaris L., AY029625; C. compressus (R. Br.) Morrone, AY029672; C. setaceus (Forssk.) Morrone, AY029673; Chaetium bromoides (J. Presl) Benth. ex Hemsl., AY029626; Dichanthelium acuminatum (Sw.) Gould & C.A.Clark, AY188485; *D. boscii (Poir.) Gould & C.A.Clark, United States, Missouri, Cherokee Pass Quadrangle, Brant 5442 (MO) KP173703; D. clandestinum (L.) Gould, AY188461; D. cumbucana (Renvoize) Zuloaga, AY188464; *D. hebotes (Trin.) Zuloaga, Brazil, Ceará, Guaramiranga, Huber s.n. (IAN); D. koolauense (H.St.John & Hosaka) Gould & C.A.Clark, AY029627; *D. linaerifolium (Scribn.) Gould, United States, Missouri, Howell County, Summers 8082 (MO) KP173704; *D. malacophyllum (Nash) Gould, United States, Missouri, Vernon County, Camp Clark, Becker 1042 (MO) KP173705; *D. oligosanthes (Schult.) Gould, United States, Missouri, Vernon County, Camp Clark, Becker 900 (MO) KP173706; D. sabulorum (Lam.) Gould & C.A.Clark, AY029654; *D. scoparium (Lam.) Gould, United States, Missouri, Stoddard County, Gust 729 (MO) KP173707; *D. stigmosum (Trin.) Zuloaga, Brazil, Santa Catarina, Uribici, Silva and Silva 26 (SI) KP173708; *D. superatum (Hack.) Zuloaga, Brazil, Santa Catarina, Dalmoli et al. 62 (SI) KP173709; Brazil, Santa Catarina, Uribici, Dalmoli et al. 75 (SI) KP173710; *D. viscidellum (Scribn.) Gould, Colombia, Norte de Santander, vicinity of Toledo, Killip and Smith 20046 (US) KP173711; Digitaria setigera Roth ex Roem. & Schult., AY029629; Echinochloa colona (L.) Link, AY029631; Eriochloa punctata (L.) Desv., AY029634; Lasiacis sorghoidea (Desv.) Hitchc. & Chase, AY029639; Megathyrsus maximus (Jacq.) B. K. Simon and S. W. L. Jacobs, AY029649; Melinis repens (Willd.) Zizka, AY029675; Moorochloa eruciformis (Sm.) Veldkamp, AY188452; Oplismenus hirtellus (L.) P.Beauv., AY029644; Panicum. Section Dichotomiflora (Hitchc.) Honda: Panicum aquaticum Poir., AY029658; P. dichotomiflorum Michx., AY188466; P. elephantipes Nees ex Trin., AY029647; P. gouinii E.Fourn., AY188467; P. pedersenii Zuloaga, AY029646; P. repens L., AY029651. Section Panicum: P. bergii Arechav., AY188457; P. fauriei Hitchc., AY029650; P. miliaceum L., AY188472; P. nephelophilum Gaudich., AY029645; P. stramineum Hitchc. & Chase, AY188489. Section Rudgeana (Hitchc.) Zuloaga: P. cervicatum Chase, AY188459; P. rudgei Roem. & Schult., AY029661. Section Urvilleana (Hitchc.) Pilg: P. chloroleucum Griseb., AY188460; P. racemosum (P.Beauv.) Spreng., AY188481. Section Virgata Hitchc. & Chase ex Pilg.: P. tricholaenoides Steud., AY188493; P. virgatum L., U21986. Ungrouped. P. mystasipus Zuloaga & Morrone, AY188474; P. olyroides Kunth, AY188475. Panicum “incertae sedis”: Section Clavelligerae Stapf: P. adenophorum K.Schum., AY188454; P. claytonii Renvoize, AY188462; *P. bullockii, Zambia, Sunzu Mountain, 20 miles S of Abercorn, Phipps and Vesey-Fitzgerald 3314 (K) KP173693; *P. ecklonii, Tanzania, Iringa, Makete, Gereau 3123 (MO) KP173694; *P. flacciflorum, Tanzania, Mpanda, Uzondo Plateau, Bidgood et al. 5501 (K) KP173695; *P. habrothix, Burundi, Bujumbura, Muhira, Reekmans 8890 (K) KP173696; *P. hymeniochilum, Tanzania, Rukwa,, Sumbawanga, Harder et al. 1257 (MO) KP173697; *P. nigromarginatum, Tanzania, Bukoba, Minziro Forest Reserve, Bayona and Festo 9244 (K) KP173698; *P. pectinellum, Zambia, Mporokoso, 28 miles east of Mporokoso, Fitzgerald 3124 (MO) KP173699; *P. peteri, Mozambique, Sofala, southern tip of Chimanimani Mts., Muller and Kelly 1103 (K) KP173700; *P. pole-evansii, Zambia, Luapula, Lake Bangweulu, Renvoize 5513 (MO) KP173701; *P. sadinii; Zambia, Mwinilunga, river Lunga at Mwinilunga, Milne-Redhead 3339 (K) KP173702; Section Monticola Stapf: P. millegrana Poir., AY029660; P. sellowii Nees, AY188484; P. trichanthum Nees, AY188492. Section Verrucosa Hitchc. & Chase ex C. C. Hsu: P. verrucosum Muhl., AY188496. Ungrouped: P. antidotale Retz., AY188456; Parodiophyllochloa cordovensis (E.Fourn.) Zuloaga & Morrone, AY188463; P. missiona (Ekman) Zuloaga & Morrone, AY188473; P. ovulifera (Trin.) Zuloaga & Morrone, AY029653; P. penicillata (Nees ex Trin.) Zuloaga & Morrone, AY18847; Pseudechinolaena polystachya (Kunth) Stapf, AY029676; Sacciolepis indica (L.) Chase, AY029677; Setaria geminata (Forssk.) Veldkamp, AY029662; S. lachnea (Nees) Kunth, AY029683; S. macrostachya Kunth, AY029678; S. palmifolia (J.König) Stapf, AY029680; S. viridis (L.) Beauv., U21976; Stenotaphrum secundatum (Walter) Kuntze, AY029684; Trichanthecium cyanescens (Lam.) Zuloaga & Morrone, AY188465. T. parvifolium (Lam.) Zuloaga & Morrone, AY188476; T. schwackeanum (Mez) Zuloaga & Morrone, AY188483; T. wettsteinii (Hack.) Zuloaga & Morrone, AY188497; Urochloa acuminata (Renvoize) Morrone & Zuloaga AY029629; U. plantaginea (Link) R.D.Webster, AY029693; Zuloagaea bulbosa (Kunth) Bess, AY029648. Tribe Paspaleae. Aakia tuerckheimii (Hack.) J.R.Grande, AY188494; Altoparadisium chapadense Filg. et al., AY029619; Anthaenantia lanata (Kunth) Benth., AY029640; Anthaenantiopsis rojasiana Parodi, AY029620; Apochloa euprepes (Renvoize) Zuloaga & Morrone, AY029657; A. subtiramulosa (Renvoize & Zuloaga) Zuloaga & Morrone, AY188490; Arthropogon villosus Nees, AY029622; Axonopus anceps (Mez) Hitchc., AY029623; Axonopus hydrolithicus (Filg. et al.) A. López & Morrone, AY029642; Canastra lanceolata (Filg.) Morrone et al., AY029621; Coleataenia anceps (Michx.) Soreng, AY188455. C. caricoides (Nees ex Trin.) Soreng, GU253330; C. longifolia (Torr.) Soreng, AY188482. C. petersonii (Hitchc. & Ekman) Soreng, AY188479. C. prionitis (Nees) Soreng, AY029652; C. stenodes (Griseb.) Soreng, GU253333; C. tenera (Beyr. ex Trin.) Soreng, AY188491; Echinolaena inflexa (Poir.) Chase, AY029633; Homolepis glutinosa (Sw.) Zuloaga & Soderstr., AY029637; Hopia obtusa (Kunth) Zuloaga & Morrone, AY029659; Hymenachne donacifolia (Raddi) Chase, AY029635; H. grumosa (Nees) Zuloaga, AY188468; H. pernambucensis (Spreng.) Zuloaga, AY188478; Ichnanthus pallens (Sw.) Munro ex Benth., AY029638; Mesosetum chaseae Luces, AY029641; Ocellochloa chapadensis (Swallen) Zuloaga & Morrone, AY188486; O. piauiensis (Swallen) Zuloaga & Morrone, AY029656; O. stolonifera (Poir.) Zuloaga & Morrone, AY18848; Oncorachis ramosa (Zuloaga & Soderstr.) Morrone & Zuloaga, AY029686; Oplismenopsis najada (Hack. & Arechav.) Parodi, AY188453; Otachyrium versicolor (Döll) Henrard, AY029643; Osvaldoa valida (Mez) J.R.Grande, AY188495; Paspalum arundinellum Mez, AY029663; P. conjugatum Bergius, AY029669; P. glaziovii (A.G.Burm.) S.Denham, AY029689; P. remotum J.Remy, AY029668; P. vaginatum Sw., AY029665; Phanopyrum gymnocarpon (Elliott) Nash, AY188469; Plagiantha tenella Renvoize, AY029674; Rugoloa hylaeica (Mez) Zuloaga, AY188470; Rugoloa pilosa (Sw.) Zuloaga, AY188480; Steinchisma decipiens (Nees ex Trin.) W. V. Br., AY188499; S. hians (Elliot) Nash, AY029685; S. laxa (Sw.) Zuloaga, AY029655; S. spathellosa (Döll) Renvoize, AY188500; Stephostachys mertensii (Roth) Zuloaga & Morrone, AY188471; Streptostachys asperifolia Desv., AY029687; Tatianyx arnacites (Trin.) Zuloaga & Soderstr., AY029688. Tribe Thysanolaenae. Thysanolaena maxima (Roxb.) Kuntze, U21984. Tribe Tristachyideae. Danthoniopsis dinteri (Pilg.) C.E.Hubb., AY029695. Tribe Zeugiteae. Zeugites pittieri Hack., U21987.
Rights and permissions
About this article
Cite this article
Zuloaga, F.O., Salomón, L. & Scataglini, M.A. Phylogeny of sections Clavelligerae and Pectinatae of Panicum (Poaceae, Panicoideae, Paniceae): establishment of the new subtribe Dichantheliinae and the genus Adenochloa . Plant Syst Evol 301, 1693–1711 (2015). https://doi.org/10.1007/s00606-014-1186-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00606-014-1186-6