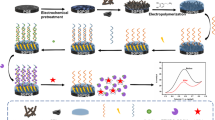
Abstract
A sandwich electrochemical sensor was fabricated based on multi-walled carbon nanotubes/ordered mesoporous carbon/AuNP (MWCNTs/CMK-3/AuNP) nanocomposites and porous core–shell nanoparticles Au@PdNPs to achieve rapid and sensitive detection of AFB1 in complex matrices. MWCNTs/CMK-3/AuNP nanocomposite, which was prepared by self-assembly method, served as a substrate material to increase the aptamer loading and improve the conductivity and electrocatalytic activity of the electrode for the first signal amplification. Then, Au@PdNPs, which were synthesized by one-pot aqueous phase method, were applied as nanocarriers loaded with plenty of capture probe antibody (Ab) and signal molecule toluidine blue (Tb) to form the Au@PdNPs-Ab-Tb bioconjugates for secondary signal amplification. The sensing system could still significantly improve the signal output intensity even in the presence of ultra-low concentration target compound due to the dual signal amplification of MWCNTs/CMK-3/AuNP nanocomposites and Au@PdNPs-Ab-Tb. The method exhibited high selectivity, low detection limit (9.13 fg/mL), and strong stability to differentiate AFB1 from other mycotoxins. Furthermore, the sensor has been successfully applied to the quantitative determination of AFB1 in corn, malt, and six herbs, which has potential applications in food safety, quality control, and environmental monitoring.
Graphical Abstract

Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Affected by climate warming, drought, and other factors, the pollution of mycotoxins in global edible and feed agricultural products is becoming more and more serious [1, 2]. In addition to corn, wheat, barley, peanuts, and other grains, the contaminated food also includes nuts, fruits, seasonings, herbs, and so on, which seriously endangers human and animal health and environmental safety [3], wherein aflatoxin B1 (AFB1) are bifuranotoxins produced by some strains, such as Aspergillus flavus and Aspergillus parasiticus, which is widely distributed in crops, food, herbs, and animal feed [4, 5]. It is extremely toxic, with strong teratogenic, carcinogenic, and mutagenic effects, and is listed as a class I carcinogen by the International Agency for Research on Cancer [6, 7]. Once AFB1 enters the human body, it can cause liver diseases, including liver damage and primary liver cancer, which seriously threatens human life and health [8]. The European Commission stipulated that the residue limit of AFB1 in edible food should not exceed 0.5 ng/mL [9]. Therefore, it is significant to develop an accurate and sensitive assay to detect trace amounts of AFB1 in raw materials or products to ensure public health and safety.
At present, chromatographic techniques, mass spectrometry techniques, and enzyme-related immunosorbent assay have been successfully used for accurate quantitative detection of AFB1 [10, 11]. However, some drawbacks still exist, including complicated pre-treatment, long analysis assay time, and costly equipment. Various analytical assay techniques have been developed and established for the accurate and quantitative analysis of AFB1, such as surface-enhanced Raman scattering [12], colorimetric assays [13], electrochemiluminescence [14], fluorescence [15], surface plasmon resonance [16], and electrochemical sensors [17], wherein electrochemical sensors have the intrinsic merits of high sensitivity, simple operation, low cost, portable devices, and easy miniaturization, which have made them one of the most appealing approaches for AFB1 assay detection [18,19,20]. Wang et al. recently established an aptasensor for rapid detection of AFB1 utilizing a methylene blue–labeled aptamer [21]. The spatial structure of the aptamer changed when the aptamer was specifically bound to the target AFB1, resulting in a corresponding change in current intensity. Ren et al. proposed a signal-switched fluorescent sensor for the rapid detection of AFB1 using DNA tetrahedra achieving a low detection limit (0.01 ng/mL) [22]. However, this way of signal output with a single signal reporting unit corresponding to a single target allowed a low sensitivity of the assay, which hindered its application in trace AFB1 monitoring [23,24,25].
In order to ensure the accurate and sensitive determination of ultra-low abundance mycotoxins in complex matrices, signal amplification based on nanomaterials is considered to be one of the valuable methods [26,27,28]. Among them, ordered mesoporous carbon (CMK-3) is a novel non-silicon-based mesoporous material, which has been widely applied in the fields of capacitive electrodes, drug carriers, and biosensors due to its high porosity and low physiological toxicity [29, 30]. In addition, multi-walled carbon nanotubes (MWCNTs), one of the most commonly employed carbon nanomaterials, are also attracting attention for its good stability and conductivity, which can be utilized as a substrate material to enhance the performance of electrodes [31]. More importantly, MWCNTs act as composite reinforcements to form nanocomposites with CMK-3 and Au nanoparticles (AuNPs). The nanocomposites MWCNTs/CMK-3/AuNPs, which integrate the excellent conductivity of MWCNTs, high surface/volume ratio of CMK-3, and the self-assembly of AuNPs with aptamers via gold-S, can effectively improve the electrochemical catalytic property and improve the AFB1 capture ability of the electrode.
In addition to improving the conductivity of the electrode to achieve signal amplification, the integration of biological recognition substances with high affinity for the target is also a valid method to improve the performance of the sensor [32]. As known, recent reports show that bioconjugates integrating antibody or aptamer with nanomaterials has resulted in high specificity and binding affinity of bioconjugates for targets [33, 34]. Although various bioconjugates have been proposed for constructing biosensor, the selection of nanomaterials in bioconjugates ultimately becomes the key to determining the activity of the final bioconjugates. An ideal bioconjugate nanomaterial in electrochemical biosensors should have the following features: (1) simple synthesis process without toxic reagents usage; (2) controllable morphology and size; (3) excellent conductivity and water dispersibility; (4) abundant binding sites for antibody or aptamer. Porous bimetallic nanoparticles with core–shell structure is the promising carriers for various recognition probes due to its larger specific area, strong catalytic ability, electrical conductivity, and stability [35, 36]. Furthermore, porous bimetallic nanocore-shell particles are also more susceptible to functional modification by biomolecules (e.g., antibodies), or load with more capture probes and signal probes, resulting in enhancement of output signal and reduction of limit of detection.
Herein, we developed a novel aptamer-antibody sandwich electrochemical sensor based on MWCNTs/CMK-3/AuNPs nanocomposites and porous Au@Pd core–shell structure for the highly sensitive AFB1 assay. MWCNTs/CMK-3/AuNP nanocomposites with a large specific surface area was first modified on glassy carbon electrode (GCE) as a substrate material to enhance the electrochemical behavior of GCE and significantly improve the loading of the aptamer. Subsequently, the aptamer was immobilized on the nanocomposites via gold-S bonding system to specifically identify AFB1 onto the GCE. Next, the porous Au@PdNP was loaded with a significant amount of antibody (Ab) and the signal molecule toluidine blue (Tb) to form the signal unit Au@PdNPs-Ab-Tb. Finally, Au@PdNPs-Ab-Tb synergistically recognized AFB1 in the sample with the aptamer, forming an aptamer-antibody sandwich structure to acquire a recognizable current signal. The detection sensitivity was remarkably enhanced due to the stepwise signal amplification using MWCNTs/CMK-3/AuNP nanocomposites and porous bimetallic core–shell nanoparticles. This study provides more possibilities for the application of MWCNTs/CMK-3/AuNPs and mesoporous bimetallic nanomaterials in electrochemical aptamer sensing, and affords strong technical support for the accurate quantitative analysis of trace AFB1 in food and medicinal materials..
Materials and methods
Reagents and instruments
The aptamer utilized in this study was synthesized by Sangon Biotech Co., Ltd. (Zhengzhou, China) and purified with HPLC. The detailed sequence of the aptamer was as follows: 5′-SH-(CH2)6-GTT GGG CAC GTG TTG TCT CTC TGT GTC TCG TGC CCT TCG CTA GGC CCA CA-3′. Aflatoxin G1 (AFG1), aflatoxin G2 (AFG2), aflatoxin B1 (AFB1), aflatoxin B2 (AFB2), and ochratoxin A (OTA) were purchased from Yuanye Bio-Technology Co., Ltd. (Shanghai, China). Gold chloride trihydrate (HAuCl4), chitosan, multi-walled carbon nanotubes (MWCNTs), hexadecylpyridinium chloride monohydrate (HDPC), potassium tetrachloropalladate (K2PdCl4), trisodium citrate, toluidine blue (Tb), and 6-mercapto-1-hexanol (MCH) were obtained from Aladdin Bio-Chem Technology Co., Ltd. (Shanghai, China). Ordered mesoporous carbon (CMK-3) was purchased from Nanjing XFNano Material Tech Co., Ltd. (Nanjing, China). Ascorbic acid (AA) was obtained from J&K Scientific Ltd. (Beijing, China). Aflatoxin B1 polyclonal rabbit antibody (Ab) was obtained from Sigma-Aldrich Trading Co., Ltd. (Shanghai, China). Corn and malt were obtained from local supermarkets (Carrefour, Zhengzhou, China). Dioscoreae Rhizoma, Achyranthis Bidentatae Radix, Rehmanniae Radix, Chrysanthemi Flos, Quisqualis Fructus and Platycladi Semen were purchased from the Third Affiliated Hospital of Henan University of Traditional Chinese Medicine (Zhengzhou, China). Ultrapure water (resistivity ≥ 18.25 mΩ) was obtained from the Milli-Q® water purification system.
Square wave voltammetry (SWV) and cyclic voltammetry (CV) determinations were conducted on a CHI 760E electrochemical workstation (Shanghai, China). Electrochemical impedance spectra (EIS) were scanned and recorded on an Autolab PGSTAT M204 electrochemical workstation (electrolyte solution was a 5 mM [Fe(CN)6]3−/4− solution containing 0.1 M KNO3, frequency range 0.1 Hz–0.1 MHz). The ultraviolet–visible spectra (UV–Vis) were measured on a Shimadzu UV-3600 Plus. Dynamic light scattering (DLS) measurements were carried out on a Malvern Zetasizer nano zsp. The surface morphology of the employed nanomaterials was characterized using a Sigma 500 scanning electron microscope (SEM) and an FEI Talos F200X G2 transmission electron microscope (TEM) and characterized by energy dispersive spectroscopy (EDS) using super-x.
Preparation of MWCNTs/CMK-3/AuNPs nanocomposites
AuNPs were prepared with slight modifications according to previous reports [37]. Firstly, 75 mL 1 mM aqueous HAuCl4 solution was placed in a three necked bottle and heated to boiling under vigorous stirring. After that, 15 mL 38.8 mM of sodium citrate was quickly added. When the color of the solution became wine red, heating was turned off and the solution was allowed to cool down. The AuNP suspension was collected and stored at 4 °C away from light. The characterization results of AuNPs are detailed in the supporting materials.
Next, chitosan (38.4 mg), CMK-3 (9.6 mg) and MWCNTs (4.8 mg) were added to HAc solution (8 mL, 2% in volume ratio), followed by stirring vigorously under 0 ℃ about 3 h. Subsequently, 8 mL AuNP suspension was slowly added under ultrasonic conditions. The whole process lasted for about 1 h to obtain MWCNTs/CMK-3/AuNPs.
Synthesis of porous Au@Pd core–shell nanoparticles (Au@PdNPs)
Au@Pd nanostructures with vertical pores were prepared via wet chemical synthesis with slight modifications following previous reports [38]. First, 0.1 g HDPC, 0.5 mL HAuCl4 (10 mM) and 2 mL K2PdCl4 (10 mM) were dispersed into 25 mL of ultrapure water and sonicated to form a homogeneous mixed solution. After that, 1.5 mL of freshly prepared aqueous AA solution (100 mM) was added to this mixed solution under constant shaking. The reaction system was allowed to stand for 3 h at 35 °C protected from light, and the resulting product Au@PdNPs was centrifuged (10,000 rpm, 10 min) and washed twice with ultrapure water. Finally, the Au@PdNP was redispersed in 10 mL of ultrapure water and stored at 4 °C away from light.
Preparation of Au@PdNPs-Ab-Tb bioconjugates
First, 20 μL of Ab solution (50 μg/mL) was added to 1 mL of Au@PdNP suspension, and the mixed solution was incubated with shaking at 37 °C for 3 h (300 rpm) to obtain Au@PdNPs-Ab conjugate solution. Then 300 μL of aqueous Tb solution (6 mM) was added to the Au@PdNPs-Ab solution and incubated with shaking at 37 °C overnight (300 rpm). Finally, the reaction mixture was centrifuged (10,000 rpm, 10 min) to remove unreacted Ab and Tb to obtain Au@PdNPs-Ab-Tb bioconjugate. The products were washed twice with PBS buffer and then redispersed in 1 mL of PBS buffer.
Fabrication of the biosensor
The procedure for the construction of this aptamer-antibody sandwich electrochemical sensor is illustrated in Scheme 1. The GCE was polished to the mirror surface with 1.0 and 0.3 μm Al2O3 slurry on the suede in turn. After each polishing, the GCE was moved into the ultrasonic water bath for cleaning for three times, and finally followed by ultrasonic cleaning with 1:1 HNO3, and distilled water, and dried in a nitrogen gas stream. Then, MWCNT/CMK-3/AuNP nanocomposite (2 μL) was added on the surface of GCE and dried in an oven. Next, the Ap solution of AFB1 (7 μL, 1 μM) was added slowly to the surface of GCE and incubated at 25 °C for 8 h. After rinsing off the unbound Ap with ultrapure water, the remaining active sites on the GCE surface were blocked with MCH (500 μL, 4 mM). Immediately afterward, AFB1 solution (5 μL) was carefully dropped onto the modified electrode. After that, 5 μL of Au@PdNPs-Ab-Tb suspension was pipetted onto the electrode and incubated for 1 h under 37 °C. Finally, the electrochemical response curve of the electrode was scanned and recorded by SWV (solution 0.1 M PBS, scan range − 0.6–0 V, quiet time 2 s, stepping potential 4 mV, frequency 25 Hz and amplitude 25 mV). The electrochemical tests in this experiment were performed using a standard three-electrode system, where AE is saturated calomel (Φ = 5.3 mm, solution: saturated KCl) and RE is platinum wire (Φ, 0.5 mm × 37 mm).
Quantitative analysis of AFB1 in real samples
2.00 g of corn, malt, and six herb test powder (through a 100 mesh sieve) were placed in a homogeneous bottle. Then, sodium chloride (3.00 g) and methanol (75 mL, 70%) were added. The mixure was stirred at the speed of 11000 r/min for 2 min and then centrifuged for 5 min (centrifugal speed 4000 r/min). The supernatant was collected, filtered (0.22-μm filter membrane), and then diluted 10 times. Finally, 2.00 pg/mL, 20.00 pg/mL and 200.00 pg/mL of AFB1 standard solution were added to the diluted sample solutions for detection and analysis through the proposed sensing system.
Results and discussion
The detection principle of sensor
This work combines MWCNTs/CMK-3/AuNP nanocomposites, Au@PdNPs, and two strong AFB1 affinity biometric probes (Ab and Ap) to construct a novel signal-enhanced electrochemical biosensor for AFB1 monitoring. The detection principle of this aptamer sensor is shown in Scheme 1.
MWCNT/CMK-3/AuNP nanocomposites could offer plenty of loading sites for the AFB1 aptamer. Au@PdNPs, which possessed numerous vertical pore channels and large specific surface area, can be used as nanocarrier for loading probe Ab and signal molecule Tb to form the signal unit Au@PdNPs-Ab-Tb. The detection of AFB1 was then achieved by precise capturing of AFB1 in the samples through the high affinity of Ab and Ap for AFB1. Chitosan, a natural polymer possessing favorable film-forming and adhesion properties, could improve the dispersibility and biocompatibility of MWCNTs and CMK-3 [39]. In addition, chitosan in an acetic acid environment contained a large amount of active amino groups and exhibited a strong positive charge [40]. It could be electrostatically bonded with negatively charged AuNPs to obtain uniform and stabilized MWCNTs/CMK-3/AuNP nanocomposites under ultrasonic conditions [32]. A stable and compact electron-conducting active layer could be formed on the GCE surface by MWCNTs/CMK-3/AuNPs, which dramatically increased the specific surface area of the electrode, enriched the immobilization amount of the aptamer, and enhanced the electrochemical activity of the electrode. Then, the AFB1 aptamer modified with thiol group was grafted onto the MWCNTs/CMK-3/AuNPs/GCE surface through the gold-S bond. Subsequently, MCH was used to seal the unbound non-specific sites. When AFB1 was added to the assay system, the aptamer specifically captured AFB1 onto the electrode surface. Finally, a significant amount of Au@PdNPs-Ab-Tb spontaneously aggregated on the surface of the modified GCE via antibody-antigen specific recognition, which dramatically increased the current intensity and thus improved the detection sensitivity of the sensor.
Characterization
Characterization of AuNPs, MWCNTs/CMK-3/AuNPs, and Au@PdNPs
Figure S1 illustrates the characterization results of AuNPs. First, the surface morphology of AuNPs was characterized using TEM. As shown in Fig. S1A, the AuNPs exhibited a uniform near spherical shape, exhibiting an average particle diameter of about 19 nm. The DLS results (Fig. S1B) were also consistent with the particle size results obtained from TEM, indicating the successful preparation of AuNPs.
SEM, TEM, and EDS were employed to characterize the surface structures and morphologies of MWCNTs, CMK-3, and MWCNTs/CMK-3/AuNP nanocomposites. As shown in Fig. 1A and Fig. S2A, MWCNTs were densely stacked with alternating tubular structures of coiling and twisting, while CMK-3 exhibited a regular ordered tubular pore structure with nanorod arrays (Fig. 1B and Fig. S2B). In addition, a great quantity of pores were formed between the carbon nanorods, which resulted in a tremendous specific surface area (Fig. 1B). Figure 1C and D illustrate that the array-like CMK-3 had been inter-stacked with the bent and coiled MWCNTS, and plenty of spherical AuNPs were scattered on its surface. Furthermore, it was further illustrated by the EDS results that the nanocomposites was composed of only Au and C elements (Fig. 1E). These results indicated that the nanocomposites has been successfully prepared.
The structure, surface morphology, and particle size distribution of Au@PdNPs were characterized by SEM, TEM, EDS, and DLS. From the SEM and TEM images, it was found that the Au@PdNPs exhibited a spherical core–shell structure with many vertical pore channels, uniform particle size distribution, and good dispersion (Fig. 2A, B). The images of high-angle annular dark-field imaging (HAADF) STEM clearly showed that the nanoparticles had a solid core structure and a large number of vertical pore channels with uniform size traits (Fig. 2C). The EDS results in Fig. 2D indicated that the nanocore-shell structure was composed of only two elements, Au and Pd. In addition, the DLS results showed that the Au@PdNPs possessed a uniform particle size distribution with the mean size of 100 nm (Fig. 2E). This was attributed to the fact that the DLS measured the equivalent hydrated mechanical radius, which would be slightly larger than the dry state particle size measured by TEM [41]. All these results indicated that the bimetallic porous nanocore-shell of Au@PdNPs was synthesized successfully.
Surface morphology of electrode
The surface morphology of different modified electrodes was investigated by SEM to monitor the modification process of the electrode. As illustrated in Fig. 3A, the unmodified bare GCE exhibited a flat and smooth surface. After MWCNTs/CMK-3/AuNPs nanocomposites modification, the electrode surface was densely packed with a large number of composites, which provided abundant attachment sites for subsequent aptamer self-assembly (Fig. 3B). As shown in Fig. 3C, when the sensor was completely modified, the electrode surface was scattered with numerous spherical Au@PdNPs-Ab-Tb, which could significantly enhance the signal output intensity and improve the sensitivity. The above results demonstrated that the electrode modification was successful.
Electrochemical characterization of the sensor
Firstly, we utilized SWV to record the current response of the modified electrode with different modification status to investigate the suitability of the electrochemical aptasensor for AFB1 detection. As exhibited in Fig. 4A, the signal probe Au@PdNPs-Ab-Tb could be successfully coupled to the electrode surface only when all modification procedures were completed, revealing a distinct Tb reduction peak at around − 0.3 V (curve d). In contrast, when the electrode lacked Ap (curve a), AFB1 (curve b), and Au@PdNPs-Ab-Tb (curve c) modified, no obvious current intensity could be recorded (except for the faint background noise). These results suggested that the “aptamer-AFB1-antibody” sandwich electrochemical aptamer sensor was suitable for AFB1 detection, and any missing step in the sensor fabrication process could not be successfully assembled and applied for AFB1 detection.
(A) SWV curves of GCE/MWCNTs/CMK-3/AuNPs/Ap/MCH/AFB1/Au@PdNPs-Ab-Tb (d) and SWV curves of unmodified Ap (a), AFB1 (b), Au@PdNPs-Ab-Tb (c), EIS (B) and CV (C) curves of bare GCE (a), GCE/MWCNTs/CMK-3/AuNPs (b), GCE/MWCNTs/CMK-3/AuNPs/Ap (c), GCE/MWCNTs/CMK-3/AuNPs/Ap/MCH (d), GCE/MWCNTs/CMK-3/AuNPs/Ap/MCH/AFB1 (e), and GCE/MWCNTs/CMK-3/AuNPs/Ap/MCH/AFB1/Au@PdNPs-Ab-Tb (f)
Next, the stepwise modification procedure of the electrode surface was characterized using EIS. As can be seen in Fig. 4B, the charge transfer resistance (Rct) decreased rapidly after modifying MWCNTs/CMK-3/AuNPs onto the electrode (~ 130.0 Ω, curve b) compared to bare GCE (~ 201.2 Ω, curve a), which was mainly attributed to the favorable electrical conductivity of MWCNTs/CMK-3/AuNPs nanocomposites. Since [Fe(CN)6]4−/3− exerted strong electrostatic repulsion with the phosphate group on the aptamer, the immobilization of the aptamer led to an increase in the Rct of the modified electrode (~ 299.8 Ω, curve c). When the nonspecific site was blocked by MCH, it formed a poorly conductive SAM on the electrode surface, which caused an increase in Rct (~ 503.1 Ω, curve d). After AFB1 was specifically captured by the aptamer, Rct further increased (~ 716.8 Ω, curve e) due to the increased steric hindrance of the electrode. Finally, the steric hindrance on GCE surface increased sharply due to the modification of Au@PdNPs-Ab-Tb, which significantly inhibited the electron transfer ability on GCE surface, and Rct increased dramatically (~ 955.7Ω, curve f). The EIS results indicated that the proposed sensing system was successfully constructed according to the expected design steps.
In addition, the electrochemical characterization of the stepwise modified electrodes was also performed by CV (Fig. 4C). The current intensity was dramatically enhanced when the GCE was modified with MWCNTs/CMK-3/AuNPs nanocomposites with excellent electrochemical properties compared to the bare GCE (curves a and b). However, with the modification of Ap, MCH, AFB1, and Au@PdNPs-Ab-Tb on the electrode layer by layer, the electron transfer efficiency on the surface of electrode was gradually suppressed, leading to a slow decrease in the current intensity (curves c to f). The obtained experimental result of CV was compatible with those of SWV and EIS, which further verified the feasibility of the sensing system.
Optimization of experimental parameters
In this section, a series of optimizations of key parameters in the construction procedure were carried out, including the modification volume of MWCNTs/CMK-3/AuNPs nanomaterials, the reaction time of AFB1, and the reaction time of Au@PdNPs-Ab-Tb, to achiveving the the best performance.
The properties of the conductive thin film layer formed by the nanocomposites on the electrode surface were strongly related to the output signal performance of this sensor. Therefore, we investigated the correlation between the drop volume of the nanocomposites and the current response of the modified electrode. As depicted in Fig. 5A, the current intensity rose as the volume of nanocomposites increased and reaches a maximum at 1.5 μL. However, once the volume of nanocomposites was higher than 1.5 μL, the current intensity of the modified electrode gradually decreased. This was probably due to the fact that when the modified volume of the nanocomposite was too large, the conductive thin film layer formed on the GCE surface was too thick for the self-assembly of the aptamer [42]. Therefore, the optimal volume of nanocomposites for modification was 1.5 μL.
Selecting the appropriate AFB1 reaction time would maximize the amount of antibody modification, thereby achieving a significant increase in the output signal. The changing curve of the current intensity of the modified GCE with AFB1 incubation time is shown in Fig. 5B. The current intensity increased sharply with increasing reaction time from 10 to 30 min and reached a maximum after 30 min. This implied that as the reaction time increased, more and more AFB1 was captured by the modified GCE. While as the incubation time of AFB1 reached 30 min, the AFB1 on the modified GCE surface reached saturation. Thus, the optimal incubation time of AFB1 was 30 min, which was used for the subsequent research.
In addition, the effect of different reaction times of Au@PdNPs-Ab-Tb on the peak current of this sensor was also investigated. The peak current gradually enhaced when the reaction time of Au@PdNPs-Ab-Tb grew from 10 to 60 min and stopped changing after 60 min (Fig. 5C). It showed that the combination of Au@PdNPs-Ab-Tb with the target AFB1 had been completed and reached the saturation state after 60 min. As a consequence, the optimal reaction time of Au@PdNPs-Ab-Tb was 60 min.
Based on the above experimental results, the optimal experimental condition for this sensing system was 1.5 μL of MWCNTs/CMK-3/AuNP nanocomposites, 30 min of AFB1 reaction time, and 60 min of Au@PdNPs-Ab-Tb reaction time.
Linear range and detection limit of the sensor
Under the optimized detection conditions, SWV experiments were performed using MWCNTs/CMK-3/AuNPs and Ap-modified GCE in different concentrations of AFB1 solution. The reduction potential peak of Tb around − 0.3 V can be clearly observed in the concentration-peak current standard curve of AFB1 (Fig. 6A) [39, 42]. In addition, the peak current intesnity of Tb became progressively higher accordingly as the AFB1 concentration increased from 20 fg/mL to 2 ng/mL. Notably, a high linear relationship between AFB1 concentration and the corresponding current of modified GCE was observed in the range of 20 fg/mL to 2 ng/mL. Through fitting and calculation, the linear equation is I (μA) = 9.836 × lg(CAFB1) + 7.346 (R2 = 0.999), and the detection limit is 9.13 fg/mL (S/N = 3), based on the 3σ/m criterion, where σ is the standard deviation of the blank (n = 3) and m is the slope of the calibration curve. In order to further illustrate the advantages of this method, we compared the analytical performance of this sensing system with currently developed AFB1 detection method (Table 1). The proposed sensor exhibited a wider detection range and a lower detection limits, having better detection performance compared with other detection methods, which suggested that the MWCNTs/CMK-3/AuNP nanocomposite could improve the conductivity and electrocatalytic activity of the electrode and Au@Pd core–shell nanocarrier could load plenty of Ab and Tb to realize signal amplification.
Selectivity, reproducibility, and stability assay
The selectivity of the MWCNTs/CMK-3/AuNPs/GCE to AFB1 was assessed by comparing the current response curves of the modified GCE to TE buffer (blank group), AFB1, AFB2, AFG1, AFG2, and OTA five mycotoxins (2 ng/mL). The electrochemical response values of AFB2, AFG1, AFG2, OTA, and blank groups were significantly lower than those of AFB1, with peak currents of 23.30%, 25.76%, 23.17%, 24.61%, and 21.94% of AFB1 assay results, respectively (Fig. 7A). This was mainly attributed to the powerful affinity recognition of AFB1 by the two recognition elements (aptamer and antibody), which allowed the sensor to precisely identify the target AFB1. Since the sensor could accurately distinguish between AFB1 and other interferents, this approach had excellent selectivity for detecting AFB1 in complex matrices.
The reproducibility of the MWCNTs/CMK-3/AuNPs and Ap-modified GCE was explored by the relative standard deviations (RSDs) of intra- and inter-batch assays. The intra- and inter-batch RSDs were 2.65% and 3.09% by repeated incubation of 2 ng/mL AFB1 solution on five intra- and inter-batch GEC modified with MWCNTs/CMK-3/AuNPs and Ap respectively, indicating the excellent reproducibility of this method. In addition, to investigate the stability of the sensing method, the fabricated GEC was kept in a refrigerator at 4 °C for 2 weeks and then subjected to electrochemical detection again. The detection results showed that the peak current intensity was still retained 97.76% of the initial value after 2 weeks of placement, indicating that the sensor had favorable stability (Fig. 7B).
Analysis of AFB1 in real samples
The electrochemical sensor employing MWCNTs/CMK-3/AuNPs and Au@Pd core–shell structure was used to quantitate AFB1 in corn, malt, and six herbs by standard addition recovery test to verify the feasibility of the sensor in practical application. It is worth noting that after alcohol extraction and centrifugation, eight kinds of actual samples could be directly added to the sensing system for analysis and detection without separation, indicating that this method could shorten the detection time. It can be seen from Table 2 that the recoveries of AFB1 in eight kinds of actual samples ranged from 93.83 to 106.29%, and the average recovery rate was 99.57. The RSD ranged from 0.42 to 5.5%, and with the average RSD was 2.34%. The actual sample test results show that the sensing system can achieve high sensitivity and specificity detection of AFB1 in complex matrices.
Conclusion
An AFB1 sandwich electrochemical sensing platform employing MWCNTs/CMK-3/AuNP nanocomposites and porous Au@PdNPs for synergistic signal amplification were successfully fabricated. MWCNTs/CMK-3/AuNP nanocomposites with large specific surface area, excellent electron transfer capability, and abundant binding sites could be used as electrode substrate materials to load more aptamers. In addition, Au@PdNPs had favorable stability and a large number of vertical pore channels, which could be served as nanocarriers to enhance the loading of antibodies and electroactive substances Tb. With their synergistic effect, the electrochemical intensity of the sensor was significantly enhanced. Under the optimal conditions, the sensor had a detection range of 20 fg/mL–2 ng/mL and a LOD of 9.13 fg/mL. In addition to possessing good selectivity, stability, and reproducibility, the as-prepared biosensor also exhibited the following features: (1) environmentally friendly and mild synthesis process of MWCNTs/CMK-3/AuNPs nanocomposites and Au@PdNPs; (2) easy to operate and fast response; (3) improved detection sensitivity by two signal amplifications; (4) rapid detection of trace AFB1 in actual samples. Further study will concentrate on improving the convenience of the sensing system, which can achieve rapid on-site detection of actual samples. This study provides a novel and effective platform for the monitoring of trace AFB1 in complex matrices, which holds promising application prospects in assessment of food and herb quality.
Data availability
Data will be made available upon request.
The data that support the findings of this study are available on request from the corresponding author, upon reasonable request.
References
Sohrabi H, Majidi M, Arbabzadeh O, Khaaki P, Pourmohammad S, Khataee A, Orooji Y (2022) Recent advances in the highly sensitive determination of zearalenone residues in water and environmental resources with electrochemical biosensors. Environ Res 204:112082. https://doi.org/10.1016/j.envres.2021.112082
Chhaya R, Nag R, Cummins E (2024) Quantitative risk ranking of mycotoxins in milk under climate change scenarios. Environ Res 245:117979. https://doi.org/10.1016/j.envres.2023.117979
Tang X, Zuo J, Yang C, Jiang J, Zhang Q, Ping J, Li P (2023) Current trends in biosensors for biotoxins (mycotoxins, marine toxins, and bacterial food toxins):principles, application, and perspective. TrAC Trend Anal Chem 165:117144. https://doi.org/10.1016/j.trac.2023.117144
Zhang M, Guo X, Wang J (2023) Advanced biosensors for mycotoxin detection incorporating miniaturized meters. Biosens Bioelectron 224:115077. https://doi.org/10.1016/j.bios.2023.115077
Wang G, Miao Y, Sun Z, Zheng S (2018) Simultaneous adsorption of aflatoxin B1 and zearalenone by mono- and di-alkyl cationic surfactants modified montmorillonites. J Colloid Interf Sci 511:67–76. https://doi.org/10.1016/j.jcis.2017.09.074
Gao Y, Wei J, Li X, Hu Q, Qian J, Hao N, Wang K (2022) Region separation type bio-photoelectrode based all-solid-state self-powered aptasensor for ochratoxin A and aflatoxin B1 detection. Sens Actuators B 364:131897. https://doi.org/10.1016/j.snb.2022.131897
Adedara I, Atanda O, Sant’Anna Monteiro C, Rosemberg D, Aschner M, Farombi E, Rocha J, Furian A, Emanuelli T (2023) Cellular and molecular mechanisms of aflatoxin B1-mediated neurotoxicity: the therapeutic role of natural bioactive compounds. Environ Res 237:116869. https://doi.org/10.1016/j.envres.2023.116869
Zhou S, Guo L, Shi X, Ma L, Yang H, Miao M (2023) In situ synthesized eRAFT polymers for highly sensitive electrochemical determination of AFB1 in foods and herbs. Food Chem 421:136176. https://doi.org/10.1016/j.foodchem.2023.136176
Liao X, Li Y, Long N, Xu Q, Li P, Wang J, Zhou L, Kong W (2023) Multi-mycotoxin detection and human exposure risk assessment in medicinal foods. Food Res Int 164:112456. https://doi.org/10.1016/j.foodres.2023.112456
Magdalena Pisoschi A, Iordache F, Stanca L, IonescuPetcu A, Purdoiu L, IonutGeicu O, Bilteanu L, Iren Serban A (2023) Comprehensive overview and critical perspective on the analytical techniques applied to aflatoxin determination – a review paper. Microchem J 191:108770. https://doi.org/10.1016/j.microc.2023.108770
Vargas Medina D, BassolliBorsatto J, Maciel E, Lanças F (2021) Current role of modern chromatography and mass spectrometry in the analysis of mycotoxins in food. TrAC Trend Anal Chem 135:116156. https://doi.org/10.1016/j.trac.2020.116156
Martinez L, He L (2021) Detection of mycotoxins in food using surface-enhanced Raman spectroscopy: a review. ACS Appl Bio Mater 4:295–310. https://doi.org/10.1021/acsabm.0c01349
Qian J, Ren C, Wang C, An K, Cui H, Hao N, Wang K (2020) Gold nanoparticles mediated designing of versatile aptasensor for colorimetric/electrochemical dual-channel detection of aflatoxin B1. Biosens Bioelectron 166:112443. https://doi.org/10.1016/j.bios.2020.112443
Tian D, Wang J, Zhuang Q, Wu S, Yu Y, Ding K (2023) An electrochemiluminescence biosensor based on graphitic carbon nitride luminescence quenching for detection of AFB1. Food Chem 404:134183. https://doi.org/10.1016/j.foodchem.2022.134183
Zuo J, Yan T, Tang X, Zhang Q, Li P (2023) Dual-modal immunosensor made with the multifunction nanobody for fluorescent/colorimetric sensitive detection of aflatoxin B1 in maize. ACS Appl Mater Interfaces 15:2771–2780. https://doi.org/10.1021/acsami.2c20269
Hu W, Chen H, Zhang H, He G, Li X, Zhang X, Liu Y, Li C (2014) Sensitive detection of multiple mycotoxins by SPRi with gold nanoparticles as signal amplification tags. J Colloid Interf Sci 431:71–76. https://doi.org/10.1016/j.jcis.2014.06.007
Yao H, Du S, Yang L, Ding Y, Shen H, Qiu Y, Dai G, Mo F (2024) A magnetic graphene oxide and UiO-66 based homogeneous dual recognition electrochemical aptasensor for accurate and sensitive detection of aflatoxin B1. Talanta 273:125915. https://doi.org/10.1016/j.talanta.2024.125915
Evtugyn G, Belyakova S (2021) Biomembrane mimetic electrochemical sensors. Curr Opin in Electrochem 28:100722. https://doi.org/10.1016/j.coelec.2021.100722
Kunene K, Sayegh S, Weber M, Sabela M, Voiry D, Iatsunskyi I, Coy E, Kanchi S, Bisetty K, Bechelany M (2023) Smart electrochemical immunosensing of aflatoxin B1 based on a palladium nanoparticle-boron nitride-coated carbon felt electrode for the wine industry. Talanta 253:124000. https://doi.org/10.1016/j.talanta.2022.124000
Luo X, Zhao J, Li M, Zhao X, Wei X, Luo Z, Gu W, Du D, Lin Y, Zhu C (2023) Single-atom materials for food safety. Mater Today 64:121–137. https://doi.org/10.1016/j.mattod.2023.02.010
Wang C, Zhao Q (2020) A reagentless electrochemical sensor for aflatoxin B1 with sensitive signal-on responses using aptamer with methylene blue label at specific internal thymine. Biosens Bioelectron 167:112478. https://doi.org/10.1016/j.bios.2020.112478
Ren W, Pang J, Ma R, Liang X, Wei M, Suo Z, He B, Liu Y (2022) A signal on-off fluorescence sensor based on the self-assembly DNA tetrahedron for simultaneous detection of ochratoxin A and aflatoxin B1. Anal Chim Acta 1198:339566. https://doi.org/10.1016/j.aca.2022.339566
Han D, Yang K, Sun S, Wen J (2023) Signal amplification strategies in electrochemiluminescence biosensors. Chem Eng J 476:146688. https://doi.org/10.1016/j.cej.2023.146688
Liu Y, Liu Y, Qiao L, Liu Y, Liu B (2018) Advances in signal amplification strategies for electrochemical biosensing. Curr Opin Electrochem 12:5–12. https://doi.org/10.1016/j.coelec.2018.05.001
Hu Q, Gan S, Bao Y, Zhang Y, Han D, Niu L (2020) Controlled/“living” radical polymerization-based signal amplification strategies for biosensing. J Mater Chem B 8:3327–3340. https://doi.org/10.1039/C9TB02419K
Suo Z, Niu X, Liu R, Xin L, Liu Y, Wei M (2022) A methylene blue and Ag+ ratiometric electrochemical aptasensor based on Au@Pt/Fe-N-C signal amplification strategy for zearalenone detection. Sens Actuators B 362:131825. https://doi.org/10.1016/j.snb.2022.131825
Wu M, Liu S, Qi F, Qiu R, Feng J, Ren X, Rong S, Ma H, Chang D, Pan H (2022) A label-free electrochemical immunosensor for CA125 detection based on CMK-3(Au/Fc@MgAl-LDH)n multilayer nanocomposites modification. Talanta 241:123254. https://doi.org/10.1016/j.talanta.2022.123254
Dong T, Matos Pires N, Yang Z, Jiang Z (2023) Advances in electrochemical biosensors based on nanomaterials for protein biomarker detection in saliva. Adv Sci 10:2205429. https://doi.org/10.1002/advs.202205429
Deshagani S, Das A, Nepak D, Deepa M (2020) Efficient energy storage by an asymmetric poly(3,4-propylenedioxythiophene)//CMK-3 supercapacitor. ACS Appl Polym Mater 2:1190–1202. https://doi.org/10.1021/acsapm.9b01081
Jiang Y, Wu F, Ye Z, Li C, Zhang Y, Li L, Xie M, Chen R (2021) Fe2VO4 nanoparticles anchored on ordered mesoporous carbon with pseudocapacitive behaviors for efficient sodium storage. Adv Funct Mater 31:2009756. https://doi.org/10.1002/adfm.202009756
Ackermann J, Metternich J, Herbertz S, Kruss S (2022) Biosensing with fluorescent carbon nanotubes. Angew Chem Int Ed 61:e202112372. https://doi.org/10.1002/anie.202112372
Huang Z, Chen H, Ye H, Chen Z, Jaffrezic-Renault N, Guo Z (2021) An ultrasensitive aptamer-antibody sandwich cortisol sensor for the noninvasive monitoring of stress state. Biosens Bioelectron 190:113451. https://doi.org/10.1016/j.bios.2021.113451
Tao X, Wang X, Liu B, Liu J (2020) Conjugation of antibodies and aptamers on nanozymes for developing biosensors. Biosens Bioelectron 168:112537. https://doi.org/10.1016/j.bios.2020.112537
Gomez Cardoso A, Rahin Ahmed S, Keshavarz-Motamed Z, Srinivasan S, Rajabzadeh R (2023) A recent advancements of nanomodified electrodes-towards point-of-care detection of cardiac biomarkers. Bioelectrochemistry 152:108440. https://doi.org/10.1016/j.bioelechem.2023.108440
Gajjala R, Naveen B, Suresh Kumar P (2021) Cu@Pd core-shell nanostructures on pencil graphite substrates as disposable electrochemical sensors for the detection of biological amines. ACS Appl Nano Mater 4:5047–5057. https://doi.org/10.1021/acsanm.1c00530
Feng Y, Xu Y, Liu S, Wu D, Su Z, Chen G, Liu J, Li G (2022) Recent advances in enzyme immobilization based on novel porous framework materials and its applications in biosensing. Coord Chem Rev 459:214414. https://doi.org/10.1016/j.ccr.2022.214414
Sonia SA, Shivangi KR, Kukreti S, Kaushik M (2022) Probing multifunctional azure B conjugated gold nanoparticles with serum protein binding properties for trimodal photothermal, photodynamic, and chemo therapy: biophysical and photophysical investigations. Biomater Adv 134:112678. https://doi.org/10.1016/j.msec.2022.112678
Ivandini T, Luhur M, Khalil M, Einaga Y (2021) Modification of boron-doped diamond electrodes with gold–palladium nanoparticles for an oxygen sensor. Analyst 146:2842–2850. https://doi.org/10.1039/D0AN02414G
Rabai S, Teniou A, Catanante G, Benounis M, Marty J, Rhouati A (2022) Fabrication of AuNPs/MWCNTS/chitosan nanocomposite for the electrochemical aptasensing of cadmium in water. Sensors 22:105. https://doi.org/10.3390/s22010105
Guo Y, Qiao D, Zhao S, Liu P, Xie F, Zhang B (2024) Biofunctional chitosan-biopolymer composites for biomedical applications. Mater Sci Eng R 159:100775. https://doi.org/10.1016/j.mser.2024.100775
Vries W, Niehues M, Wissing M, Würthwein T, Mäsing F, Fallnich C, Studer A, Ravoo B (2019) Photochemical preparation of gold nanoparticle decorated cyclodextrin vesicles with tailored plasmonic properties. Nanoscale 11:9384–9391. https://doi.org/10.1039/C9NR02363A
Liu Z, Wang H, Li J, Wang M, Yang H, Si F, Kong J (2021) Detection of exosomes via an electrochemical biosensor based on C60-Au-Tb composite. Microchem J 170:106772. https://doi.org/10.1016/j.microc.2021.106772
Singh A, Lakshmi G, Fernandes M, Sarkar T, Gulati P, Singh R, Solanki P (2021) A simple detection platform based on molecularly imprinted polymer for AFB1 and FuB1 mycotoxins. Microchem J 171:106730. https://doi.org/10.1016/j.microc.2021.106730
Zhang H, Mao W, Hu Y, Wei X, Huang L, Fan S, Huang M, Song Y, Yu Y, Fu F (2022) Visual detection of aflatoxin B1 based on specific aptamer recognition combining with triple amplification strategy. Spectrochim Acta A 271:120862. https://doi.org/10.1016/j.saa.2022.120862
Liu X, Wen Y, Wang W, Zhao Z, Han Y, Tang K, Wang D (2020) Nanobody-based electrochemical competitive immunosensor for the detection of AFB1 through AFB1-HCR as signal amplifier. Microchim Acta 187:352. https://doi.org/10.1007/s00604-020-04343-2
Li M, Yue Q, Fang J, Wang C, Cao W, Wei Q (2022) Au modified spindle-shaped cerium phosphate as an efficient co-reaction accelerator to amplify electrochemiluminescence signal of carbon quantum dots for ultrasensitive analysis of aflatoxin B1. Electrochim Acta 407:139912. https://doi.org/10.1016/j.electacta.2022.139912
Funding
This work was supported by the Project of Tackling of Key Scientific and Technical Problems in Henan Province (232102321119) and Henan College Students’ Innovation and Entrepreneurship Training Program Fund (X202310471023).
Author information
Authors and Affiliations
Contributions
Liang Guo: investigation, methodology, validation, data curation, writing—original draft. Shijin Zhou: methodology, validation, writing—original draft. Jinyan Xue: data curation, formal analysis, resources. Zenghui Liu: investigation, validation. Shuqing Xu: data curation, resources. Zhangxu He: resources. Huaixia Yang: conceptualization; supervision; writing—review and editing; funding acquisition.
Corresponding author
Ethics declarations
Ethical approval
This research did not involve human or animal samples.
Competing interests
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Guo, L., Zhou, S., Xue, J. et al. Signal-enhanced electrochemical sensor employing MWCNTs/CMK-3/AuNPs and Au@Pd core–shell structure for sensitive determination of AFB1 in complex matrix. Microchim Acta 191, 594 (2024). https://doi.org/10.1007/s00604-024-06665-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00604-024-06665-x