Abstract
A user-friendly, cost-effectively, portable, and environmentally friendly colorimetric sensor for the quantitative determination of formaldehyde was developed based on the combining of microfluidic paper-based analytical device (μPAD), headspace microextraction (HSME), and digital image colorimetry. Coupling HSME and μPAD led to enhancements in selectivity and sensitivity of the sensor through sample cleanup and analyte enrichment. To construct the μPAD-HSME device, two pieces of paper as the sample and detection zone were placed facing each other so that a small common and sealed space was created between them. The color change occurred when the analyte in the gaseous form crossed this gap and reached the detection zone. Colorimetric sensing in the detection zone was performed based on the Hantzsch reaction. The color change in the detection zone was recorded by a smartphone and digital images were processed using image analysis software based on the RGB model. The influence of some key variables on the sensitivity of the method including derivatization reagent composition, sample volume, extraction temperature, and extraction time was studied and optimized. The linear dynamic range of the method was obtained in two ranges of 0.10–0.75 and 0.75–5.0 mg L−1 with a limit of detection of 0.03 mg L−1. The recoveries were in the range 80–126% for the quantification of formaldehyde in textile, milk, and wastewater samples.
Graphical abstract

Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Since the initial work on microfluidic paper-based analytical devices (μPADs) in 2007 [1], numerous paper-based sensing devices have been fabricated in various fields including environmental monitoring, biomedical diagnostics, water, and food safety [2]. μPAD has been an alternative to the complex analytical instruments due to its unique advantages consist of user-friendly, consumption of the low amount of reagents, low cost, easy to fabrication, portability, and environmentally friendly. As well, fluids can be transferred in the fibrous and porous network of the cellulose paper through capillary effect without need to an external pumping system [3]. Until now, various detection methods have been coupled to μPAD such as colorimetric, electrochemistry, fluorescence, and luminescence-based sensing [2].
In general, digital image colorimetry (DIC) is a popular detection technique in PADs because the white color of paper offers proper support for the color discrimination via the naked eye. Additionally, colorimetric assay in paper strips is rapid, inexpensive, and can be performed on-site without using complex equipment. DIC helps to quantitative measurement through detection of color change using image acquisition tools such as smartphones, digital cameras, and scanners. Compare to other acquisition tools, smartphone is widely used due to its portability, lightness, significant improvements in its functions, equipped with various applications, and network sharing capability [4,5,6].
In most colorimetric-based detections on μPADs, the sample is loaded directly on the sample zone and flows through a channel towards the detection zone. This method is not suitable for analyzing colored, turbid, and complex samples. Besides, since no preconcentration takes place in the μPAD during the analysis, detection limits often are high. These drawbacks can be eliminated by combining headspace microextraction (HSME) and μPAD for the analysis of volatile and semi-volatile compounds [7]. HSME leads to enhancement of the selectivity and sensitivity of μPAD sensors through high levels of sample cleanup and analyte enrichment.
In most of the previously reported coupling PAD and HSME, a piece of impregnated cellulose paper with a colorimetric reagent is hung on the top of sample solution. In this case, since the paper is placed horizontally in the vial, it is difficult to capture the photos directly from the paper in the vial [8,9,10,11]. Therefore, to record the digital images, the colored paper is transferred to a photographic box or desktop scanner. In this manner, total analysis from the preparation to detection step takes a long time that consequences a reduction in sample throughput. Also, performing HSME in the vial requires a relatively large amount of samples and reagents in milliliter range, as well as equipment such as a stirrer.
In the current work, μPAD, HSME, and DIC were combined to develop a method for the determination of formaldehyde (FA) as a volatile organic compound. In μPAD-HSME, in addition to using a paper as the detection zone, a separate paper was used as the sample container instead of the vial. In this way, the memory effect will be eliminated using disposable cellulose papers. In the design of μPAD-HSME device, two pieces of filter paper were placed facing each other in a way that a small common and sealed space was created between them. One of the papers acted as a sample zone and the other as a detection zone. Two paper layers were attached together in the form of bottom and top arrangement. The color change occurred when the vapor of analyte crossed the gap between two layers and reached the detection zone. Colorimetric determination of FA was performed based on the Hantzsch reaction [12]. The color change on the detection zone was recorded by a smartphone and color information was obtained by the image processing software. The feasibility and applicability of the proposed method were demonstrated by measuring FA in milk, textiles, and industrial wastewaters.
Experimental
Chemicals and materials
FA solution (37%), glacial acetic acid, acetylacetone (99.5%), and ammonium acetate were bought from Merck (Darmstadt, Germany). The Stock standard solution of FA (2000 mg L−1) was prepared weekly. Working standard solutions of FA were freshly prepared by diluting the stock solution with an appropriate of DI water.
The derivatization reagent solution containing acetylacetone (0.2% v/v), ammonium acetate (150 g L−1), and acetic acid (3% v/v) was daily prepared and stored in an amber glass bottle to protect against light. Grade 44 cellulose filter paper (Whatman, Maidstone, UK) was employed to make the μPAD.
Apparatus
An on-off temperature controller (W1209 model) and ceramic thermal resistor heating element (2 × 4.5 cm) were provided from local electronic markets. A smartphone with a 13-megapixel camera (Samsung, J5) was used as the acquisition tool to capture the images. A pH meter (Metrohm 827 pH meter, Switzerland) was used to control the solutions pH. Single beam UV-vis spectrophotometer (Jenway 6300-UV-vis spectrophotometer, UK) was used as the detection system for the standard method analysis.
Fabrication of μPAD
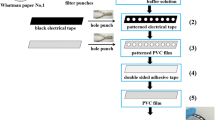
The procedure of μPAD fabrication is schematically represented in Fig. 1. The patterns of the bottom and top paper layers were designed by CorelDraw X6 and cut using a CO2 laser. The dimensions of the paper layers are shown in Fig. 1a. The bottom layer consisted of sample and donor zone connected together using a 0.8-cm channel. As shown in Fig. 1b, the paper layers were positioned on the polymethyl methacrylate (PMMA) sheets. The bottom paper layer was fixed on the PMMA sheet (10 × 30 mm) using a double-sided adhesive tape. A square paper was fixed in a laser engraved square shape (5 × 5 mm with a depth of 1 mm) on the top PMMA sheet and used as detection zone. When these two PMMA sheets were stuck together, the donor and acceptor (detection) regions are spaced 1 mm apart (Fig. 1c). The adhesive tape helps to seal the space between donor and acceptor zones.
Fabrication of photographic dark box
A lab-made box (120 × 80 × 90 mm, 2.8 mm thickness) was built with black Plexiglas sheets. The outside and inside of photographic box is shown in Figs. 1d and 1e. All sheets were stuck together with epoxy glue. A thermal resistor heating element equipped with an on/off temperature controller was placed on the bottom of the box where the chip was situated. A 10 × 10 mm observation window was created on the top of the box by the laser cutting. A white light-emitting diode lamp was embedded on the side wall of the box to illuminate inside the box. The optimal distance of 8 cm between the camera lens and the chip was considered to capture clear images.
Sample preparation
Wastewater samples were collected from petroleum refinery factory (wastewater #1), resin manufacturing (wastewater #2), and textile industry (wastewater #3). Cow milk samples were obtained from a dairy farmer (milk #1) and a local supermarket (milk #2). Three textile samples consisting of crepe, nylon, and cotton fabric were purchased from local markets. Wastewater samples were directly analyzed by μPAD-HSME method without pretreatments. In order to pretreatment of cow milk, the proteins were denatured before analyzing. For this end, 1.4 mL of HCl (4.0 mol L−1) was added to the 7.0 mL of milk sample and were mixed manually. The mixture was centrifuged at 3500 rpm for 10 min. Then, 290 μL of NaOH (10 mol L−1) was poured into 5.0 mL of the supernatant to reach the pH value to 5. The centrifugation was performed again at 3500 rpm for 5 min. Finally, the supernatant was separated from the precipitate for further analysis. To prepare textile samples, 3 g of chopped textile was soaked in 100 mL of water in a container with a lid. The samples were transferred to the oven at 40 °C for 1 h and shaken manually every 5 min [13].
Standard procedure for FA determination
After filtration, 5.0 mL of pretreatment sample solution was poured into a vial containing 3.0 mL of the derivatization reagent. The vial was sealed and heated in the oven at 40 °C for 30 min. Then, the vial was kept at room temperature for 30 min. The absorbance of the solutions was measured at the wavelength of 412 nm [14].
μPAD-HSME procedure
Prior to analysis, the filter papers were soaked in methanol and water to take out the impurities. To prepare the detection zone, 3 μL of reagent was loaded to the detection zone and allowed to dry at 25 °C for 5 min. Then, the detection zone was placed on the top PMMA sheet. Also, the bottom paper layer was placed in the bottom PMMA sheet. The two PMMA sheets were attached together. The device was placed inside the box on the resistance heater at the temperature of 70 °C. As can be seen in Fig. 1d, sample zone was out of the box. Then, 10 μL of the sample solution was loaded in the sample zone. After 3 min, the created color in the detection zone was recorded using a smartphone. Digital images were processed using image analysis software based on the RGB model. The inverse of the blue value (B-value) of the selected area was considered as the analytical response. All the reported signals [ΔB] were based on the difference between the standard or sample solution signal and the blank signal.
Result and discussion
As a small molecule, FA evaporates easily from the formalin solution. Temperature has a major effect on the escape of volatile compounds from the surface of samples. In the current work, the temperature of the donor zone was raised and controlled using a heating element and on-off controller to evaporate FA molecules. FA molecules in the gaseous form reached the detection zone, above the donor zone, where the derivatization reagent has already been pre-deposited to perform a chemical reaction. The colorimetric determination of FA was based on the Hantzsch pyridine synthesis as a reference method [12]. The Hantzsch pyridine synthesis is a multi-component organic reaction between an aldehyde such as FA, two equivalents of a β-keto ester such as acetylacetone and a nitrogen donor such as ammonium acetate or ammonia. In this method, dihydropyridine: 3, 5-diacetyl-1, 4-dihydrolutidine (DDL)—the yellow product—is produced from cyclization of acetylacetone, ammonium acetate, and FA (Fig. S1, Supplementary Material) [15]. In the standard process, the absorbance of solution was measured by spectrophotometer. In colorimetric method, the color intensity of yellow product (DDL) was measured by DIC.
Optimization of photography setting
In order to have the least amount of noise and the highest resolution of images, imaging parameters were studied and optimized. In photography, creating a balance between three essential settings including shutter speed, ISO number, and white balance is necessary to create a well-exposed image. Shutter speed is defined as the time that the shutter is open to expose the sensor to the light. Shutter speed is usually measured in seconds and fractions of a second. In photography, ISO is defined as the sensor sensitivity to the light. ISO is measured in numbers, usually multiples of 100. White balance mentions to the effects of color temperature and tints in photos. Different light sources emit a variety of color temperatures that vary between orange and blue in the spectrum. In order to have the least amount of noise and the highest resolution of images, shutter speed, ISO number, and white balance were considered in the range of 1–1/4000 s, 100–800, and 1000–10,000 K, respectively. The studies have shown that shutter speed, ISO number, and white balance provide the clearest image in 1/4000 s, 100, and 5000 K, respectively.
The effect of derivatization composition on the signal intensity
For acquiring the maximum sensitivity, the effects of main factors on the formation of derivatization reagent including pH, concentration of acetylacetone, and ammonium acetate were studied. In all experiments, other conditions such as standard solution volume, FA concentration, temperature, and extraction time were kept constant at 15 μL, 1.0 mg L−1, 50 °C, and 5 min, respectively. All experiments were repeated three times.
The effect of acetylacetone concentration on the color intensity was evaluated in the range of 0.5 to 3% v/v. According to the results in Fig. S2a (Supplementary Material), maximum color intensity was obtained at the concentration of 1%. Addition of a higher concentration of acetylacetone decreased the response due to the increase of blank signal [16]. So, concentration of acetylacetone was set at 1% v/v for further tests.
The effect of ammonium acetate concentration on the colorimetric response was estimated in the range of 0.5–4% w/v. As shown in Fig. S2b (Supplementary Material), the colorimetric response increased with increasing ammonium acetate up to 2%, and no significant change was observed at the higher amounts. Therefore, 2% w/v of ammonium acetate was nominated for the subsequent experiments.
The effect of derivatization solution pH on the FA determination was investigated between 5 and 6.5 by adding appropriate amounts of acetic acid. Fig. S2c (Supplementary Material) illustrates the effect of derivatization solution pH on the sensor response. According to the result, pH = 6 produced the maximum response.
The effect of extraction variables on the signal intensity
To maximize the method sensitivity, the effects of extraction variables including extraction temperature, extraction time, and sample volume were studied and optimized.
Amount of sample solution volume loaded on the μPAD depends on the paper capacity, which also depends on the paper surface. Loading a large sample volume on the μPAD requires more time to completely absorb on the paper and demands stepwise injection that is not suitable for field applications. On the other hand, a large sample volume causes droplet formation on the top layer and reduces the signal intensity. The minimum volume required to fill the whole area of the PAD was 5 μL. Therefore, the effect of sample volume on the signal intensity was investigated between 5 and 20 μL. As can be seen in Fig. S3a (Supplementary Material), the signal amount raised with increasing sample volume up to 10 μL. The signals were almost the same for 10 and 15 μL, and it was reduced for 20 μL. So, the sample volume of 10 μL was chosen as the optimum value.
Extraction temperature has two effects on the μPAD-HSME process, including effect of temperature on the derivatization reaction kinetic and mass transfer of analyte from the donor zone to the headspace. Temperature dependence of response was studied by varying the heater temperature between 40 and 80 °C. According to Fig. S3b (Supplementary Material), the color intensity increased with elevating the temperature from 40 to 70 °C. When a higher temperature was applied, the color suddenly disappeared from the paper. Degradation of the colored product (DDL) at high temperatures caused the color to fade [17]. Consequently, 70 °C was selected as the extraction temperature in further experiments.
Extraction time is specified as the time interval from loading the sample on the μPAD to record the image. In fact, this time is the time required to flow the solution through the channel, evaporate the analyte, and complete the derivatization reaction in the detection zone. Color changes were recorded by the smartphone at different times from 0 to 15 min. As shown in Fig. S3c (Supplementary Material), the color appeared after 3 min and remained constant for 6 min. After 9 min, the color of the detection zone disappeared. Therefore, 3 min was selected as the extraction time.
Analytical performance
The optimal conditions were used to obtain the analytical figures of merit of the μPAD-HSME method for the determination of FA. Linear dynamic ranges were observed in two ranges of 0.10–0.75 and 0.75–5.0 mg L−1 with determination coefficients (R2) of 0.9903 and 0.9984, respectively (Fig. 2). The limit of detection (LOD) and limit of quantification (LOQ) were estimated based on the 3 and 10 times the standard deviation of a blank sample (n = 3) relative to the slope of the calibration curve in the low concentration region. The LOD and LOQ were 0.03 and 0.10 mg L−1, respectively. The method precision was evaluated by measuring relative standard deviation (RSD) values of standard solutions at 0.30 and 2.5 mg L−1 concentration levels. Intra-day RSDs were 9% and 5% and inter-day RSDs were 10% and 8% for the low and high concentration levels, respectively.
Some characteristics of the current method were compared with other colorimetric procedures used for the determination of FA and tabulated in Table S1 (Supplementary Material). Compared to the other methods, this method consumes a very low volume of sample solution (10 μL) and reagent (3 μL). Compared with the majority of the methods listed in the table, the present method has relatively wide linear range and short extraction/detection time (3 min). Since μPAD was integrated with HSME, it can be used for colored and turbid water samples without the need for filtration. In addition, the analysis is fast and the required equipment and tools are affordable. Also, compared to the standard method, the present method consumes very small amounts of samples and reagents. The analysis is also done in a shorter time. Therefore, this method has achieved the goals of green analytical chemistry more than the standard method.
Real sample analysis
The applicability of the current method was studied by determination of FA in industrial wastewaters, textiles, and milk. The accuracy of the method was assessed by calculating spiking recoveries at two concentration levels in real samples. Real samples were analyzed by the present and standard methods for the determination of FA and the results are summarized in Table 1. The standard addition method was used for FA measurement in real samples. As shown in Table 1, FA was detected in wastewater #1 and #2 at the concentrations of 0.19 and 0.15 mg L−1, respectively. Also, FA was found in the textiles in the concentrations range of 2.4–3.6 mg kg−1. FA was detected in milk #1 and milk #2 at the concentration levels of 1.3 and 7.1 mg L−1, respectively. All the calculated recoveries were between 80 and 126% (with RSDs lower than 16% for n = 3), indicating the method is satisfactory for quantifying FA.
The statistical procedure (two-tailed Student’s t-test, at p = 0.05) was applied to compare the results of present and standard methods. This test revealed that there is no significant difference between the two sets of observations.
According to the literature, the natural concentration of FA in the milk samples is usually between 1.7 and 8.7 mg L−1 [18]. The obtained results showed that FA was at the normal concentration level in both milk samples.
The maximum residue limit of FA in textiles in direct contact with skin is specified at 75 mg kg−1 based on Japan’s law, which is reduced to 20 mg kg−1 for children’s clothing [13]. In the present work, FA concentration was found less than 14.7 mg kg−1 in textile samples, which is lower than the permissible concentration level of FA in textiles.
Interference study
The influence of various ions and organic compounds that commonly found with FA in the aqueous environment was tested using the standard solution of FA (1.00 mg L−1). A relative error of ±5% on the concentration of FA was considered tolerable. No interference was observed from ions Na+, K+, Ca2+, Cu2+, Pb2+, Al3+, Fe2+, and NH4+ at 2500-fold excess. However, interference was observed from acetaldehyde (fivefold) and methanol (20-fold). Interference studies of FA in the presence of interfering compounds are shown in Fig. S4 (Supplementary Material).
Conclusion
In this study, a simple, low-cost, sensitive, and portable colorimetric sensor was developed for FA quantification based on the derivatization reaction in the detection zone. In this method, μPAD, HSME, and DIC were combined. Combining μPAD with HSME resulted in enhancement in the sample cleanup and analyte preconcentration. Thanks to the use of μPAD, this sensor consumes a very low volume of reagents and samples. Turbid and color water samples can be handled by the method without any additional pretreatment. The memory effect was eliminated because of using disposable papers. The μPAD-HSME device can be used as an on-site diagnostic tool due to its simplicity, use of portable electronics as well as the smartphone as the detection system, cost-effectiveness, reduction of total analysis time, user-friendliness, and no need for specific skills. The method exhibited satisfactory accuracy and precision for the determination of FA in wastewater, textile, and milk samples. Wastewater samples were analyzed without the need for sample treatment.
References
Martinez AW, Phillips ST, Butte MJ, Whitesides GM (2007) Patterned paper as a platform for inexpensive, low-volume, portable bioassays. Angew Chemie 119:1340–1342. https://doi.org/10.1002/ange.200603817
Agarwal C, Csóka L (2019) Recent advances in paper-based analytical devices: a pivotal step forward in building next-generation sensor technology. In: Sustainable polymer composites and nanocomposites. Springer International Publishing, Cham, pp 479–517
Gutorova SV, Apyari VV, Kalinin VI et al (2021) Composable paper-based analytical devices for determination of flavonoids. Sens Actuators B Chem 331:129398. https://doi.org/10.1016/j.snb.2020.129398
Morbioli GG, Mazzu-Nascimento T, Stockton AM, Carrilho E (2017) Technical aspects and challenges of colorimetric detection with microfluidic paper-based analytical devices (μPADs)—a review. Anal Chim Acta 970:1–22. https://doi.org/10.1016/j.aca.2017.03.037
Fan Y, Li J, Guo Y et al (2021) Digital image colorimetry on smartphone for chemical analysis: a review. Meas J Int Meas Confed 171:108829. https://doi.org/10.1016/j.measurement.2020.108829
Fernandes GM, Silva WR, Barreto DN et al (2020) Novel approaches for colorimetric measurements in analytical chemistry—a review. Anal Chim Acta 1135:187–203. https://doi.org/10.1016/j.aca.2020.07.030
Bagheri N, Saraji M (2019) Combining gold nanoparticle-based headspace single-drop microextraction and a paper-based colorimetric assay for selenium determination. Anal Bioanal Chem 411:7441–7449. https://doi.org/10.1007/s00216-019-02106-4
Saraji M, Bagheri N (2018) Paper-based headspace extraction combined with digital image analysis for trace determination of cyanide in water samples. Sens Actuators B Chem 270:28–34. https://doi.org/10.1016/j.snb.2018.05.021
Pena-Pereira F, Villar-Blanco L, Lavilla I, Bendicho C (2018) Test for arsenic speciation in waters based on a paper-based analytical device with scanometric detection. Anal Chim Acta 1011:1–10. https://doi.org/10.1016/j.aca.2018.01.007
Jaikang P, Paengnakorn P, Grudpan K (2019) Simple colorimetric ammonium assay employing well microplate with gas pervaporation and diffusion for natural indicator immobilized paper sensor via smartphone detection. Microchem J:104283. https://doi.org/10.1016/j.microc.2019.104283
Cho YB, Jeong SH, Chun H, Kim YS (2018) Selective colorimetric detection of dissolved ammonia in water via modified Berthelot’s reaction on porous paper. Senss Actuators B Chem 256:167–175. https://doi.org/10.1016/j.snb.2017.10.069
Nash T (1953) The colorimetric estimation of formaldehyde by means of the Hantzsch reaction. Biochem J 55:416–421. https://doi.org/10.1042/bj0550416
Shahvar A, Saraji M, Shamsaei D (2018) Headspace single drop microextraction combined with mobile phone-based on-drop sensing for the determination of formaldehyde. Senss Actuators B Chem 273:1474–1478. https://doi.org/10.1016/j.snb.2018.07.071
Dvořák P, Ramos RM, Vyskočil V, Rodrigues JA (2020) A new electroanalytical methodology for the determination of formaldehyde in wood-based products. Talanta 217:121068. https://doi.org/10.1016/j.talanta.2020.121068
Mariano S, Wang W, Brunelle G et al (2010) Colorimetric detection of formaldehyde: a sensor for air quality measurements and a pollution-warning kit for homes. Procedia Eng 5:1184–1187. https://doi.org/10.1016/j.proeng.2010.09.323
Li Q, Sritharathikhun P, Motomizu S (2007) Development of novel reagent for Hantzsch reaction for the determination of formaldehyde by spectrophotometry and fluorometry. Anal Sci 23:413–417. https://doi.org/10.2116/analsci.23.413
Kochetkova M, Timofeeva I, Bulatov A (2021) A derivatization and microextraction procedure with organic phase solidification on a paper template: spectrofluorometric determination of formaldehyde in milk. Spectrochim Acta - Part A Mol Biomol Spectrosc 263:120160. https://doi.org/10.1016/j.saa.2021.120160
Mostafapour S, Mohamadi Gharaghani F, Hemmateenejad B (2021) Converting electronic nose into opto-electronic nose by mixing MoS2 quantum dots with organic reagents: application to recognition of aldehydes and ketones and determination of formaldehyde in milk. Anal Chim Acta 1170:338654. https://doi.org/10.1016/j.aca.2021.338654
Acknowledgements
The research council of Isfahan University of Technology (IUT) and the Center of Excellence in Sensor and Green Chemistry are acknowledged for financial support of the project.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
ESM 1
(DOCX 810 KB)
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Mohammadi, V., Saraji, M. Development of a colorimetric sensor based on the coupling of a microfluidic paper-based analytical device and headspace microextraction for determination of formaldehyde in textile, milk, and wastewater samples. Microchim Acta 191, 66 (2024). https://doi.org/10.1007/s00604-023-06139-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00604-023-06139-6