Abstract
A sensitive electrochemical strategy for carcinoembryonic antigen 15–3 (CA15-3) detection is reported using CTAB-Co-MOFs@AuPt NPs as signal probes. The electrochemical strategy was designed as follows: First, the graphene aerogel@gold nanoparticles (GA@Au NPs) nanocomposites were employed to modify the sensing surface for promoting electron transfer rate and primary antibody (Ab1) immobilization due to GA possesses a large specific surface area, eminent conductivity, and a 3D network structure. Cobalt metal–organic frameworks (CTAB-Co-MOFs) synthesized were then used as a carrier for AuPt NPs and secondary antibody (Ab2) immobilization (notes: labelled-Ab2). With sandwich immunoreaction, the labelled-Ab2 was captured on the surface of the GA@Au NPs nanocomposites. Finally, differential pulse voltammetry (DPV) was employed to register the electrochemical signal of the immunosensor at the potential of − 0.85 V (vs SCE) in phosphate buffer saline (PBS) containing 2.5 mM H2O2. It was verified that the electrochemical reduction signal from Co3+ to Co2+ was recorded. The AuPt NPs could catalyze the reaction of H2O2 oxidizing Co2+ to Co3+, resulting in the amplification of the electrochemical signal. Under the selected conditions, the immunosensor can detect CA15-3 in the range 10 µU/mL to 250 U/mL with a low detection limit of 1.1 µU/mL. In the designed strategy, the CTAB-Co-MOFs were not only employed as carriers for AuPt NPs, but also acted as signal probes. The CTAB-Co-MOFs were investigated including SEM, TEM, XPS, and XRD. The application ability of the immunosensor was evaluated using serum sample, demonstrating the immunosensor can be applied to clinic serum analysis.
Graphical Abstract

Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Breast cancer (BC) has always been one of the most significant death causes of women, and it becomes the first of cancer patient in the world according to the latest statistics from the World Health Organization [1]. As is well known, early diagnosis and treatment in time would greatly improve the survival rate of patients. Fortunately, detecting cancer biomarkers provides a new future for early diagnosis of BC. Currently, carbohydrate antigen 15–3 (CA15-3) is recognized as the most significant breast tumor markers [2], which was used to evaluate the human normal and diseased states. Usually, the serum CA15-3 levels below 30 U/mL are regarded as healthy states, and above 30 U/mL hints a high risk of BC [3]. Therefore, developing a rapid and sensitive detection technique of CA15-3 is very crucial for early diagnosis and later treatment.
Currently, the detection methods of CA15-3 biomarker have photoelectrochemical (PEC) [4], chemiluminescence [5], fluorescence [6], and enzyme-linked immunosorbent assay (ELISA) [7]. Nevertheless, the above methods suffer from complex operations, expensive equipment, and trained personnel. ELISA needs enzyme to partake, and the activity of enzyme is affected from pH and temperature, resulting in low repeatability. Hence, it is still challenging to develop a method for detecting CA15-3 with simple, sensitive, and high repeatability. Electrochemical immunosensors have been widely applied to cancer biomarkers detection owing to these virtues of sensitivity, high selectivity, quick readout, and easy to miniaturize [8,9,10]. For example, Feng’s group [9] reported a sandwich-type electrochemical immunosensing of CYFRA 21–1 cancer marker.
As is well known, cancer biomarkers in clinical samples are low abundance, in order to detect lower level of cancer biomarkers; signal amplification strategies are often introduced to augment the sensitivity of immunosensors. From the literature, nanomaterials, such as graphene and Au NPs are often employed to enhance sensing sensitivity [11]. However, the sensitivity of immunosensors reported is not high enough and lacks of real sample application. So, it is also challenging to develop higher sensitive electrochemical immunosensors to detect low abundance of serum cancer biomarkers. Recently, graphene aerogel (GA), featuring a 3D hollow network structure and rich porosity, large specific surface area, and ultra-low density [12], has been causing a great deal of attention. The GA not only inherits the good physical and chemical peculiarities of graphene [13], but also stability, ion diffusion, and electron transport rate have been improved clearly [14]. As a result, GA shows good application ability in energy storage and sensing fields [15,16,17].
Metal–organic frameworks (MOFs), a new type of material, have attracted extensive interest owing to their virtues of large specific surface area, adjustable porosity, abundant functional groups, and unsaturated metal sites [18, 19]. Furthermore, different functional materials or signal molecules, catalysts, and signal probes [20] can be loaded on MOFs to improve the sensitivity of sensor. For example, Guan and his coworkers [21] employed Co-MOFs as signal probes and toluidine blue (TB) as reference probes to fabricate a dual-signal ratiometric aptasensor for ochratoxin A detection. Liu and his coworkers [22] employed Ag-MOFs as signal probes to detect cancer biomarker CEA. Inspired by the advantages of MOFs and literature previously, in this work, we developed a sensitive electrochemical immunosensor for detecting the cancer biomarker CA15-3 using CTAB-Co-MOFs materials as signal probes. The designed immunosensor possesses the following advantages. Firstly, GA@Au NPs, 3D nano-network structure, high conductivity, and large specific surface area are used as a sensing matrix to amplify electrochemical signal. Secondly, CTAB-Co-MOFs not only serve as carriers for AuPt NPs and Ab2 immobilization, but also act as signal probes. AuPt NPs have catalysis ability to the system of H2O2-Co2+, resulting in sensing signal amplification clearly. As a result, the immunosensor demonstrated prominent performance, including good sensitivity and selectivity.
Experimental section
Chemicals, reagents, and instruments
Related chemicals, reagents, and instruments are indicated in the Supplementary Materials (Section S1 and Section S2).
Synthesis of materials
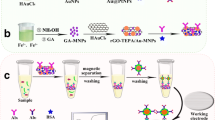
Au NPs [23], Pt NPs [24], CTAB-Co-MOFs [25], GA@Au NPs, and CTAB-Co-MOFs@AuPt NPs materials were synthesized with slight modification according to the reported literature. The related specific synthesis steps can be sought out in the Supplementary Materials (Section S3).
Preparation of labelled-Ab2 bioconjugates
The preparation of labelled-Ab2 bioconjugates is shown in the Supplementary Materials (Section S4).
Fabrication of the electrochemical immunosensor
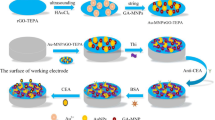
The building process of the immunosensor is shown in Scheme 1. Simple, the glassy carbon electrode (GCE) was first handled by polishing, washing ultrasonically, and drying in a flow of nitrogen [26]. The following materials were sequentially assembled on the surface of GCE. Firstly, 10 µL of GA@Au NPs (1 mg/mL) suspension solution was dropped on GCE and dried in a 37 °C vacuum oven. Secondly, 6 µL of CA15-3 antibody (Ab1, 20 µg/mL) was coated and incubated at 4 °C overnight to anchor the antibody. At each subsequent step, the modified electrode was washed with PBS (0.01 M, pH = 7.4) solution to avoid analyte adsorption on the electrode surface. Subsequently, the electrode was blocked with 6 µL bovine serum albumin solution (BSA, 1 wt%) and incubated at room temperature for 30 min. After that, 6 µL CA15-3 solution with a series of concentrations was added and incubated at 37 °C for 1 h. Finally, 6 µL of CTAB-Co-MOFs@AuPt NPs-Ab2 solution was added dropwise on the electrode to specifically bind by antibody-antigen interactions to construct a sandwich-type immunosensor. The immunosensor was incubated at 37 °C for 1 h and stored at 4 °C when not in use.
Electrochemical measurements
Electrochemical measurements are revealed in the Supplementary Materials (Section S5).
Results and discussion
Design principle of immunosensor
GA@Au NPs were selected for sensing matrix to connect with Ab1 vs Au-NH2 bond. CTAB-Co-MOFs, larger specific surface area, were employed as a carrier for AuPt NPs and Ab2 immobilization. In the presence of CA 15–3, a sandwich-type immune complex was formed on the electrode surface by use of the specific interaction of Ab and Ag. H2O2 can catalyze the Co2+/Co3+ reaction process, meanwhile, AuPt NPs on the surface of CTAB-Co-MOFs can catalyze the reaction of H2O2 oxidizing Co2+ to Co3+. The electrochemical reduction response of Co3+/Co2+ in CTAB-Co-MOFs self was used for the sensing signal. The signal intensity was related to CA15-3 level, so CA15-3 can be quantified via measuring the signal intensity change. The multiple amplification improves the sensitivity of immunosensor. The mechanism is shown as follows:

Characterization of materials
Characterization of GA@AuNPs nanocomposites
Figure 1A and Fig. S1 show photographs and SEM images of GA at different magnifications. It can be seen that GA consists of randomly arranged and fluffy wrinkled graphene sheets and interconnected with each other to present a porous 3D network structure, which improves the stability of the material and enhances the electrical conductivity [14]. Figure 1B shows the SEM of GA @Au NPs; the Au NPs with a size of 16.72 ± 0.91 nm are consistently spread on the surface of GA, indicating that GA@Au NPs nanocomposites were successfully prepared.
A SEM image of GA. B SEM images of GA@Au NPs at different magnifications (inset size distribution). C SEM image of CTAB-Co-MOFs (inset size distribution), D TEM image of CTAB-Co-MOFs, E XRD, F XPS spectra of CTAB-Co-MOFs. G High-resolution XPS fitting spectra of Co 2p. H FT-IR patterns of CTAB-Co-MOFs and Co-MOFs
Characterization of CTAB-Co-MOFs
SEM and TEM were applied to characterize the morphology of the CTAB-Co-MOFs (Fig. 1C, D). The CTAB-Co-MOFs exhibit a regular polyhedral structure and the average size is about 142.68 ± 12.76 nm. Figure 1E shows the XRD diffraction peak of CTAB-Co-MOFs, the distinctly sharp and intense characteristic peaks at 5° to 35° indicate high crystallinity of CTAB-Co-MOFs [25]. XPS was employed to investigate the surface composition of it. It is clear that the product contains Co, C, N, and O elements (Fig. 1F), which is in accordance with the results of EDS (Fig. S2). Furthermore, the high-resolution Co 2p XPS spectra show that peaks of Co 2p3/2 and Co 2p1/2 appear at 781.18 and 796.68 eV [27], and satellite peaks emerge at 786.58 ~ 789.48 eV and 801.48 ~ 803.98 eV (Fig. 1G). The FT-IR technique was used to verify relevant functional groups in MOFs (Fig. 1H), the stretching vibration characteristic peaks of Co – N at 424.1 cm−1, and C – N and C = N at 1142.3 cm−1 and 1579.1 cm−1 are observed in CTAB-Co-MOFs, respectively [27]. All investigations confirmed the successful synthesis of the CTAB-Co-MOFs.
In addition, the difference between CTAB-Co-MOFs and Co-MOFs was investigated, using SEM (Fig. S4A-B), TEM (Fig. S4C-D), FT-IR (Fig. 1H) and XRD (Fig. S5). No significant difference was observed from these figures above, indicating that CTAB could not change the morphology, functional groups, and crystal structure. What is more, CTAB-Co-MOFs have better stability than Co-MOFs (Fig. S6). This suitable explanation is that the hydrophobic cetyl chain of CTAB molecules improve the water stability of CTAB-Co-MOFs [28].
Characterization of CTAB-Co-MOFs@AuPt NPs nanocomposites
TEM was used to investigate Au NPs and Pt NPs obtained (Fig. 2A, B), and it was found that Au NPs and Pt NPs were uniformly spherical with an average size of 17.5 ± 0.78 nm and 3.90 ± 0.47 nm, respectively. From the SEM and TEM images (Fig. 2C, D), it can be seen that the Au NPs and Pt NPs appeared as small clusters on the surface of the CTAB-Co-MOFs. The CTAB-Co-MOFs@AuPt NPs also exhibit the polyhedral structure similar to CTAB-Co-MOFs. Furthermore, the EDS result confirms the existence of C, N, O, Co, Au, and Pt elements (Fig. 2E). The XRD pattern displays that the CTAB-Co-MOFs@AuPt NPs composites still own an excellent crystalline state (Fig. 2F), and the AuPt NPs exhibit four characteristic peaks from 35 to 80°. Notably, the peak intensity at 38.1° is much higher than that of other planes, which manifests that Pt atoms exist in the Au matrix [29]. These results above confirm the successful preparation of CTAB-Co-MOFs@AuPt NPs.
Construction investigation of the immunosensor
Figure 3A demonstrates current density in 1.0 mM [Fe(CN)6]3−/4− solution at the different stepwise process of this immunosensor. Because of the larger specific surface area and bonzer conductivity of GA@Au NPs, the GA@Au NPs/GCE (curve b) exhibited a higher current density in contrast to bare GCE (curve a). As Ab1, BSA, CA15-3 antigen, and labelled-Ab2 were orderly assembled on electrode surface, the current densities were reduced continuously (curves c–f) due to their nonconductivity, indicating the immunosensor was successfully prepared.
Figure 3B shows the EIS curves of 5.0 mM [Fe(CN)6]3−/4− at different step of immunosensor preparation. The GA@Au NPs/GCE (curve b) presented a smaller semicircle in contrast to the bare GCE (curve a). After immobilization with Ab1, BSA, and CA15-3 antigen, respectively, the semicircle diameter was increased sequentially (curve b–f). When labelled-Ab2 was captured on the electrode surface, the diameter of the semicircle was further aggrandized due to antigen–antibody complex formation. The EIS results obtained were consistent with that of CV, revealing the successful manufacture of the immunosensor.
Investigation of the signal amplification and feasibility of CA15-3 analysis
In order to verify signal amplification from AuPt NPs and H2O2, DPV response at the same concentration of CA15-3 (100 µU/mL) was recorded for this evaluation at different conditions (seen Fig. 4A). In the absence of the labelled Ab2 (curve a), no any signal response was observed and in the presence of CTAB-Co-MOFs@Au NPs/BSA-Ab2, a clearly DPV signal was obtained (curve b) due to a sandwich structure formation. If the support electrolyte containing H2O2, the increasing DPV signal was obtained clearly (curve c), when AuPt NPs existed (curve d), the DPV signal was further increased, indicating that AuPt NPs had good catalytic ability to the system of H2O2—Co2+.
A DPV responses of immunosensors recorded at different signal probes. (a) No labelled Ab2 probe; (b) CTAB-Co-MOFs@Au NPs-Ab2 probe; (c) CTAB-Co-MOFs@Au NPs-Ab2 probe in the presence of H2O2; (d) CTAB-Co-MOFs@AuPt NPs-Ab2 probe in the presence of H2O2. Condition: The concentration of CA15-3 is 100 µU/mL, incubation time of 60 min. B DPV response of the immunosensor recorded in the absence of CA15-3 antigen (curve a) and in the presence of CA15-3 antigen (curve b). Condition: The concentration of CA15-3 is 10 mU/mL, incubation time of 60 min
The feasibility of CA15-3 analysis was investigated, and the results are presented in Fig. 4B. In the absence of CA15-3 (curve a), no obvious response signal was received; however, a larger response signal was obtained when existed CA15-3 (curve b). The signal intensity was related to the concentration of CA15-3. Thus, the concentration of CA15-3 can be quantified based on the peak current intensity changes, indicating the feasibility of immunosensor to detect the serum CA15-3.
Optimization of experimental condition
Herein, experimental conditions (e.g., pH, incubation temperature and time, and the concentration of Ab1) were optimized to improve the analytical performance of the immunosensor. The results obtained are shown in the Supplementary Materials (Section S8). Briefly speaking, the optimized results are listed as follows: pH 7.4; incubation temperature, 37 °C; incubation time, 60 min; the concentration of Ab1, 20 µg/mL; and the concentration of H2O2, 2.5 mM, and the amount of GA@Au NPs, 10 µL.
Analytical performance
Under the optimal conditions, the designed immunosensor was employed to detect different concentrations of CA15-3 and recorded their DPV response. As shown in Fig. 5A, the currents are increased gradually at the potential about − 0.85 V (vs SCE) with the increase of CA15-3 concentration, and a good working cure was obtained in the range of 10 µU/mL to 250 U/mL (Fig. 5B) with the limit of detection (LOD) was 1.1 µU/mL (at the 3σ/S, σ is the standard deviation of the blank and S is the slope of the analytical curve). The linear regression equation was y = 4.4500 + 3.9063 lg x (y is the peak current value (µA), x is the concentration of CA15-3 (µU/mL), R2 = 0.999). Compared with other immunosensors reported previously (shown in Table 1), the immunosensor shows a wider linear range and lower LOD. The reasons of good performance can be explained as follows: (1) GA@Au NPs, good conductivity and larger surface area, were employed for sensing matrix to enhance electron transfer rate. (2) CTAB-Co-MOFs, larger specific surface area, were employed as a carrier for improving the amount of AuPt NPs and Ab2. (3) AuPt NPs catalyze the reaction of H2O2 oxidizing Co2+ to Co3+, resulting in the significant amplification of the sensing signal.
A DPV response recorded at various CA15-3 concentrations. B The working curves of peak current (I) vs the logarithmic of CA15-3 concentration. Conditions (U/mL): C1, 0; C2, 0.00001; C3, 0.0001; C4, 0.001; C5, 0.01; C6, 0.1; C7, 1; C8, 10; C9, 100; C10, 250. The datum dot is average value of three times
Selectivity, reproducibility, and stability
To assess the selectivity of the immunosensor, some interferences (e.g., CA19-9, IgG, PSA, HGB, HSA, Mb, and BSA) were selected for this investigation. Specifically, the immunosensor was employed to detect 0.01 U/mL CA15-3 antigen in the presence of single interfering substance and recorded DPV response corresponding to different conditions. As shown in Fig. 6A, no apparent current change was observed, hinting at the good selectivity of immunosensor.
A Specificity of the immunosensor (condition: 0.01 U/mL CA15-3; interference substances: 1 U/mL CA19-9, 10 µg/mL IgG, 10 µg/mL PSA, 100 µg/mL HGB, 100 µg/mL HSA, 100 µg/mL Mb and 100 µg/mL BSA; n = 3). B Reproducibility of the immunosensor (0.1 U/mL CA15-3; n = 3); C Stability of the immunosensor (100 µU/mL CA15-3; n = 3)
Reproducibility was evaluated using six immunosensors to detect CA15-3 (0.1 U/mL) under the same conditions and record their DPV response (shown in Fig. 6B). The relative standard deviation (RSD) is 0.35%. Additionally, the stability of the immunosensor was assessed by depositing it in the refrigerator (4 °C) for 7 days and testing it once a day. The results are presented in Fig. 6C, and it can be seen that the current retained 94.7% of its initial value on the 7th day, indicating the good stability of the immunosensor.
Serum sample analysis
To verify the feasibility of the proposed immunosensor, human serum sample was employed and the recovery test was adopted. According to the method described in this experiment, the sample was parallelly detected five times. The results are shown in Table 2 according to the equation (\(\overline{x}\pm t\frac{s}{\sqrt{n} }\), s is the standard deviation). The recoveries obtained are within 98.33 to 102.08% with RSD values within 2.49 to 2.67%, indicating that the proposed immunosensor has good feasibility in clinical analysis.
Conclusion
In this work, a sensitive sandwich electrochemical CA15-3 immunosensor was developed using GA@Au NPs and CTAB-Co-MOFs@AuPt NPs. The proposed immunosensor shows excellent analytical performance, including wider linear range (10 µU/mL to 250 U/mL) and lower LOD of 1.1 µU/mL. Satisfactory, the immunosensor has potential application in the early clinical diagnosis of BC via clinical serum analysis. Furthermore, the immunosensor also provides a feasible strategy for detecting other cancer biomarkers only adjust corresponding to antibody.
References
Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, Bray F (2021) Global Cancer Statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA A Cancer J Clin 71(3):209–249
Xiong X, Zhang Y, Wang YF, Sha HF, Jia NQ (2019) One-step electrochemiluminescence immunoassay for BC biomarker CA 15–3 based on Ru(bpy)62+-coated UiO-66-NH2 metal-organic framework. Sens Actuat B Chem 297:126812
Ribeiro JA, Sales MGF, Pereira CM (2021) Electrochemistry-assisted surface plasmon resonance biosensor for detection of CA 15–3. Anal Chem 93(22):7815–7824
Peng MX, Lin SY, Lin ZB, Zheng DL, Song YB, Lu FS, Chen YW, Gao WH (2022) Rationally constructed ZnCdS-HDCs@In2S3-HNRs double-hollow heterojunction with promoted light capture capability for photoelectrochemical biosensing. Biosens Bioelectron 201:113957
Jiang XY, Wang HJ, Yuan R, Chai YQ (2015) Sensitive electrochemiluminescence detection for CA15-3 based on immobilizing luminol on dendrimer functionalized ZnO nanorods. Biosens Bioelectron 63:33–38
Wu YW, Chen XL, Wang XF, Yang M, Xu FL, Hou CJ, Huo DQ (2021) A fluorescent biosensor based on prismatic hollow Metal-polydopamine frameworks and 6-carboxyfluorescein (FAM)-labeled protein aptamer for CA15-3 detection. Sensors Actuat B-Chem 329:129249
Ambrosi A, Airo F, Merkoci A (2010) Enhanced gold nanoparticle based ELISA for a BC biomarker. Anal Chem 82(3):1151–1156
Ge XY, Feng YG, Cen SY, Wang AJ, Mei LP, Luo XL, Feng JJ (2021) A label-free electrochemical immnunosensor based on signal magnification of oxygen reduction reaction catalyzed by uniform PtCo nanodendrites for highly sensitive detection of carbohydrate antigen 15–3. Anal Chim Acta 1176:338750
Feng YG, He JW, Chen DN, Jiang LY, Wang AJ, Bao N, Feng JJ (2022) A sandwich-type electrochemical immunosensor for CYFRA 21–1 based on probe-confined in PtPd/polydopamine/hollow carbon spheres coupled with dendritic Au@Rh nanocrystals. Microchim Acta 189:271
Thangapandi K, Arumugam S, Amalesh N, Ajeet K, Saikat K (2021) Bio-nanocomposite based highly sensitive and label-free electrochemical immunosensor for endometriosis diagnosticsapplication. Bioelectrochemistry 139:107740–107749
Jin H, Mo K, Wen F, Li Y (2019) Preparation and pervaporation performance of CAU-10-H MOF membranes. J Membrane Sci 577:129–136
Zhi D, Li T, Li J, Ren H, Meng F (2021) A review of three-dimensional graphene-based aerogels: Synthesis, structure and application for microwave absorption. Compos Part B-Eng 211:108642
Sahoo BB, Kumar N, Panda HS, Panigrahy B, Sahoo NK, Soam A, Mahanto BS, Sahoo PK (2021) Self-assembled 3D graphene-based aerogel with Au nanoparticles as high-performance supercapacitor electrode. J Energy Storage 43:103157
Santos-Gómez LD, García JR, Montes-Morán MA, Menéndez JA, García-Granda S, Arenillas A (2021) Ultralight-weight graphene aerogels with extremely high electrical conductivity. Small 17(41):2103407
Liu XT, Pang K, Yang H, Guo XZ (2020) Intrinsically microstructured graphene aerogel exhibiting excellent mechanical performance and super-high adsorption capacity. Carbon 161:146–152
Maleki H (2016) Recent advances in aerogels for environmental remediation applications: A review. Chem Eng J 300:98–118
Öner E, Öztürk A, Yurtcan AB (2020) Utilization of the graphene aerogel as PEM fuel cell catalyst support: effect of polypyrrole (PPy) and polydimethylsiloxane (PDMS) addition. Int J Hydrogen Energy 45:34818–34836
Indra A, Song T, Paik U (2018) Metal organic framework derived materials: progress and prospects for the energy conversion and storage. Adv Mater 30:1705146
Li Z, Xi Y, Zhao A, Jiang J, Li B, Yang X, He J, Li F (2021) Cobalt-imidazole metal-organic framework loaded with luminol for paper-based chemiluminescence detection of catechol with use of a smartphone. Anal Bioanal Chem 413(13):3541–3550
Liu SY, Lai C, Liu XG, Li BS, Zhang C, Qin L, Huang DL, Yi H, Zhang MM, Li L, Wang WJ, Zhou XR, Chen L (2020) Metal-organic frameworks and their derivatives as signal amplification elements for electrochemical sensing. Coordin Chem Rev 424:213520
Guan Y, Si PB, Yang T, Wu Y, Yang TH, Hu R (2023) A novel method for detection of ochratoxin A in foods—Co-MOFs based dual signal ratiometric electrochemical aptamer sensor coupled with DNA walker. Food Chem 403:134316
Liu JB, Shang YH, Zhu QY, Zhang XX, Zheng JB (2019) A voltammetric immunoassay for the carcinoembryonic antigen using silver(I)-terephthalate metal-organic frameworks containing gold nanoparticles as a signal probe. Microchim Acta 186:509
Li MY, Zhang TT, Zhang YZ (2023) Ultrasensitive electrochemical sensing platform for miRNA-21 detection based on manganese dioxide-gold nanoparticle nanoconjugates coupled with hybridization chain reaction and horseradish peroxidase signal amplification. Analyst 148(9):2180–2188
Liu JB, Fu SH, Yuan B, Li YL, Deng ZX (2010) Toward a universal “adhesive nanosheet” for the assembly of multiple nanoparticles based on a protein-induced reduction/decoration of graphene oxide. J Am Chem Soc 132(21):7279–7281
Zhu XF, Yuan S, Ju SY, Yang J, Zhao C, Liu H (2019) Water splitting-assisted electrocatalytic oxidation of glucose with a metal-organic framework for wearable nonenzymatic perspiration sensing. Anal Chem 91(16):10764–10771
Zhang TT, Ma Y, Zhang YZ (2023) A simple electrochemical strategy for the detection of the cancer marker CA19-9 by signal amplification using copper organic framework nanocomposite. Analyst 148(23):5905–5914
Zhang LX, Xian JX, Xiang XX, Ouyang H, Wang L, Fu ZF (2022) Surfactant-assisted synthesis of water-stable cobalt-imidazole metal organic frameworks as signal probes for chemiluminescent immunoassay. Sensors Actuat B-Chem 373:132774
Huang Q, Guo Y, Chen D, Zhang L, Li TT, Hu Y, Qian J, Huang S (2021) Rational construction of ultrafine noble metals onto carbon nanoribbons with efficient oxygen reduction in practical alkaline fuel cell. Chem Eng J 424:130336
Oladipo AO, Iku SII, Ntwasa M, Nkambule TTI, Mamba BB, Msagati TAM (2020) Doxorubicin conjugated hydrophilic AuPt bimetallic nanoparticles fabricated from phragmites australis: characterization and cytotoxic activity against human cancer cells. J Drug Deliv Sci Technol 57:101749
Ke H, Zhang X, Huang CS, Jia NQ (2018) Electrochemiluminescence evaluation for carbohydrate antigen 15–3 based on the dual-amplification of ferrocene derivative and Pt/BSA core/shell nanospheres. Biosens Bioelectron 103:62–68
Wang AJ, Zhu XY, Chen Y, Luo XL, Xue YD, Feng JJ (2019) Ultrasensitive label-free electrochemical immunoassay of carbohydrate antigen 15–3 using dendritic Au@Pt nanocrystals/ferrocene-grafted-chitosan for efficient signal amplification. Sens Actuators B, Chem 292:164–170
Funding
This work is supported by the Nature Science Foundation of the Education Department of Anhui Province (No KJ2016A848).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Ethics approval
All experiments were performed in accordance with the relevant laws and institutional guidelines of China, and approved by the ethics committee at Anhui Normal University (AHNU-ET2023051).
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Zhang, T., Ma, Y., Tian, R. et al. CTAB-Co-MOFs@AuPt NPs as signal probes for the electrochemical detection of carcinoembryonic antigen 15–3. Microchim Acta 191, 176 (2024). https://doi.org/10.1007/s00604-024-06254-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00604-024-06254-y