Abstract
Surface-enhanced Raman scattering (SERS) has been recognized as one of the most sensitive analytical methods by adsorbing the target of interest onto a plasmonic surface. Growing attention has been directed towards the fabrication of various substrates to broaden SERS applications. Among these, flexible SERS substrates, particularly paper-based ones, have gained popularity due to their easy-to-use features by full contact with the sample surface. Herein, we reviewed the latest advancements in flexible SERS substrates, with a focus on paper-based substrates. Firstly, it begins by introducing various methods for preparing paper-based substrates and highlights their advantages through several illustrative examples. Subsequently, we demonstrated the booming applications of these paper-based SERS substrates in abiotic and biological matrix detection, with particular emphasis on their potential application in clinical diagnosis. Finally, the prospects and challenges of paper-based SERS substrates in broader applications are discussed.
Graphical abstract

Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Raman spectroscopy is a powerful methodology for identifying, characterizing, and quantifying unknown objects [1]. It is a fast, nondestructive, and noninvasive analytical technique that provides unique fingerprint spectral and chemical information about substances in nearly in any state [2]. Compared with other spectral detection methods such as infrared spectroscopy, Raman spectroscopy offers distinct advantages since it shows no interference from water and requires little sample pretreatment. However, its wider application is limited due to the relatively low sensitivity, which results from the low intensity of Raman signal [1, 2].
The discovery and wide application of surface-enhanced Raman scattering (SERS) have largely overcome the aforementioned limitations. With the advantages of high sensitivity, minimal background interference from water, short testing time, and less need for sample pretreatment, SERS is considered to be one of the most promising analytical methods for specific detection of chemical and biological molecules in different matrix with ultra-high sensitivity [3, 4]. In recent years, it has received extensive attention as an important tool for detecting and sensing trace targets in the fields of analytical chemistry, biomedical diagnosis, food quality control, environmental monitoring, and national security [5,6,7,8]. When molecules are adsorbed onto rough noble metal surfaces, such as silver (AgNPs) or gold nanoparticles (AuNPs), local plasmon resonance effects on their surfaces can enhance the Raman signal via an electromagnetic mechanism. SERS performance largely depends on the plasmonic properties of metal nanostructures, as well as their morphology, dimension, aggregation state, and surrounding environment [8,9,10]. Therefore, the elaborate design and preparation of SERS substrates with high enhancement have become one of the crucial topic in this field [11].
Over the years, various strategies have been developed to prepare functional noble metal SERS substrates. The sensitivity of SERS detection has been greatly improved by utilizing three-dimensional (3D) structures in combination with substrates, which could be obtained by directly employing plasmonic nanostructures or integrating plasmonic nanostructures with non-plasmonic frames [4]. Traditional rigid SERS substrates, such as glass, silicon, and anodic aluminum oxide, are widely used for highly sensitive detection with good reproducibility, whereas they are fragile and usually difficult to bend, which limits the applications for extracting analytes from uneven surfaces. In addition, the preparation of these substrates is costly and time-consuming, requiring specialized operators, which severely weakens the applications in conventional laboratory, on-site analysis, and in general medical and public safety fields [3, 8].
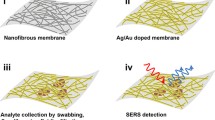
Flexible materials such as plastic polymers, paper, graphene/graphene oxide (GO), and cellulose [12] are superior in making close contact with various shaped surface. These materials have been applied to prepare SERS substrates, generally referred to as paper-based SERS substrates due to their soft properties like paper. Great attention has been paid to the paper-based SERS substrates because of the notable superiorities, including low cost, ease of fabrication, and user-friendliness [4]. The preparation of paper-based SERS substrates typically involves the assembly of metal nanoparticle arrays on flexible materials through methods such as immersion coating, in situ growth, inkjet printing, screen printing, and others. Compared with the traditional rigid SERS substrates, paper-based SERS substrates exhibit the following outstanding characteristics: (I) Mostly composed of biodegradable materials, the functional sensors constructed by paper-based SERS substrates are environmentally friendly. (II) The flexibility of paper-based SERS substrates enables full contact with the analyte. It is also easy to collect samples to the maximum extent from irregular surface by “wipe,” “paste,” and “peel,” which makes the sensor suitable for on-site detection. (III) Paper-based SERS substrates could be easily split into pieces of different shapes and sizes to suit particular applications. They are lightweight, portable, and can be worn on the body for the detection of substances on the surface or secreted by the human body [13]. (IV) The folds and fibrils of the flexible material promote the uniform distribution of metal nanoparticles and the formation of “hot spots,” thus serving as an effective basis for routine SERS analysis and showing excellent efficiency and durability even after a lot of mechanical stimulation [14]. (V) With the inexpensive and cost-effective performance, paper-based substrates hold great potential for practical testing outside the academic field [3, 8, 15].
In this review, we provide a comprehensive overview of the latest advancements in flexible, especially the paper-based SERS substrates. We review the various preparation methods and unique characteristics of these substrates and summarize their applications in environmental, food, and drug analysis in vitro. We then discuss the use of paper-based SERS substrates in biomolecular detection in biological samples, with emphasis on their value in medical diagnostics. Finally, the future development in biomedicine and forensic medicine is prospected.
Several different preparation methods of paper-based SERS substrate
As various flexible materials have been included to fabricate paper-based SERS substrates, researchers have developed a variety of methods over the years to effectively attach noble metal nanoparticles to these flexible materials to form hot spots and obtain better Raman enhancement [16]. Among these, filter papers (FPs) and membranes have been widely used as the flexible substrates with the integral functions of filtration and preconcentration [17]. In this section, we will highlight a selection of the most widely used methods for paper-based SERS substrates, including drop coating, immersion, in situ growth, inkjet printing, and screen printing.
Paper-based SERS substrates prepared by drop coating and immersion methods
Drop coating and immersion methods are the most common and traditional methods used to prepare paper-based SERS substrates [18]. Both methods share similar principles and offer convenient applications. In these approaches, a flexible material capable of absorbing water is brought into contact with the colloidal solution containing noble metal nanoparticles, either through drop coating or immersion. As the liquid is absorbed by the flexible material, the noble metal nanoparticles will be absorbed on the fiber of the flexible material [19]. These two methods are straightforward and efficient, allowing for control over the dimensions and morphology of the deposited nanoparticles [20]. McGillivray et al. [21] incorporated AuNPs onto FP to enable SERS detection of polystyrene nanoparticles. Kah et al. [22] used crystal violet (CV) as a Raman probe molecule and deposited colloidal gold nanostars (AuNS) treated with sodium citrate on common laboratory FP for multiple drops to prepare an optimized SERS substrate. Subsequently, CV was dropped on the pre-dried AuNS-FP substrate to obtain SERS spectra. This approach achieved a limit of detection (LOD) of 1 nM for CV with the enhancement factor (EF) of 1.2 × 107. These FP-based substrates offer simple and cost-effective platform for rapid in situ detection of trace substances. In recent years, gold-shelled starch microbeads were fabricated as soft template with size-controllable features. Using a drop coating method, a paper-based SERS method sensor was hereby developed for the detection of 4-mercaptobenzoic acid [23]. Zhou et al. [24] used the dropping method to prepare a silver nanowire (AgNW)-based paper substrate for SERS sensing. Furthermore, 3D silver nanostructures were synthesized via a simple liquid-phase reduction method and then evaporate to dry on the surface of bacterial nanocellulose (BNC) membranes [25]. This flexible substrate was thus prepared for ultrasensitive SERS detection. Chen et al. [26] coated the FP with polydimethylsiloxane (PDMS) to enhance the hydrophobicity and followed by a drop-casting method for the fabrication of paper-based SERS substrate. Zhang et al. utilized the coffee ring pattern to construct uniform nanoarchitectures on a PVDF film through an infiltration induced particle packing [27]. Mesoporous polydopamine (PDA) particles were employed as the core of nanoarchitecture and enabled the growth of AuNPs on the outer surface. This uniform patterned SERS substrate exhibited high repeatability and boosted the detection performance. Singamaneni et al. [28] prepared a flexible SERS substrate based on gold nanorod (AuNR) adsorption by immersion method for the first time. Chen et al. [29] prepared the SERS substrate by directly immersing FP in an Au@AgNPs solution. The dialysis method was also reported by doping AuNPs on the cellulose dialysis membrane to prepare the SERS substrate [30]. Hydrophilic PVDF membranes could be introduced as a flexible substrate to enable the adsorption of multi-shaped AgNPs under suction filtration [31]. By repeatedly immersing the cellulosic film into an Ag-SiO2 Janus nanoparticles solution, a SERS active substrate was prepared for RB detection with an enhancement of ∼5 × 104 [32]. As shown in Fig. 1A, Thu et al. [33] reported the high enhancement effect of interconnected plasmonic nanostructures. AgNPs with varying diameters were used for immersing and dropping on cellulosic paper, which resulted in the preparation of plasmonic array-based SERS substrate. By immersing the cellulose paper into a biosynthesized AuNPs colloid, Yoshimura et al. [34] fabricated a flexible SERS substrate for amino acid and melamine detection. Moreover, Chen et al. [35] reported the preparation of BNCs-based SERS substrate by coating nanocomposites. Specifically, AuNPs were immobilized on 2D hexagonal boron nitride and then mixed with BNCs to prepare the flexible SERS substrate, enabling a femtomole level LOD for toxic pollutants. Compared with traditional rigid substrate, these proposed flexible paper-based SERS substrates could make close contact with the irregularly shaped surface, thus significantly improving the sample collection efficiency.
While both drop coating and immersion methods are similar, the area where the nanoparticle binding to the flexible fiber is usually smaller on the substrate when using the drop coating method, and the influence of the coffee ring effect may be greater. Therefore, the substrate prepared by immersion method seems to show higher uniformity and better repeatability. For example, Lu et al. [20] used immersion method to modify FP with AgNPs after chemisorbing the FP with chloride ions to overcome electrostatic repulsion to AgNPs. This obtained paper-based SERS substrate showed good uniformity, with the relative standard deviation (RSD) of Raman strength obtained at different locations being 8.8%. Furthermore, Zhou et al. [36] utilized an “impregnation method” to assemble nanomaterials onto a polyester fiber membrane to prepare a flexible SERS substrate, utilizing 3D ZnO@Ag nanoflowers for membrane coating to generate hot spots for highly sensitive SERS sensing.
However, the volume of nanoparticle solution required by immersion method is generally greater than that for drop coating method. To address this, researchers have combined these two methods to immerse flexible substrates in nanoparticle droplets, which can not only save the amount of nanoparticle solution but also maintain good uniformity. Zhang et al. [37] prepared GO cellulose paper by drying a mixed solution of GO and paper pulp in the air, and then immersed it into droplets of AgNP solution to produce a paper-based SERS substrate. This substrate can be prepared on a large scale, offering strong stability and an RSD less than 10%. Similarly, by introducing aqueous colloid of AuNPs into the cellulose pulp during the dissolution process, researchers prepared Au cellular fibers substrates for SERS sensing [38]. Han et al. [39] demonstrated an improved method for assembling AgNPs onto the FP. Silver sol with a few drops of inducer were added into organic solution, forming a silver film after shaking and standing. The silver nanolayer became denser under the Marangoni force and was then transferred onto a FP. This substrate exhibited uniform and stable features, which enabled the qualitative and quantitative SERS detection. Liu et al. [40] used the tip of an arrow-shaped paper to absorb droplets containing AuNPs through capillary force and generated an intelligent and multifunctional paper-based SERS sensor card. Notably, this card is cost-effective, simple to prepare, and offers rapid detection of 5 × 10−17 M substances from only 10 μL sample, which could be further used as a portable sensor for environmental and food analysis. The RSD of Raman signal strength obtained at separate locations using this card is 8.7%. In addition, in order to reduce the coffee ring effect, attention has been paid to drop nanoparticles onto hydrophobic flexible substrates. For instance, Yu et al. [41] coated a hydrophobic poly(l-lactic acid) film made of electrospinning with AuNPs droplets. This substrate exhibited a detection limit of 0.1 nM for Rhodamine 6G (R6G) with an RSD of 8%. In recent years, cellulose nanocrystals (CNCs) have emerged as a multifunctional support for the preparation of SERS substrate. Electrostatic adsorption enabled the assembling of gold nanosphere (AuSph) onto CNC surface through a straightforward mixing process, providing the one-dimensional hot spots for SERS biosensing [42]. Moreover, through further sulfhydryl functionalization on CNC, researchers achieved the self-assemble of AuSph on CNC and formation of ultra-stable SERS substrate [43]. In short, the drop coating and immersion methods are known as the most straightforward and convenient approaches for developing flexible paper-based SERS sensors.
Paper-based SERS substrates prepared by in situ growth method
The in situ growth method involves synthesizing nanoparticles directly on the substrate through redox reaction or electrical replacement. Over the last decades, a variety of functional paper-based substrates have been fabricated using this method. For example, nanocrystalline cellulose, isolated from discarded cigarette filter, was reported to serve as both reducing and stabilizing agent [44]. A thermal decomposition method was employed for the fabrication of SERS substrate and the synthesized AgNPs were in controllable diameters from 4.6 to 19.9 nm. Further, SiO2 was used as efficient support for the substrate fabrication [45]. Lu’s group [46] grew silver in situ on CNCs to prepare composite material (CNC-Ag) integrated with a FP, enhancing nanoparticles adhesion and improving the reproducibility of SERS sensor. The LODs of phenylethanolamine A and metronidazole are down to 5 nM and 200 nM, respectively, indicating that it offers great promise for label-free detection of trace substances in situ. Zhang et al. [47] introduced the dopamine self-polymerization on cellulose FP. The reduction capability of PDA allowed for the direct growth of AuNPs on the paper surface for SERS detection. Ahmed et al. [48] employed multiple growth steps to precisely control the size of the nanoparticles and improve the nanoparticle density. Iterative seeding method was developed for in situ growth of AuNPs onto pseudo-paper films. The obtained SERS substrate exhibited uniform structures and flexible features for on-site application [49]. Noh et al. [50] presented a layer-by-layer reduction method to maximize the AgNPs coating of cellulose paper, establishing a paper-based SERS sensing platform by introducing multiple silver/chitosan nanocomposite layers. Other flexible matrix such as carbon cloth (CC) was also used as the substrate for AgNPs coating. Jiang et al. [51] employed such CC substrate to introduce TiO2 framework with shrubby-like morphology that enabled the in situ reduction of silver ions onto the surface (Fig. 1B). Typically, this CC/TiO2/Ag substrate was used for the quercetin monitoring with high sensitivity. Kong et al. [52] developed a multifunctional SERS substrate by in situ growing AgNPs on one side of the FP. With the sample added on the unmodified side of the FP and then flowed onto the AgNPs side, this multifunctional SERS sensor allowed the direct detection of thiram in ketchup without any pretreatment. Additionally, the silver mirror reaction has been employed for in situ growth of silver due to its cost-effective and minimal requirement for complicated instruments or substrate restrictions. For instance, Yang et al. [53] prepared a flexible SERS substrate by doping AgNP on FP via silver mirror reaction, further utilized it for the detection of tyrosine in aqueous solution. The SERS signal of this paper-based substrate was enhanced by 50 times compared with traditional glass substrate, and the reproducibility was significantly improved. Dwivedi et al. [54] developed a one-step method for the in situ growth of AgNS on FP through silver mirror reaction that was further utilized as high sensitive SERS substrate. Nanda et al. [55] also prepared an AgNPs/FP substrate by silver mirror reaction, achieving detection limits of 10−12 M for R6G and 10−9 M for Rhodamine B (RB), respectively.
To further improve the reinforcing effect of the substrate, multiple growth of different kinds of nanoparticles has been adopted for substrate preparation. Yu et al. [56] established a paper-based SERS platform by employing a cooperative enhancement method. They first attached a biomimetic recognizer (BR) to the silver coated paper, and then facilitated the in situ growth of AgNPs layer on the Ag/BR paper surface, creating a sandwich structure as shown in Fig. 1C. This platform holds the characteristics of high sensitivity, high selectivity, easy operation, and high efficiency. The LOD for the detection of tartrazine in children’s snacks was as low as 0.1138 μg mL−1, well below the concentration limit (100 μg mL−1) in the food additive standard. Recently, they combined the biomimetic recognizer polymer (BRP) with 3D dendritic gold (DAu) to fabricate flexible SERS substrates on the cellulosic matrix [57].
In situ chemical reduction method was employed to synthesize DAu on paper slips. Corresponding BRP, wrapped with urchin-like nanoparticles, was used to immobilize the DAu area to further establish a SERS sensor. Thiram detection was realized within the range of 0.1–100 nM with the LOD of 27.5 pM. Tu et al. [14] developed a flexible SERS sensor by in situ growth of silver on polyimide (PI), followed by encapsulation of gold core-shell nanoparticles to form Ag@AuNPs, which could achieve efficient and repeatable sampling. The sensor exhibited high signal uniformity and stability for the detection of trace substances, with the EF as high as 1.07 × 107 and LOD as low as 10−9 M. Zhao’s team [6] printed the gold seeds synthesized by sodium borohydride on paper through inkjet printing. Placing the paper in the growth solution allowed for the in situ growth of AuNPs, resulting in a paper-based SERS substrate with good homogeneity and stability. Since then, they have improved the method to achieve a 1.356 × 103-fold higher enhancement through secondary growth of AgNPs [58]. Electrochemical deposition method was also reported for the preparation of cellulose nanofiber (CNF) paper-based SERS substrates. AgNWs coated on CNF nanopaper were employed as the working electrode to induce the electrodeposition of mesoporous Au film. The thickness of Au film could be precisely controlled via the electrochemical deposition process, resulting in high performance SERS substrate [59]. Lovera et al. employed the electrochemical deposition method to further deposit silver onto Gum Arabic monomer, producing a cluster complex that enabled the sensitive pesticide detection [60]. The in situ growth method mentioned above provides superior and well-controlled features, enabling the deposition of nanoparticles of various types in specific locations on flexible paper-based SERS substrate. The preparation steps are generally rapid and cost-effective, making paper-based SERS sensors promising candidates for efficient biochemical identification.
Paper-based SERS substrates prepared by inkjet printing method
In addition to the methods mentioned above, inkjet printing represents one of the common techniques for preparing paper-based SERS substrates. It involves printing inks made from colloidal solutions of noble metal nanoparticles onto flexible materials. White et al. [61] firstly modified the surface chemistry of cellulose paper and printed AgNPs on this substrate by a consumer inkjet printer. The sensing arrays were thus formed for the detection of R6G at 10 fM with reproducibility. Lee et al. [62] directly deposited a layer of AgNPs on the surface of chitosan-modified office paper by using inkjet microreactor. A flexible SERS substrate was thus created with low LOD of 10.7 pM and high EF of 7.4 × 108, showing excellent sensitivity. Hulse et al. [63] optimized the inkjet printing process to achieve the optimal SERS performance that includes the colloid ink formulations, printing conditions, and the pretreatment of the sensors. Huang et al. [64] performed gravure printing on the sulfonated reduced graphene film, then inkjet printed nano-silver on the surface of weighing paper and brought the two together. The LODs of this flexible SERS substrate for malachite green (MG) and R6G are 10−7 M, with the EF of 109. As shown in Fig. 2A, Mazali et al. [9] demonstrated a paper-based SERS substrate by inkjet printing of AuSph of a specific size on hydrophobic chromatographic paper. This substrate showed excellent detection abilities for CV and thiram, with quantitative threshold as low as 10−11 M. White’s team [65,66,67] has also worked on developing and improving methods for preparing flexible substrates by inkjet printing. They prepared flexible SERS substrate by embedding nanoparticles into cellulose substrate for trace chemical detection. The separation of components in the sample was combined with SERS detection using polymer membrane, and the LOD was further improved using lateral flow concentration. By using dendrimer-stabilized Au:Ag nanoalloys for the preparation of stable inks for inkjet printing, Trindade et al. [68] reported the paper-based SERS substrate for thiram detection. Polystyrene was used beforehand to improve the hydrophobicity of the paper. These SERS substrates prepared via inkjet printing enable simple and fast preparation of various substrate patterns with mass production. This facilitates the design of structured arrays for multichannel detection.
Inkjet printing (A) [9] and screen printing (B) [69] based method for preparation of paper-based SERS substrate. C Deposition of silver nanowire ink on paper by an automatic writing method [86]. D Assemble of gold nanoparticles at a three-phase interface for the preparation of flexible SERS substrate [88]
Paper-based SERS substrates prepared by screen printing method
Screen printing is widely recognized for rapid, repeatable, and low-cost production of sensor chips. This technique mainly uses photography to print patterns on screen printing substrate. It can achieve not only mass production but also a high level of quality control, which is crucial to the arrival of the sensor era. Long’s team [69] presented a straightforward approach for mass production of flexible SERS substrates using screen printing technology. Fig. 2B shows the fabrication of the SERS substrates and the prepared SERS array exhibits high reproducibility, stability, and cost-effectiveness. After that, the team fabricated a bimetal microfluidic dumbbell type SERS sensor on cellulose paper with a similar approach and improved the sensing ability [70]. This sensor has an EF of 8.6 × 106 for R6G and can be utilized to detect aromatic pollutants in wastewater. The mass production characteristics of these substrates above further broaden their application prospects. Fodjo et al. [71] printed AgNPs and GO ink on cellulose paper with screen printing technology, thus prepared a large number of controllable substrates. These substrates were used for pesticides detection on fruit surface, exhibiting much lower detection limit than the national standard of the USA. This highlights the practical potential of screen printing paper-based SERS substrates.
Paper-based SERS substrates prepared by other methods
Among the various reduction methods for nanoparticles deposition, laser-based photoreduction is particularly useful for silver deposition. It has been employed to generate morphologies such as spherical and planar structures on a hydrophilic cellulose surface, and the controllable planar showed high SERS activity [72]. On this basis, a photothermal method facilitated the modulation of the surface morphology of metal films on paper. Specifically, KrF laser illumination was used to crush the metal film, leading to the formation of nanoparticle arrays. Quasi-3D paper-based substrates were thus fabricated for the SERS detection with attomolar sensitivity [73]. Wu et al. [74] reported a direct photochemical synthesis of AgNPs on nanocellulose substrate without the need for any reductants. This method enabled the loading of silver nanotubes with well-defined morphology on the cellulose film to form the SERS substrate. Additionally, other methods are employed for the preparation of paper-based SERS substrates, such as spraying method [75, 76], brushing method [77], textured method [78], physical vapor deposition method [79, 80], laser induction method [73], wax printing method [81], magnetron sputtering method [82], electrostatic interaction method [83], and microwave synthesis [84]. For instance, in situ sputtering system was employed for deposition of plasmonic AgNPs on PI nanopillars, resulting in the SERS substrate with high uniformity and controllability [72]. Vacuum evaporation was used to deposit nano-silver layer on the surface of cotton fabrics-based substrates, exhibiting hydrophobic or super hydrophobic features [85]. These substrates significantly improve the SERS performance. In recent years, a rapid spray coating method was introduced to fabricate paper-based substrate with AuNPs. This method showed improved loading efficiency compared with dip coating or drop coating method, exhibiting a higher SERS sensing quality [75]. To further simplify the preparation process, a convenient writing approach was recently developed for the preparation of paper-based SERS substrates. As shown in Fig. 2C, it was reported that an automatic writing method was used to deposit AgNW ink on a drawing paper to prepare a paper-based SERS substrate, which was further used for the detection of CV residues in fingerprints [86]. Another user-friendly “pen-on-paper” method was described by using an ordinary fountain pen backfilled with arbitrary plasmonic nanoparticles as ink. It allowed for precise control of the size and pattern of the substrate region during the direct writing process, which facilitated high-throughput SERS sensing [87]. Furthermore, a microcontact imprinting method was recently used for the preparation of flexible SERS substrate by combining the graphics printing with self-assemble technique. AuNPs assembled on a three-phase interface were effectively transferred onto a PDMS film to function as the SERS substrates for the highly sensitive detection of microRNA (Fig. 2D) [88].
Further simulation methodologies are becoming increasingly valuable in the design and development of paper-based SERS substrates, particular in understanding the plasmonic enhancement on these substrates [89]. Generally, there are a large number of methods for the preparation of flexible paper-based SERS substrate, and their advantages and disadvantages are shown in Table 1.
Application of paper-based SERS substrate in the detection of abiotic samples
Paper-based SERS sensors hold significant promise for detecting harmful chemicals in industrial wastewater, pesticide residues on agricultural products, food additives, and other trace chemicals in vitro. These research directions are gaining unprecedented popularity at present.
Application of paper-based SERS substrate in detecting industrial wastewater
Water quality management has grown to be a critical and challenging problem for human beings. The water-soluble inorganic pollutants such as chloride, nitrate, phosphate, and sulfate produced by factories can cause eutrophication of aquatic ecosystem, proliferation of aquatic plants, and death of aquatic animals like fish and shrimp due to hypoxia and suffocation, resulting in serious ecological imbalance [90]. Additionally, dissolved organic substances, like dyes used in the textile industry, contribute significantly to water pollution. For example, dyes such as R6G and methyl orange (MO) commonly used in textiles and leather processing eventually find their way into the environment, posing substantial threats to ecosystems [91]. The pH monitoring also serves as an important indicator for water pollution. Li et al. [92] presented a flexible paper-based SERS method for continuous pH monitoring in liquid environment by immobilizing AuNPs onto a PVDF membrane. This flexible SERS substrate was subsequently modified with a probe molecule 4-mercatobenzaldehyde (4-MBA), where the sensitive response of carboxyl group on 4-MBA to different pH conditions facilitated the rapid pH sensing. This SERS-based method enabled pH sensing in less than 10 s, promoting the broad applications in dynamic water monitoring.
SERS method has been extensively employed in chemical and biological sensing. The huge potential of flexible SERS substrates in the field of sustainable energy and environment also makes them increasingly used in the field of environmental detection in recent years. Qureshi et al. [90] prepared a graphene nano-mesh-Ag-ZnO metal (GNMM) nanohybrid as a flexible SERS substrate for highly sensitive detection. The LOD for MO and R6G dyes reached to 10 pM and 0.1 pM, respectively. The substrate can be self-cleaned through photocatalytic degradation of adsorbed R6G, making it recyclable. Additionally, the substrate exhibited high antibacterial activity against Escherichia coli. GNMM nanohybrid paper-based SERS substrate offers the advantages of cost-effective, ease of preparation, high sensitivity, and reusability, which could be further utilized for detecting various environmental pollutants and antibacterial agents. As shown in Fig. 3A, Le et al. [93] used a simple immersion method to prepare AgNPs coated paper-based SERS substrate. With a “dip and dry” method, this flexible substrate enabled the detection of methylene blue in river water with a LOD of 0.1 nM and recovery rate of 93–104%. Nanocellulose-based Cu2O/Ag nanostructures were prepared as flexible SERS substrates for the detection of trace methylene blue in water [94]. Another study by Xiao et al. [95] involved the preparation of a AuNPs@cellulose diacetate flexible SERS substrate for thiram pesticide detection in water. Choi’s group [91] developed a paper-based SERS sensor that uniformly distributes synthetic AuNPs on paper, enabling direct and label-free analysis of components in wastewater. The LOD of R6G is down to 10−10 M with the high EF of 2.8 × 107. When 4-aminobenzoic acid and catechol are used to simulate wastewater, the LODs of the two substances are 10−9 M and 10−5 M, respectively. By mixing silver nanocrystals with CNFs and filtered in vacuum, Li et al. [96] prepared a flexible SERS substrate for the detection of MG in water. Due to the suitability of the SERS method for aqueous sample analysis and the paper-based substrate shows the filtering capabilities, this paper-based SERS substrate shows extensive applications in the analysis of industrial wastewater.
A “Dip and dry” method for the detection of MB in river water using a filter paper-based SERS substrate [93]. B Schematic illustration of the thiram detection in apple skin by using a heat-shrink film-based SERS substrate [111]. C AgNPs coated carboard platform for the SERS detection of tetracycline in milk samples [125]. D The CRISPR/Cas based SERS method for the detection of target DNA of pathogenic bacteria [135]. 4. Application paper-based SERS substrates in biological sample detection
Application of paper-based SERS substrate in detecting pesticide residues in environmental and agricultural products
Flexible paper-based SERS substrate shows encouraging applications for nondestructive food detection [97] that particularly suited for the detection of pesticide residues. Pesticides are widely used in agriculture to protect crops and enhance product quality. However, these various pesticides used in modern agriculture cause a large number of residues in agricultural products, posing a great threat to human health [98]. Current analytical methods for pesticide residues detection, such as gas chromatography (GC), gas chromatography-mass spectrometry (GC-MS), high-performance liquid chromatography (HPLC), and liquid chromatography-mass spectrometry (LC-MS), are often time-consuming and require complex sample preparation. Therefore, there is still a great need to develop a simple, reliable, and efficient way for the determination of trace pesticide residues [99].
SERS method has shown wide applications in the field of agricultural [100]. As an inexpensive method for trace pesticides detection, paper-based SERS sensors have recently become the focus of research [101]. These paper-based SERS sensors have the advantage of direct collecting agriculture residues by wiping the peel surface with the flexible substrate. For example, they can detect thiram and its analogues, which are commonly used as fungicides in agriculture but can be toxic to humans. Huang et al. [102] built a flexible SERS substrate based on in situ growth of Ag nanoparticles on nanocellulose fiber, which enabled highly sensitive detection of pesticide at ppm level. A paste-detect method was used for the detection of hazardous residues on real-world samples, such as fish and pears. Jiang et al. [98] developed a paper-based microfluidic SERS device to quantitatively detect trace residual thiram, reaching a LOD as low as 1.0 × 10−9 M. Wang et al. [103] used polymethyl methacrylate tape to paste paper-based substrate onto a hydrophobic PDMS surface. They developed a dual-function flexible SERS sensor that could quantify thiram on fruit surface and in fruit juice. Sample enrichment could be achieved due to the hydrophobicity of PDMS, thus improving the sensitivity of the sensor. At the same time, this approach also prevented the loss of noble metal nanoparticles during extraction, greatly improving the accuracy and repeatability of detection. Lin et al. [104] showed a facile method for the preparation of CNFs functionalized with AuNPs. This nanocomposite served as a flexible SERS substrate for the ultrasensitive detection of thiram in juice, with a LOD of 52 ppb. Jeong et al. [101] hydrophobically modified raw hydrophilic FP with alkyl ketene dimer (AKD) and developed a paper-based SERS sensor. This sensor exhibited advantages of simple preparation, cost-effectiveness, high sensitivity, and reproducibility and reached the detection limits of 0.46 and 0.49 nM for thiram and ferbam, respectively. Lin et al. [105] utilized quartz paper for surface coating with two different nanoparticles to establish a SERS biosensor. CNFs-based substrates were thus prepared for rapid detection of pesticide residues in vegetables, achieving the ability to detect ferbam in kale at a low concentration of 50 μg/kg in a few minutes. You et al. [106] developed a SERS sensor based on nanoporous cellulose paper. It can simultaneously detect thiram, tricyclazole, and carbaryl in a mixed solution, with an EF up to 1.4 × 107 and the LODs were 10−9, 10−7, and 10−6 M, respectively. The sensor can also be used for extracting and rapidly detecting these three pesticides on fruit surface, with detection limits of 6, 60, and 600 ng cm−2, respectively, which are far below the maximum residue requirement on apple surface. Wang et al. [13] developed a paper-based fluidic SERS sensor to quantitatively analyze thiram in unpretreated orange juice samples, demonstrating its application in the quantitative analysis of contaminants in complex samples. By directly irradiation of the AgNO3 on a carboxyl methyl cellulose film, they prepared a flexible SERS substrate for thiram quantification with a LOD of 10−8 M and R2 of 0.998. [107] Ma’s team [108] assembled Ag@SiO2 on FP to produce a flexible substrate with a detection limit of 10−9 M for thiram. Lin et al. [109] presented a AgNPs coated cellulose fiber as the flexible SERS substrate for the detection of thiabendazole pesticides in apples. Hwang et al. [110] developed a dielectric coated plasmonic paper-based SERS substrate by assembling Ag@SiO2 nanotubes on Fe-TiO2 nanosheet modified paper. This substrate achieved a LOD of 19 μg/L for p-thiabendazole solution, and the Raman signal on apple surface could be recognized at 15 ppb. It demonstrated significantly improved sensitivity compared to unmodified plasmonic paper. Recently, Jaiswal et al. [111] deposited Au@Ag nanorods onto the surface of a heat-shrink film to fabricate effective SERS substrates. The film surface became wrinkled under heat treatment, which enabled a rearrangement of the surface nanomaterials and greatly enhanced the Raman signals of target analyte. This method was used for the detection of thiram on the apple surface at a concentration of 1 ppm by swabbing with this shrink film-SERS substrate (Fig. 3B). These methods show high potential for trace pesticides in a real-world environment.
Parathion, malathion, dimethoate, trichlorfon, and other organophosphorus pesticides are among the most commonly used organophosphorus pesticides in plant pest control. These pesticides tend to adhere to the surface of fruits and vegetables, resulting in nerve toxicity upon human consumption. In a study by Ma et al. [112], a paper-based SERS substrate was used to detect methyl parathion standard solution with the detection limit of 0.011 μg/cm2. This substrate was also used to extract methyl parathion residue from apple surface through a wiping method, with the recovery rate up to 98.72%. In a separate study, Wang et al. [113] prepared a SERS substrate for separation channel with AKD modified paper, which was used for simultaneous separation and detection of thiuram and dimethoate, with LODs of 19.16 μg/L and 54.57 μg/L, respectively. Yang et al. [114] deposited AgNPs on FP via silver mirror reaction and prepared a paper-based SERS substrate on a large scale, which was further used for rapid and portable detection of pesticide residues on various fruit peels. The LODs for thiram and paraoxon were 0.72 ng cm−2 and 0.23 μg cm−2, respectively. These above substrates not only show the advantages of flexible substrates in extracting complex surface samples but also prove the feasibility in the field of pesticide residue testing.
MG is generally employed as a fungicide and antigenic agent in aquaculture, but its toxic triphenylmethane structure can induce a range of potential carcinogenic, mutagenic, teratogenic, and other pathological effects. Although MG is considered as a banned drug, it is still often abused illegally due to the lack of cost-effective alternatives. In a study by Zhang et al. [8], they grew AgNPs in situ on PDA template FP and prepared FP@PDA@AgNPs bands. It further served as an excellent SERS platform for the rapid collection and detection of MG residues on fish scales, crab shells, and shrimp skins, with LODs of 0.04635 pg/cm2, 0.06952 pg/cm2, and 0.09270 pg/cm2, respectively. Shao et al. [115] embedded Ag nanowire suspension into PDMS film and made a flexible SERS substrate for quantitative MG detection in fruit juice, achieving a LOD of 10 nM. Chen et al. [49] grew AuNPs in situ on pseudo-paper film and prepared a flexible SERS substrate that could detect three pesticides residues of thiram, parathion-methyl, and MG on the apple surface through simple wiping. The detection limits were below the maximum pesticide residue limits of China and European Union. Wu et al. [116] developed a nanocellulose-based flexible SERS substrate by precisely loading two types of plasmonic nanoparticles on the surface. Pesticide residues on the apple surface were detected by simple wiping, with the LODs of 4.1 and 10.7 μg/L for omethoate and acetamiprid, respectively. Qu et al. [3] prepared a large number of disposable SERS substrates using FP via a spraying method. The LODs of MG, methylene blue, and CV were 4.3 nM, 20 nM, and 81 nM, respectively. These substrates significantly improved the efficiency of drug residues collection and exhibited excellent SERS sensitivity and reliability, enabling rapid on-site detection of drug residues in agricultural products. Ma et al. [117] presented an expanded graphite-covered cellulose film as a flexible and hydrophobic SERS substrate for convenient detection. The use of AuNR in the evaporation process increased the number of hot spots, resulting in substantial enhancement. Trace residues on the surface of shrimp and grapes were determined, with LODs of 1 ppb for CV and 50 ppb for thiram.
Neonicotinoids are extensively employed insecticides worldwide, which are agonists of nicotinic acetylcholine receptors and could cause paralysis and death. Consequently, neonicotinoids residues in agricultural products are potential risks to consumers. Using a multi-layer SERS-enhanced strategy, Yu et al. [10] established a paper-based sensor to real-time detect various neonicotinoid pesticide components. The sensor demonstrated high sensitivity and specificity, with a LOD down to 0.02811 ng mL−1 for imidacloprid, a neonicotinoid insecticide. In another development, Wang et al. [99] prepared a super-hydrophobic FP-based SERS substrate, which could detect nitenpyram (NIT) with high sensitivity to the LOD of 1 nM. Due to the high polarity of NIT and low solubility in organic solvents, this method is significantly superior to traditional detection methods as it is more effective, simpler, and without the need for sample pretreatment. In recent years, 2,4-dichlorophenoxyacetic acid (2,4-D) residue was effectively detected in green tea by coating cellulose paper with citrate functionalized AgNPs as flexible SERS substrate [118]. This presented sensor enabled the detection of 2,4-D at 104 μg/g with the RSD value < 5%, providing a reliable means of analysis.
Furthermore, researchers have made significant progress in developing flexible paper-based SERS substrates for the detection of additional pesticides. Jung et al. [119] constructed a paper-based SERS sensor by synthesizing AuNPs on cellulose FP. This substrate exhibited a LOD of 10−7 M for methylene blue solution, and a remarkable detection limit of 1 ppm for diquat and paraquat on the surface of apple peels. Ekgasit et al. [120] prepared a biodegradable BNC flexible SERS substrate by vacuum filtration for on-site detection of methomyl, a carbamate pesticide residue on fruit peel. All the above examples collectively illustrate that flexible SERS substrate can be successfully utilized for the rapid and sensitively in situ detection of agricultural residues in complex samples. The ongoing development of such flexible paper-based SERS substrates holds great promise in meeting the health and safety needs of consumers.
Application of paper-based SERS substrate in food quality supervision and inspection
With the improvement of living standards, the presence of illegal or excessive additives in the food market poses a great risk to people’s health. SERS technology has attracted considerable attention and has been successfully applied to the on-site detection of food additives in complex samples [121], contributing to the growing field of food quality analysis [122]. Flexible paper-based substrates, in particular, have shown emerging applications in food detection [97] and safety [123, 124]. For instance, by coating carboard substrate with AgNPs, Fortunato et al. [125] used this nanoplasmonic SERS substrate for the detection of antibiotic residues in milk sample. This approach required only a few microliters of milk for the detection of tetracycline, and the concentrations were determined based on peak intensities (Fig. 3C). Yin et al. [126] hydrophobically treated FP and established a SERS sensor based on AgNPs, enabling the detection of melamine in diluted milk with a low LOD of 1 ppm. Chen et al. [127] combined a gas diffusion paper-based microfluidic device with SERS for the determination of sulfite in wine, achieving a detection limit of 2 μg mL−1. The device shows features of portability and ease of operation, which promote it as a valuable tool in the field monitoring of sulfites.
Nitrite (NO2−) is not only generally used as a preservative for meat or meat products to maintain their bright color and enhance the flavor, but it also poses a significant threat to human health. Conventional detection methods for nitrite often require complicated sample pretreatment procedures, which resulted in the detectable concentration of NO2− being in micromole level. Therefore, a method for the analysis of ultra-trace NO2− in complex matrices is still greatly needed. Consequently, Li et al. [121] developed a paper detection tape based on colorimetric/fluorescence/SERS three-mode sensor to detect NO2− in food. For NO2− solution, the LODs for the colorimetric and fluorescence readouts were 50 nM and 10 nM, respectively, while with SERS readout, the LOD for nitrite in meat products or human urine was dramatically down to 0.8 nM. This highly sensitive sensor enables the reliable quantification of NO2−, addressing the need for detecting ultra-trace levels of food matrices.
Over the years, illegal traders have mixed dyes into food or herbs to enhance their appearance and drive up the prices [128]. Lu et al. [129] prepared a self-assembled paper-based SERS substrate to detect RB added in pepper powder. With simple sample pretreatment, the LOD for RB could be as low as 10−6 g g−1. Santhanam et al. [130] prepared a flexible SERS swab using an inkjet printing method to directly detect two types of pigments, artemisinin yellow (MY) and MG, which are illegally added in vegetables at nanomolar level. Lu et al. [128] combined AuNRs with mono-6-thio-cyclodextrin (HS-β-CD) and assembled them on FP cellulose via electrostatic adsorption and hydrogen bonding. A CD-AuNR paper-based SERS substrate was thus prepared to detect Sudan III and Sudan IV. The LODs for these dyes were 0.1 μM and 0.5 μM, respectively, demonstrating the much higher sensitivity compared to conventional AuNR paper-based SERS substrate.
Heavy metals are elements known for their significant biological toxicity. Difficult to be biodegraded, they are biomagnified by hundreds or thousands of times in the food chain and eventually accumulated in human organs and causing chronic poisoning. While heavy metals in food may not be intentionally added, they are a critical concern for food safety. For the heavy metals detection using paper-based SERS method, Wang et al. [131] established a paper-based SERS tandem chromatography separation/detection platform, showing the detection limit of 1 μM for Cd2+, Cu2+, and Ni2+ in rice. Acrylamide (AAm) is a substance formed when starchy food is cooked at high temperature above 120 °C. Animal studies have shown that AAm has reproductive toxicity, genetic toxicity, neurotoxicity, and possible carcinogenic effect. Zhu et al. [132] constructed a SERS substrate to detect AAm in food by immersing FP into strawberry-like SiO2/Ag nanocomposites, achieving a LOD of 0.02 nM. These results show that paper-based SERS substrates can be used to detect target analytes in complex food matrices. With the advantages of cost-effective and portability, they facilitate on site product checks and inspection by relevant authorities.
In addition to illegal food additives, the freshness is also an essential evaluation indicator in the field of food safety. Putrescine and cadaverine, two volatile gases produced through microbial decarboxylation of amino acids, have been considered as one of the indicators of food spoilage. Kang’s team [133] developed a metal organic framework coated SERS paper substrate, functionalized with 4-MBA, to detect these two gas molecules with detection limits of 76.99 and 115.88 ppb, respectively. We believe that this paper-based SERS platform, as exemplified by the above-mentioned substrates, holds great potential to detect a variety of substances and can find applications in food quality assessment.
Application of paper-based SERS substrate in explosive detection
Due to the rapidity and high sensitivity of SERS technology, it has become one of the most preferred means for explosives detection in recent years. For instance, SOMA et al. [11] loaded AuNPs of different shapes on FP to prepare a paper-based SERS substrate, which was used for the detection of military explosive picric acid (PA) with a LOD of 5 μM. Dou et al. [134] impregnated silver nanotriangle on FP to produce a flexible SERS substrate for PA detection with LOD as low as 10−6 M. Fierro-Mercado and Hernández-Rivera [1] used inkjet printing method to prepare flexible substrates for the detection of 2,4,6-trinitrotoluene, 2,4-dinitrotoluene, 1,3,5-trimethylbenzene, and other explosives with high sensitivity and good repeatability, demonstrating its direct application in forensic science and homeland security.
Application paper-based SERS substrates in biological sample detection
The detection of small molecules within complex biological samples, such as plasma, urine, and saliva, has been a longstanding problem. While as the advancement of paper-based biosensors, the capabilities of point-of-care testing have expanded greatly [136]. SERS technology has been applied to the fields of microorganism and their products, in vivo drug analysis, disease diagnosis. The development of related paper-based SERS substrates is poised to further enhance the capabilities of point-of-care testing and facilitate the detection of small molecules in biological samples.
Application of paper-based SERS substrates in the detection of microorganism and their products
The application of paper-based SERS substrates in the rapid and reliable detection of microorganisms and their product is a promising advancement in various fields, including in medicine, forensic medicine, food, or environmental science. For example, Kaul et al. [137] performed the SERS analysis and stoichiometric evaluation on seven kinds of meat-related microorganisms using a paper-based SERS substrate. This method offers a significant advantage on the discrimination error of lower than 2.5%, without requiring complex sample pretreatment. It is timesaving and more suitable for the application of food regulatory authorities. Poppi et al. [138] prepared a SERS substrate using AuNPs and FP by an immersion coating method, and combined with partial least squares discriminant analysis for label-free identification of bacteria. This method has been proved to provide comparable results to 16S rRNA gene sequence analysis. Jung et al. [139] coupled a paper-based SERS substrate composed of AgNWs with polymerase chain reaction (PCR), which enabled the detection of respiratory bacterial DNA with LOD of 3.12 pg/μL. White et al. [140] used paper-based SERS sensor and portable Raman spectrometer for the phenotypic identification of multidrug resistance of bacteria. This method simplifies the cultivation steps compared to traditional microbiology, thus enabling clinicians to make timely clinical decisions when treating infection. Moreover, the detection of pathogenic bacteria can also be accomplished through the detection of specific nucleic acid. As shown in Fig. 3D, Ma et al. [135] demonstrated a CRISPR/Cas-based platform on microfluidic paper substrate for the SERS detection of interest DNA. This approach utilized AuNS functionalized with both linker ssDNA and 4-mercaptobenzoic acid, which served as the SERS signal molecules. This platform activated the trans-cleavage of CRISPR-Cas 12a in the presence of target DNA, enabling the detection of Salmonella typhimurium in food with LOD of 3–4 CFU/mL.
Endotoxins, composed of proteins, phospholipids, and lipopolysaccharides, serve as important components of the cytoderm of Gram-negative bacteria. Endotoxins are released when the bacteria die, triggering a severe immune response once inside the body. The limulus amoebocyte lysate test is currently considered as the gold standard for endotoxins detection, yet susceptible to interference from enzymes and impurities. To achieve real-time in situ detection of endotoxin, Xu et al. [141] constructed a flexible SERS sensor that combined nanostructure of biological scaffold with cicada wings-templated SERS monitoring strategy. This sensor enabled the detection endotoxin in 100 s, with a detection limit of 6.25 ng/mL. This SERS method provided reliable results, which were in agreement with that obtained from a gold standard ELISA method. Furthermore, other bacterial signaling molecules were detected via a flexible SERS substrate. Park et al. [142] detected and monitored the concentration of pyocyanin directly by Au deposition onto a paper-based substrates. The pyocyanin secreted from the cultured P. aeruginosa could be rapidly detected with the LOD of 0.12 ppm. These paper-based SERS methods offer a convenient and sensitive means of monitoring bacterial products and signaling molecules, making them invaluable tools in the field of microorganism sensing.
Application of paper-based SERS substrate in drug and poison analysis
Paper-based SERS substrate shows widespread applications in the analysis and detection of drugs and poisons in vivo. In the practical field of clinical medicine and forensic medicine [143], rapid and in situ analysis of the types and quantities of drugs and poisons contained in blood, urine, and other body fluids plays a very important role in clinical emergency, determination of drug dosage, and criminal investigation. With the development of precision medicine, real-time monitoring of drug levels in patients is becoming increasingly important for the safe and effective use of therapeutic drugs, a process known as therapeutic drug monitoring. To this end, Bamrungsap et al. [144] prepared a paper-based SERS substrate by combining GO and AuNRs on cellulose substrate, enabling the detection of mitoxantrone with a LOD of approximately 5 μM. Antibacterial drugs include antibiotics and certain chemosynthetic drugs, known for their ability to inhibit and kill bacteria and other microorganisms. Measuring the antibiotics levels in the blood is crucial for the doctors to make rapid treatment decisions for patients and to prevent bacteria and fungi from developing drug resistance. Huang et al. [145] grew AgNPs on FP fiber to detect moxifloxacin with a detection limit of 1 μM. White et al. [146] employed an inkjet printing method to prepare a vertical flow membrane SERS sensor, which facilitated the quantitative detection of fluorocytosine, an antifungal drug, in undiluted human serum. This method took only 15 min to detect fluorocytosine at a concentration of 10 μg mL−1 in serum, much faster than the current gold standard. Buurman et al. [147] sprayed AgNPs on fiberglass paper for the detection of enoxacin and enrofloxacin antibacterial agents with a detection limit of 10 μM.
Dexmedetomidine hydrochloride (DH) is a selective α2 adrenergic receptor antagonist, which is mainly used for intraoperative and postoperative sedation. In recent years, DH has been abused in criminal cases such as robbery, rape, and others, increasing the demand for DH detection. Nowadays, DH is mainly determined by LC and LC-MS, which offer high accuracy, but require long experimental time, large scale of instruments, and professional operators. Therefore, they are probably unsuitable for quick on-site detection. In Zhang’s work [148], a paper-based SERS substrate was prepared by self-assembling silver colloids on FP, enabled the quantitative determination of DH in aqueous solution, urine and serum. The LODs for DH in urine and serum were determined to be 10 μg mL−1 and 12 μg mL−1, respectively. This approach owns the advantages of rapidity, accuracy, and nondestructiveness and is suitable for DH field testing. Sofebuvir is a drug for the treatment of chronic hepatitis C, and its metabolite is 2′-deoxy-2′-fluoro-2′-C-methyluridine (PSI-6206). The determination of PSI-6206 in biological fluids is of great significance for the clinical pharmacology and pharmacokinetics of sofebuvir. Ayoko et al. [149] developed a A4 paper-based non-interfering technique that combined HPLC with SERS for the quantitative detection of PSI-6206. This method was fast and sensitive and eliminated the false-positive results caused by “memory effect” in traditional HPLC-SERS method.
As the main component of aspirin, acetylsalicylic acid has antipyretic, analgesic, and anti-inflammatory properties and is one of the most commonly used drugs over the world. Low-dose daily aspirin intake is effective in preventing recurrence of cardiovascular disease, but excessive aspirin intake increases the risk of gastrointestinal disease. Therefore, quantitative detection of acetylsalicylic acid in biological samples is important. Carneiro et al. [150] developed a paper-based SERS method by incorporating AgNPs into FP for the quantification of acetylsalicylic acid. This method demonstrated impressive accuracy, with a relative error of 2.06% when compared to HPLC-UV. In brief, the paper-based SERS method offers a versatile and effective approach for the analysis and detection of various drugs and poisons in complex biological matrices. It delivers rapid and accurate results with potential applications in clinical and forensic contexts.
Application of paper-based SERS substrates in clinical diagnosis
The determination of small molecules in biologic fluid is of vital significance in clinical diagnosis, such as blood, tears, urine, saliva, and sweat. Typically, most of the existing analytical methods require large volumes of samples, expensive equipment, skilled personnel, and high costs. In recent years, SERS technology has attracted wide attention in medical diagnosis application with the advantages of user-friendliness, high sensitivity, and selectivity [151]. At the same time, paper-based SERS substrate also shows the prospect of low-cost diagnostic platform [152]. Various biomarkers in blood samples are recognized to be closely related with specific disease, and the paper-based SERS biosensors are well developed for this purpose. For example, Li et al. [153] developed a SERS biosensor named enPSERS by modifying the GO/plasmonic AuNS hybrid on FP with enrichment ability. It was employed for sensitive and label-free detection of free bilirubin in serum, so as to accurately diagnose jaundice and related diseases. The detection limit of free bilirubin in serum was 0.436 μM, which was comparable with current general detection method. Tamer et al. [154] constructed the microfluidic channel using nitrocellulose membrane as SERS substrate by wax printing method. This sensor has been shown to detect glucose molecules in the blood without any pretreatment. With the blood dropping into the hydrophilic channel, cells and protein molecules were immobilized on the nitrocellulose membrane, while glucose molecules flowed to SERS detection region through the channel, achieving a recovery rate up to 88%. Bamrungsap et al. [155] immersed ordinary FP in AuNR solution to prepare a plasmonic paper for SERS detection of epithelial cell adhesion molecules highly expressed in colorectal cancer cells HT-29. This method was used to distinguish HT-29 cells from fibroblasts and blood cells with the sensitivity of 84.8% and 96.4%, respectively, along with the specificity of 82.6% and 100%, respectively.
Early and precise cancer diagnosis increases the chances of successful treatment and improves survival rates for cancer patients. Serum samples are generally employed for cancer detection and the bottleneck is to develop a new cancer screening diagnostic tool with high sensitivity and specificity. At present, ELISA, PCR, local surface plasmon resonance, and chemiluminescence immunoassay are generally employed for the detection of tumor associated proteins, with the specific interactions between antibody and antigen playing a key role in these methods. Specifically, Garnier et al. [152] functionalized AuNPs on paper substrate and developed a SERS platform for detecting antigen-antibody reaction. This platform rapidly identified the bindings between antigens and antibodies even at low concentrations, offering a cost-effective method that is suitable for clinical, forensic, and biological laboratory applications. Cao et al. [156] developed a sandwich immunoassay based on SERS technology using hydrophobic FP with gold nanoflowers as the capture base and silver-gold nanofibers as the label. It was employed for simultaneous determination of squamous cell carcinoma antigen and osteopontin in serum from cervical cancer, with LODs of 8.628 pg/mL and 4.388 pg/mL, respectively. The feasibility of this method in clinical sample detection was proved by the gold standard ELISA method. Driskell et al. [157] developed a SERS rapid vertical flow (SERS-RVF) immunoassay for the detection of mouse IgG. Quantitative verification showed that the LOD of SERS-RVF was 3 ng mL−1, which is comparable with traditional detection method. As shown in Fig. 4A, Jiang et al. [158] demonstrated a hydrophilic-hydrophobic polymer band with Raman standard, which was used for quantitative SERS analysis of ferritin (FER) with ultrasensitivity. This sensor has a low detection limit of 0.41 pg/mL for FER. Aptamer-based specific identification was also employed in this test strip. Chen et al. [159] developed a paper-based SERS test band, which enabled the quantification of cancer marker Mucin-1 in whole blood.
A The typical schematic of the improved lateral flow strip based on hydrophilic–hydrophobic SERS substrate for ultrasensitive and quantitative immunoassay [158]. B Plasmonic Schirmer strip for the collection and detection with tear fluid [166]. C Schematic of fabrication and analysis processes for detection of biomarkers in human sweat using a hand-held Raman spectrometer [168]. D Conceptual illustration of a wearable plasmonic paper fluidic device for sweat collection, storage, and in situ analysis using SERS [169]. E The pH-adjusted cellulose SERS sensor for the evaluation of vasospasm and hydrocephalus after subarachnoid hemorrhage (SAH) [170]
With the increasing incidence of cardiovascular and cerebrovascular diseases, atherosclerosis (AS), as the pathological basis of many of the above diseases, has attracted extensive attention. Wang et al. [160] designed a sandwich SERS detection platform based on nanoporous network membrane, which was used for the monitoring the status of IL-10 and MCP-1 (chemokine), two key cytokines involved in the AS progression. This method further allowed for the early diagnosis of AS, with the minimum detection concentration of these two substances in human serum being 0.1 pg mL−1. Khor et al. proposed a paper-based microfluidic SERS device to quantitatively detect GPBB, CK-MB, and cTnT simultaneously, which is helpful for the early diagnosis and prognosis evaluation of acute myocardial infarction. The LODs of GPBB, CK-MB, and cTnT were 8, 10, and 1 pg mL−1, respectively, which were far below the clinical threshold value. Therefore, the above methods exhibit substantial clinical potential, enabling physicians to make rapid clinical decisions and improve the treatment effect of patients.
Urine samples are a noninvasive source for the diagnosis and prediction of target disease. Uric acid is recognized as a crucial urine biomarker for many diseases such as gout. Poppi et al. [161] developed an approach for on-site quantification of uric acid in urine with a LOD as low as 0.11 mM using AuNPs coated paper as a substrate. E. coli is known to be related with a wide variety of infections like urinary tract infections and other disease. By establishing a paper-based lateral flow immunoassay (LFIA), E. coli in urine samples was detected with the dual readouts. AuNPs, modified with Raman label DTNB, served not only as the SERS reporter but also as the colorimetric source. E. coli could thus be detected in a linear range of 103 and 106 CFU mL−1 with the LOD of 45 CFU mL−1 [162]. Further integrated with a magnetic enrichment, a paper-based LFIA enabled the E. coli detection with a promising LOD of 0.52 CFU mL−1 in urine samples. Specific antibody modified magnetic gold (Fe3O4@Au) nanoparticles were firstly included for the E. coli extraction and enrichment. Afterwards, casein, as a selective substrate for rennet enzyme, was immobilized on the polyethyleneimine modified magnetic gold (Fe3O4/Au-PEI) that could be cleaved from these nanoparticles in the presence of rennet enzyme to develop the paper-based LFIA [163]. Adenine detection shows importance in various diseases resulting from the alteration of guanine and adenine nucleotides in DNA. Hwang et al. [164] modified silver nanocubes onto the surface of a flexible hydrophobic FP to prepare a high reproducible SERS substrate. This substrate enabled the detection of adenine at a low concentration of 0.89 nM with the EF of 6.55 × 106. SERS analysis in real urine samples was presented that showed the characteristic absorption bands of adenine at 733 cm−1 and 1331 cm−1, which were attributed to the C-H ring breathing and N-C vibrational modes, respectively. The nanoparticles coated paper-based substrate could be further modified to establish highly sensitive SERS biosensors. By self-assembling a monolayer of AuSph onto FP, the strong affinity of chloride ion with the gold surface was adapted to greatly enhance the SERS signals. Fentanyl citrate in urine samples were detected with the LOD of 0.59 g mL−1 [165].
Biomarkers in tear fluid are known to play significant role in daily healthcare and disease monitoring. Jeong et al. [166] utilized the hierarchical structures of cellulose fiber to establish plasmonic Schirmer strip for the efficient collection and detection with tear fluid. Gold nanoislands were evaporated on the top surface of cellulose fibers, serving as a SERS substrate. The detection of uric acid at 25 to 150 μM was achieved by using SERS method, which covers the physiological levels (Fig. 4B). Choi et al. [167] prepared AuNPs into viscous ink and made a single metal paper-based SERS sensor by screen printing technology. Tears collected from patients with adenovirus conjunctivitis and herpes simplex conjunctivitis were utilized to evaluate the performance of paper-based SERS platform. The EF of the platform is 1.8 × 104, and the variation from point to point is less than 5%.
It is worth mentioning that the paper-based SERS substrate has also achieved certain results in the development of wearable sensors for the detection of biomarkers in sweat. Hong et al. [168] developed a sensor consisting of silver nanosnowflakes and hydrophobic FP that facilitates the simultaneous detection of creatinine and cortisol in small volumes of human sweat using a hand-held Raman spectrometer (Fig. 4C). With the development of microfluidic technique, Tian et al. [169] developed a paper-based microfluidic system for continuous and simultaneous quantitative analysis of sweat loss, sweat rate, and metabolites in sweat (Fig. 4D). The synthesized AuNRs were uniformly adsorbed onto chromatography paper and composed as plasmonic sensor, which enabled the detection of uric acid using SERS. And the paper microfluidics facilitated the quantification of the sweat loss and sweat rate. Although this is a newly arisen field, we believe it will become another pivotal area of research within the study of paper-based SERS substrate.
Cerebrospinal fluid (CSF) is the important medium between the brain and spinal cord, playing vital role in maintaining the neural structures. Choi et al. [170] developed a pH-adjusted cellulose SERS sensor for the evaluation of vasospasm and hydrocephalus after subarachnoid hemorrhage (SAH). As shown in Fig. 4E, pH controlled positively charged AuNPs were adsorbed onto the surface of cellulose fibers to construct the flexible SERS substrate. It enabled the identification and prediction of SAH-induced complications.
In addition to the disease markers in body fluids mentioned above, the ultrasensitive quantification of trace chemicals and biomolecules in cells is also crucial for disease diagnosis. However, when these substances in cells are released into body fluids, the analyte molecules diffuse freely in highly diluted solutions, resulting in reduced detection sensitivity and repeatability. To address these problems, Qu et al. spun the lubricant onto commercial paper and prepared a hydrophobic SERS substrate that could concentrate nanoparticles and analytes. The sensor was used for the detection of cytochrome C with a LOD of 7.2 × 10−9 M, showing good repeatability. Lee et al. [171] grew silver nanoplates on paper for quantitative detection of hydrogen sulfide (H2S) gas released by living cancer cells in colorimetric and SERS dual-mode. The LOD of the colorimetric method was 520 nM, and SERS method demonstrated an elevated sensitivity with a linear range of 82–330 nM. Addition to the above applications, paper-based SERS substrates also exhibit wide applications in the detection of cosmetics [2], botany [172], etc.
Indeed, paper-based SERS substrates provide a rapid and highly sensitive platform for the detection of biomarkers in various body fluids, such as in blood, urine, tear fluid, sweat, and even CSF, enabling early diagnosis and effective monitoring of many types of diseases. Furthermore, these cost-effective paper-based SERS substrates are available in resource-limited regions, being valuable in wearable sensors for continuous monitoring and disease screening. The potential applications expand to various fields, making it a promising technology for improving healthcare and diagnostics.
Conclusions and prospects
The flexible paper-based SERS substrates have attracted increasing attention in recent decades due to their diversified applications in environmental monitoring, food safety analysis, clinical diagnosis, and others. This is an ever-growing field, especially superior in the point-of-sample applications in resource-constrained area without access to laboratory facilities or skilled technicians [173, 174]. The future of paper-based SERS method, especially when integrated with portable hand-held instruments, provides great possibilities in the analytical field. This review paper provides a comprehensive overview of the paper-based SERS substrates, covering various preparation methods and the fundamental applications. Methods including in situ growth, inkjet printing, and screen printing are summarized for the preparation of flexible paper-based substrates. Further, we exemplify and discuss the detections of industrial wastewater and pesticide residues, and in food quality supervision and inspection. The application of paper-based SERS substrates in biological sample detection is highlighted, emphasizing their potential in the clinical applications.
Though the paper-based SERS substrate has witnessed great progress in the past few decades, there are still challenges such as the sensor reproducibility and quantification performance. On the one hand, the stable performance from batch to batch is widely recognized as being crucial for real-world applications. Thus, it is still highly needed for large-scale production of paper-based SERS substrate with high stability and reproducibility to overcome the discrepancy of used environment and maintain consistency between batches. Advances in nanomaterials and nanoscience offer promising solutions to address this challenge, thereby unveiling the great potential of paper-based SERS substrates in practical applications. On the other hand, the precise quantification of target species is another key point, particularly in clinical diagnosis, where a threshold value is generally employed for specific disease diagnosis. While the SERS-based quantitative analysis remains challenging, in recent years, the integration of microfluidic methods with paper-based SERS substrates has significantly reduced the experimental operator error. The automation of measurement process could improve the repeatability of flexible SERS sensor and enable the quantification of analyte molecules [175, 176]. Moreover, multimodal readouts for paper-based SERS sensors have been developed by incorporating colorimetric or fluorescence results to further verify the detection and enable the advantages of convenience and high sensitivity. These integrated approaches could be further utilized for simultaneous quantification of multiple analyte molecules.
The increasing demand of rapid identification in complex samples requires instant detection but often involves tedious and complicate analysis of acquired spectra. Thus, there is a growing need for advanced data processing for the flexible paper-based SERS sensors [177, 178]. In recent years, researchers have focused on the development of advanced algorithms for SERS analysis, such as the Savitzky-Golay filter for baseline smoothing, to better identify the Raman signals, machine learning algorithms for multivariate SERS spectra, etc. The booming of these advanced algorithms simplifies the analysis of SERS spectroscopic data, enabling rapid identification and quantification of target components, particularly in complex biological samples with low signal-to-noise ratio. With the continuous efforts from multidisciplinary researchers, a lot can be expected from the flexible paper-based SERS substrates that promise a wide range of applications. On-site testing for environmental monitoring, food safety control, and drug testing could be easily foreseen with the portable paper-based SERS method. Further combination with the lateral flow assays, dual or multiple readout of biosensors, could provide convincing results and show possibilities in point-of-care testing with clinical applications. The development of flexible paper-based SERS methods into standardized procedures holds great potential for various real-world applications.
References
Fierro-Mercado PM, Hernández-Rivera SP (2012) Highly sensitive filter paper substrate for SERS trace explosives detection. Int J Spectrosc 2012:1–7. https://doi.org/10.1155/2012/716527
Sallum LF, Soares FLF, Ardila JA, Carneiro RL (2014) Optimization of SERS scattering by Ag-NPs-coated filter paper for quantification of nicotinamide in a cosmetic formulation. Talanta 118:353–358. https://doi.org/10.1016/j.talanta.2013.10.039
Yang G, Fang X, Jia Q et al (2020) Fabrication of paper-based SERS substrates by spraying silver and gold nanoparticles for SERS determination of malachite green, methylene blue, and crystal violet in fish. Microchim Acta 187. https://doi.org/10.1007/s00604-020-04262-2
Rajapandiyan P, Yang J (2014) Photochemical method for decoration of silver nanoparticles on filter paper substrate for SERS application. J Raman Spectrosc 45:574–580. https://doi.org/10.1002/jrs.4502
Vo-Dinh T (1995) SERS chemical sensors and biosensors: new tools for environmental and biological analysis. Sensors Actuators B Chem 29:183–189. https://doi.org/10.1016/0925-4005(95)01681-3
Weng G, Yang Y, Zhao J et al (2018) Preparation and SERS performance of Au NP/paper strips based on inkjet printing and seed mediated growth: the effect of silver ions. Solid State Commun 272:67–73. https://doi.org/10.1016/j.ssc.2018.01.014
Desmonda C, Kar S, Tai Y (2016) Formation of gold nanostructures on copier paper surface for cost effective SERS active substrate — effect of halide additives. Appl Surf Sci 367:362–369. https://doi.org/10.1016/j.apsusc.2016.01.154
Zhang L, Liu J, Zhou G, Zhang Z (2020) Controllable in-situ growth of silver nanoparticles on filter paper for flexible and highly sensitive sers sensors for malachite green residue detection. Nanomaterials 10. https://doi.org/10.3390/nano10050826
Godoy NV, García-Lojo D, Sigoli FA et al (2020) Ultrasensitive inkjet-printed based SERS sensor combining a high-performance gold nanosphere ink and hydrophobic paper. Sensors Actuators B Chem 320:128412. https://doi.org/10.1016/j.snb.2020.128412
Zhao P, Liu H, Zhang L et al (2020) Paper-based SERS sensing platform based on 3D silver dendrites and molecularly imprinted identifier sandwich hybrid for neonicotinoid quantification. ACS Appl Mater Interfaces 12:8845–8854. https://doi.org/10.1021/acsami.9b20341
Moram SSB, Byram C, Soma VR (2020) Gold-nanoparticle- and nanostar-loaded paper-based SERS substrates for sensing nanogram-level Picric acid with a portable Raman spectrometer. Bull Mater Sci 43. https://doi.org/10.1007/s12034-019-2017-8
Li S, Chen H, Liu X et al (2022) Nanocellulose as a promising substrate for advanced sensors and their applications. Int J Biol Macromol 218:473–487. https://doi.org/10.1016/j.ijbiomac.2022.07.124
Lin S, Lin X, Han S et al (2020) Flexible fabrication of a paper-fluidic SERS sensor coated with a monolayer of core–shell nanospheres for reliable quantitative SERS measurements. Anal Chim Acta 1108:167–176. https://doi.org/10.1016/j.aca.2020.02.034
Liu X, Ma J, Jiang P et al (2020) Large-scale flexible surface-enhanced Raman scattering (SERS) sensors with high stability and signal homogeneity. ACS Appl Mater Interfaces 12:45332–45341. https://doi.org/10.1021/acsami.0c13691
Restaino SM, White IM (2019) A critical review of flexible and porous SERS sensors for analytical chemistry at the point-of-sample. Anal Chim Acta 1060:17–29. https://doi.org/10.1016/j.aca.2018.11.057
Li Y, Zhang K, Zhao J et al (2016) A three-dimensional silver nanoparticles decorated plasmonic paper strip for SERS detection of low-abundance molecules. Talanta 147:493–500. https://doi.org/10.1016/j.talanta.2015.10.025
Xu F, Xuan M, Ben Z et al (2021) Surface enhanced Raman scattering analysis with filter-based enhancement substrates: a mini review. Rev Anal Chem 40:75–92. https://doi.org/10.1515/revac-2021-0126
Villa JEL, Santos DP d, Poppi RJ (2016) Fabrication of gold nanoparticle-coated paper and its use as a sensitive substrate for quantitative SERS analysis. Microchim Acta 183:2745–2752. https://doi.org/10.1007/s00604-016-1918-0
Weng G, Feng Y, Zhao J et al (2019) Size dependent SERS activity of Ag triangular nanoplates on different substrates: glass vs paper. Appl Surf Sci 478:275–283. https://doi.org/10.1016/j.apsusc.2019.01.142
Hasi WLJ, Lin X, Lou XT et al (2015) Chloride ion-assisted self-assembly of silver nanoparticles on filter paper as SERS substrate. Appl Phys A Mater Sci Process 118:799–807. https://doi.org/10.1007/s00339-014-8800-x
Kihara S, Chan A, In E et al (2022) Detecting polystyrene nanoplastics using filter paper-based surface-enhanced Raman spectroscopy. RSC Adv 12:20519–20522. https://doi.org/10.1039/d2ra03395j
He S, Chua J, Tan EKM, Kah JCY (2017) Optimizing the SERS enhancement of a facile gold nanostar immobilized paper-based SERS substrate. RSC Adv 7:16264–16272. https://doi.org/10.1039/c6ra28450g
Luo K, Wang K, Lim MC et al (2022) Synthesis of starch-based plasmonic core-shell microparticles for SERS applications. ACS Sustain Chem Eng 10:10268–10274. https://doi.org/10.1021/acssuschemeng.2c02092
Sun H, Li X, Hu Z et al (2021) Hydrophilic-hydrophobic silver nanowire-paper based SERS substrate for in-situ detection of furazolidone under various environments. Appl Surf Sci 556:149748. https://doi.org/10.1016/j.apsusc.2021.149748
Zhang S, Xu J, Liu Z et al (2022) Facile and scalable preparation of solution-processed succulent-like silver nanoflowers for 3D flexible nanocellulose-based SERS sensors. Surfaces and Interfaces 34:102391. https://doi.org/10.1016/j.surfin.2022.102391
Sridhar K, Inbaraj BS, Chen BH (2022) An improved surface enhanced Raman spectroscopic method using a paper-based grape skin-gold nanoparticles/graphene oxide substrate for detection of rhodamine 6G in water and food. Chemosphere 301:134702. https://doi.org/10.1016/j.chemosphere.2022.134702
Zhou M, Wang Z, Xia D et al (2022) Hybrid nanoassembly with two-tier host-guest architecture and regioselective enrichment capacity for repetitive SERS detection. Sensors Actuators B Chem 369:132359. https://doi.org/10.1016/j.snb.2022.132359
Lee CH, Tian L, Singamaneni S (2010) Paper-based SERS swab for rapid trace detection on real-world surfaces. ACS Appl Mater Interfaces 2:3429–3435. https://doi.org/10.1021/am1009875
Zhu A, Ali S, Xu Y et al (2022) SERS-based Au@Ag NPs solid-phase substrate combined with chemometrics for rapid discrimination of multiple foodborne pathogens. Spectrochim Acta - Part A Mol Biomol Spectrosc 270. https://doi.org/10.1016/j.saa.2021.120814
Gomez-Caballero LF, Pichardo-Molina JL, Basurto-Islas G (2022) Cellulose dialysis membrane tubing doped with gold nanoparticles as SERS substrate. Mater Lett 313:131718. https://doi.org/10.1016/j.matlet.2022.131718
Ying CC, Cheung CC, An CX et al (2021) High sensitivity enhancement of multi-shaped silver-nanoparticle-decorated hydrophilic PVDF-based SERS substrates using solvating pretreatment. Sensors Actuators B Chem 347:130614. https://doi.org/10.1016/j.snb.2021.130614
Panwar K, Jassal M, Agrawal AK (2017) Ag–SiO 2 Janus particles based highly active SERS macroscopic substrates. Appl Surf Sci 411:368–373. https://doi.org/10.1016/j.apsusc.2017.03.105
Trang TNQ, Vinh LQ, Doanh TT, Thu VTH (2021) Structure-adjustable colloidal silver nanoparticles on polymers grafted cellulose paper-based highly sensitive and selective SERS sensing platform with analyte enrichment function. J Alloys Compd 867:159158. https://doi.org/10.1016/j.jallcom.2021.159158
Satheeshkumar E, Karuppaiya P, Sivashanmugan K et al (2017) Biocompatible 3D SERS substrate for trace detection of amino acids and melamine. Spectrochim Acta - Part A Mol Biomol Spectrosc 181:91–97. https://doi.org/10.1016/j.saa.2017.03.040
Wang C, Zhou S, Tian Y et al (2022) Super-hydrophilic SERS sensor with both ultrahigh activity and exceptional 3D spatial uniformity for sensitive detection of toxic pollutants. Appl Surf Sci 603:154445. https://doi.org/10.1016/j.apsusc.2022.154445
Li X, Zhou H, Wang L et al (2023) SERS paper sensor based on three-dimensional ZnO@Ag nanoflowers assembling on polyester fiber membrane for rapid detection of florfenicol residues in chicken. J Food Compos Anal 115:104911. https://doi.org/10.1016/j.jfca.2022.104911
Lv P, Chen Z, Ma Z et al (2020) Ag nanoparticle ink coupled with graphene oxide cellulose paper: a flexible and tunable SERS sensing platform. Opt Lett 45:4208. https://doi.org/10.1364/ol.400131
Skwierczyńska M, Woźny P, Runowski M et al (2022) Optically active plasmonic cellulose fibers based on Au nanorods for SERS applications. Carbohydr Polym 279. https://doi.org/10.1016/j.carbpol.2021.119010
Sha X, Fang G, Cao G et al (2022) Qualitative and quantitative detection and identification of two benzodiazepines based on SERS and convolutional neural network technology. Analyst 147:5785–5795. https://doi.org/10.1039/d2an01277d
Zhang K, Zhao J, Xu H et al (2015) Multifunctional paper strip based on self-assembled interfacial plasmonic nanoparticle arrays for sensitive SERS detection. ACS Appl Mater Interfaces 7:16767–16774. https://doi.org/10.1021/acsami.5b04534
Shao J, Tong L, Tang S et al (2015) PLLA nanofibrous paper-based plasmonic substrate with tailored hydrophilicity for focusing SERS detection. ACS Appl Mater Interfaces 7:5391–5399. https://doi.org/10.1021/am508881k
Zhang Q, Zhang Y, Chen H et al (2022) One-dimensional nanohybrids based on cellulose nanocrystals and their SERS performance. Carbohydr Polym 284:119140. https://doi.org/10.1016/j.carbpol.2022.119140
Chen H, Liu X, Zhang Q et al (2022) Ultrastable water-dispersible one-dimensional gold nanoparticles@cellulose nanocrystal. Colloids Surfaces A Physicochem Eng Asp 655:130147. https://doi.org/10.1016/j.colsurfa.2022.130147
Ogundare SA, van Zyl WE (2018) Nanocrystalline cellulose as reducing- and stabilizing agent in the synthesis of silver nanoparticles: application as a surface-enhanced Raman scattering (SERS) substrate. Surfaces and Interfaces 13:1–10. https://doi.org/10.1016/j.surfin.2018.06.004
Ogundare SA, van Zyl WE (2019) Amplification of SERS “hot spots” by silica clustering in a silver-nanoparticle/nanocrystalline-cellulose sensor applied in malachite green detection. Colloids Surfaces A Physicochem Eng Asp 570:156–164. https://doi.org/10.1016/j.colsurfa.2019.03.019
Xian L, You R, Lu D et al (2020) Surface-modified paper-based SERS substrates for direct-droplet quantitative determination of trace substances. Cellulose 27:1483–1495. https://doi.org/10.1007/s10570-019-02855-6
Dong J, Wang T, Xu E et al (2022) Flexible hydrophobic CFP@PDA@AuNPs stripes for highly sensitive SERS detection of methylene blue residue. Nanomaterials 12:1–13. https://doi.org/10.3390/nano12132163
Batool A, Khan GA, Ahmed W (2022) Seed-mediated growth of highly concentrated silver nanoparticles on a flexible substrate forapplications in SERS-based trace detection. Vib Spectrosc 123:103438. https://doi.org/10.1016/j.vibspec.2022.103438
Luo W, Chen M, Hao N et al (2019) In situ synthesis of gold nanoparticles on pseudo-paper films as flexible SERS substrate for sensitive detection of surface organic residues. Talanta 197:225–233. https://doi.org/10.1016/j.talanta.2018.12.099
Kang Y, Kim HJ, Lee SH, Noh H (2022) Paper-based substrate for a surface-enhanced Raman spectroscopy biosensing platform—a silver/chitosan nanocomposite approach. Biosensors 12. https://doi.org/10.3390/bios12050266
Han L, Liu H, Zhang J et al (2022) Recyclable SERS monitoring of food quality based on the shrubby morphology of titania oxide-triggered electromagnetic “hotspots.”. Appl Surf Sci 604:154456. https://doi.org/10.1016/j.apsusc.2022.154456
Hou M, Li N, Tian X et al (2023) Preparation of SERS active filter paper for filtration and detection of pesticides residue from complex sample. Spectrochim Acta - Part A Mol Biomol Spectrosc 285:121860. https://doi.org/10.1016/j.saa.2022.121860
Cheng ML, Tsai BC, Yang J (2011) Silver nanoparticle-treated filter paper as a highly sensitive surface-enhanced Raman scattering (SERS) substrate for detection of tyrosine in aqueous solution. Anal Chim Acta 708:89–96. https://doi.org/10.1016/j.aca.2011.10.013
Verma M, Naqvi TK, Tripathi SK et al (2021) Paper based low-cost flexible SERS sensor for food adulterant detection. Environ Technol Innov 24:102033. https://doi.org/10.1016/j.eti.2021.102033
Das D, Senapati S, Nanda KK (2019) “rinse, Repeat”: an efficient and reusable SERS and catalytic platform fabricated by controlled deposition of silver nanoparticles on cellulose paper. ACS Sustain Chem Eng. https://doi.org/10.1021/acssuschemeng.9b02651
Liu H, Zhao P, Wang Y et al (2020) Paper-based sandwich type SERS sensor based on silver nanoparticles and biomimetic recognizer. Sensors Actuators B Chem 313:127989. https://doi.org/10.1016/j.snb.2020.127989
Liu H, Zhao P, Xiu W et al (2022) SERS paper slip based on 3D dendritic gold nanomaterials coupling with urchin-like nanoparticles for rapid detection of thiram. Sensors Actuators B Chem 355:131264. https://doi.org/10.1016/j.snb.2021.131264
Weng G, Yang Y, Zhao J et al (2020) Improving the SERS enhancement and reproducibility of inkjet-printed Au NP paper substrates by second growth of Ag nanoparticles. Mater Chem Phys 253:123416. https://doi.org/10.1016/j.matchemphys.2020.123416
Kim D, Kim J, Henzie J et al (2021) Mesoporous Au films assembled on flexible cellulose nanopaper as high-performance SERS substrates. Chem Eng J 419:129445. https://doi.org/10.1016/j.cej.2021.129445
Yang Y, O’Riordan A, Lovera P (2022) Highly sensitive pesticide detection using electrochemically prepared Silver-Gum Arabic nanocluster SERS substrates. Sensors Actuators B Chem 364:131851. https://doi.org/10.1016/j.snb.2022.131851
Yu WW, White IM (2010) Inkjet printed surface enhanced Raman spectroscopy array on cellulose paper. Anal Chem 82:9626–9630. https://doi.org/10.1021/ac102475k
Thuy TT, Sharipov M, Lee Y et al (2020) Inkjet-based microreactor for the synthesis of silver nanoparticles on plasmonic paper decorated with chitosan nano-wrinkles for efficient on-site surface-enhanced Raman scattering (SERS). Nano Sel 1:499–509. https://doi.org/10.1002/nano.202000081
Tay LL, Poirier S, Ghaemi A, Hulse J (2022) Inkjet-printed paper-based surface enhanced Raman scattering (SERS) sensors for the detection of narcotics. MRS Adv 7:190–196. https://doi.org/10.1557/s43580-022-00257-8
Xiao G, Li Y, Shi W et al (2017) Highly sensitive, reproducible and stable SERS substrate based on reduced graphene oxide/silver nanoparticles coated weighing paper. Appl Surf Sci 404:334–341. https://doi.org/10.1016/j.apsusc.2017.01.231
Hoppmann EP, Yu WW, White IM (2013) Highly sensitive and flexible inkjet printed SERS sensors on paper. Methods 63:219–224. https://doi.org/10.1016/j.ymeth.2013.07.010
Yu WW, White IM (2013) Inkjet-printed paper-based SERS dipsticks and swabs for trace chemical detection. Analyst 138:1020–1025. https://doi.org/10.1039/c2an36116g
Yu WW, White IM (2013) Chromatographic separation and detection of target analytes from complex samples using inkjet printed SERS substrates. Analyst 138:3679–3686. https://doi.org/10.1039/c3an00673e
Fernandes T, Martins NCT, Fateixa S et al (2022) Dendrimer stabilized nanoalloys for inkjet printing of surface-enhanced Raman scattering substrates. J Colloid Interface Sci 612:342–354. https://doi.org/10.1016/j.jcis.2021.12.167
Qu LL, Li DW, Xue JQ et al (2012) Batch fabrication of disposable screen printed SERS arrays. Lab Chip 12:876–881. https://doi.org/10.1039/c2lc20926h
Qu LL, Song QX, Li YT et al (2013) Fabrication of bimetallic microfluidic surface-enhanced Raman scattering sensors on paper by screen printing. Anal Chim Acta 792:86–92. https://doi.org/10.1016/j.aca.2013.07.017
Ma Y, Wang Y, Luo Y et al (2018) Rapid and sensitive on-site detection of pesticide residues in fruits and vegetables using screen-printed paper-based SERS swabs. Anal Methods 10:4655–4664. https://doi.org/10.1039/c8ay01698d
Cañamares MV, Garcia-Ramos JV, Gómez-Varga JD et al (2007) Ag nanoparticles prepared by laser photoreduction as substrates for in situ surface-enhanced Raman scattering analysis of dyes. Langmuir 23:5210–5215. https://doi.org/10.1021/la063445v
Yu CC, Chou SY, Tseng YC et al (2015) Single-shot laser treatment provides quasi-three-dimensional paper-based substrates for SERS with attomolar sensitivity. Nanoscale 7:1667–1677. https://doi.org/10.1039/c4nr05178e
Wu J, Xi J, Chen H et al (2022) SERS-active nanocellulose substrate via in-situ photochemical synthesis. Int J Biol Macromol 215:368–376. https://doi.org/10.1016/j.ijbiomac.2022.06.036
Jang W, Byun H, Kim JH (2020) Rapid preparation of paper-based plasmonic platforms for SERS applications. Mater Chem Phys 240:122124. https://doi.org/10.1016/j.matchemphys.2019.122124
Siebe HS, Chen Q, Li X et al (2021) Filter paper based SERS substrate for the direct detection of analytes in complex matrices. Analyst 146:1281–1288. https://doi.org/10.1039/d0an02103b
Zhang W, Li B, Chen L et al (2014) Brushing, a simple way to fabricate SERS active paper substrates. Anal Methods 6:2066–2071. https://doi.org/10.1039/c4ay00046c
Zhang L, Li X, Ong L et al (2015) Cellulose nanofibre textured SERS substrate. Colloids Surfaces A Physicochem Eng Asp 468:309–314. https://doi.org/10.1016/j.colsurfa.2014.12.056
Zhang R, Bin XB, Liu XQ et al (2012) Highly efficient SERS test strips. Chem Commun 48:5913–5915. https://doi.org/10.1039/c2cc31604h
Zhao F, Wang W, Zhong H et al (2021) Robust quantitative SERS analysis with relative Raman scattering intensities. Talanta 221:121465. https://doi.org/10.1016/j.talanta.2020.121465
Chen L, Ying B, Song P, Liu X (2019) A nanocellulose-paper-based SERS multiwell plate with high sensitivity and high signal homogeneity. Adv Mater Interfaces 6:1–10. https://doi.org/10.1002/admi.201901346
Doanh TT, Duong T, Danh NC, et al (2020) Highly efficient SERS performance from the silver nanoparticles/graphene nanoribbons/cellulose paper. Sci Technol Dev J 23:First. 10.32508/stdj.v23i3.2390
Liu S, Cui R, Ma Y et al (2020) Plasmonic cellulose textile fiber from waste paper for BPA sensing by SERS. Spectrochim Acta - Part A Mol Biomol Spectrosc 227. https://doi.org/10.1016/j.saa.2019.117664
Marques AC, Pinheiro T, Morais M et al (2021) Bottom-up microwave-assisted seed-mediated synthesis of gold nanoparticles onto nanocellulose to boost stability and high performance for SERS applications. Appl Surf Sci 561:150060. https://doi.org/10.1016/j.apsusc.2021.150060
Sun C, Zhang S, Wang J, Ge F (2022) Enhancement of SERS performance using hydrophobic or superhydrophobic cotton fabrics. Surfaces and Interfaces 28:101616. https://doi.org/10.1016/j.surfin.2021.101616
Wang K, Qiu Z, Qin Y et al (2022) Preparation and SERS performance of silver nanowires arrays on paper by automatic writing method. Spectrochim Acta - Part A Mol Biomol Spectrosc 281:121580. https://doi.org/10.1016/j.saa.2022.121580
Polavarapu L, La PA, Novikov SM et al (2014) Pen-on-paper approach toward the design of universal surface enhanced Raman scattering substrates. Small 10:3065–3071. https://doi.org/10.1002/smll.201400438
Li H, Zhang H, Luo W et al (2022) Microcontact printing of gold nanoparticle at three-phase interface as flexible substrate for SERS detection of microRNA. Anal Chim Acta 1229:340380. https://doi.org/10.1016/j.aca.2022.340380
Saini RK, Sharma AK, Agarwal A, Prajesh R (2022) Near field FEM simulations of plasmonic gold nanoparticle based SERS substrate with experimental validation. Mater Chem Phys 287:126288. https://doi.org/10.1016/j.matchemphys.2022.126288
Bharadwaj S, Pandey A, Yagci B et al (2018) Graphene nano-mesh-Ag-ZnO hybrid paper for sensitive SERS sensing and self-cleaning of organic pollutants. Chem Eng J 336:445–455. https://doi.org/10.1016/j.cej.2017.12.040
Lee JC, Kim W, Choi S (2017) Fabrication of a SERS-encoded microfluidic paper-based analytical chip for the point-of-assay of wastewater. Int J Precis Eng Manuf - Green Technol 4:221–226. https://doi.org/10.1007/s40684-017-0027-9
Sun J, Zhang Z, Liu C et al (2021) Continuous in situ portable SERS analysis of pollutants in water and air by a highly sensitive gold nanoparticle-decorated PVDF substrate. Anal Bioanal Chem 413:5469–5482. https://doi.org/10.1007/s00216-021-03531-0
Mai QD, Nguyen HA, Dinh NX et al (2023) Versatile and high performance in-paper flexible SERS chips for simple and in-situ detection of methylene blue in river water and thiram on apple skin. Talanta 253:124114. https://doi.org/10.1016/j.talanta.2022.124114
Luo Y, Xing L, Hu C et al (2022) Facile synthesis of nanocellulose-based Cu2O/Ag heterostructure as a surface-enhanced Raman scattering substrate for trace dye detection. Int J Biol Macromol 205:366–375. https://doi.org/10.1016/j.ijbiomac.2022.02.102
Yu H, Guo D, Zhang H et al (2023) Facile fabrication of flexible AuNPs@CDA SERS substrate for enrichment and detection of thiram pesticide in water. Spectrochim Acta Part A Mol Biomol Spectrosc 285:121930. https://doi.org/10.1016/j.saa.2022.121930
Hu F, Li Y, Zhang Y et al (2022) Flexible Ag NCs/CNFs film for colorimetric and SERS dual-mode ultrasensitive detection of mercury ions (II). Vib Spectrosc 118:103342. https://doi.org/10.1016/j.vibspec.2022.103342
Zhang D, Pu H, Huang L, Sun DW (2021) Advances in flexible surface-enhanced Raman scattering (SERS) substrates for nondestructive food detection: fundamentals and recent applications. Trends Food Sci Technol 109:690–701. https://doi.org/10.1016/j.tifs.2021.01.058
Zhu J, Chen Q, Kutsanedzie FYH et al (2017) Highly sensitive and label-free determination of thiram residue using surface-enhanced Raman spectroscopy (SERS) coupled with paper-based microfluidics. Anal Methods 9:6186–6193. https://doi.org/10.1039/c7ay01637a
Wang Q, Liu Y, Bai Y et al (2019) Superhydrophobic SERS substrates based on silver dendrite-decorated filter paper for trace detection of nitenpyram. Anal Chim Acta 1049:170–178. https://doi.org/10.1016/j.aca.2018.10.039
Liu C, Xu D, Dong X, Huang Q (2022) A review: research progress of SERS-based sensors for agricultural applications. Trends Food Sci Technol 128:90–101. https://doi.org/10.1016/j.tifs.2022.07.012
Lee M, Oh K, Choi HK et al (2018) Subnanomolar sensitivity of filter paper-based SERS sensor for pesticide detection by hydrophobicity change of paper surface. ACS Sensors 3:151–159. https://doi.org/10.1021/acssensors.7b00782
Chen J, Huang M, Kong L (2020) Flexible Ag/nanocellulose fibers SERS substrate and its applications for in-situ hazardous residues detection on food. Appl Surf Sci 533:147454. https://doi.org/10.1016/j.apsusc.2020.147454
Lin S, Hasi W, Han S et al (2020) A dual-functional PDMS-assisted paper-based SERS platform for the reliable detection of thiram residue both on fruit surfaces and in juice. Anal Methods 12:2571–2579. https://doi.org/10.1039/d0ay00483a
Xiong Z, Lin M, Lin H, Huang M (2018) Facile synthesis of cellulose nanofiber nanocomposite as a SERS substrate for detection of thiram in juice. Carbohydr Polym 189:79–86. https://doi.org/10.1016/j.carbpol.2018.02.014
Sun L, Yu Z, Alsammarraie FK et al (2021) Development of cellulose nanofiber-based substrates for rapid detection of ferbam in kale by surface-enhanced Raman spectroscopy. Food Chem 347:129023. https://doi.org/10.1016/j.foodchem.2021.129023
Kwon G, Kim J, Kim D et al (2019) Nanoporous cellulose paper-based SERS platform for multiplex detection of hazardous pesticides. Cellulose 26:4935–4944. https://doi.org/10.1007/s10570-019-02427-8
Fan J, Fang X, Zhang Y et al (2022) Quantitative SERS sensing mediated by internal standard Raman signal from silica nanoparticles in flexible polymer matrix. Spectrochim Acta - Part A Mol Biomol Spectrosc 278:121304. https://doi.org/10.1016/j.saa.2022.121304
Sun M, Li B, Liu X et al (2019) Performance enhancement of paper-based SERS chips by shell-isolated nanoparticle-enhanced Raman spectroscopy. J Mater Sci Technol 35:2207–2212. https://doi.org/10.1016/j.jmst.2019.05.055
Liou P, Nayigiziki FX, Kong F et al (2017) Cellulose nanofibers coated with silver nanoparticles as a SERS platform for detection of pesticides in apples. Carbohydr Polym 157:643–650. https://doi.org/10.1016/j.carbpol.2016.10.031
Mekonnen ML, Chen CH, Osada M et al (2020) Dielectric nanosheet modified plasmonic-paper as highly sensitive and stable SERS substrate and its application for pesticides detection. Spectrochim Acta - Part A Mol Biomol Spectrosc 225:117484. https://doi.org/10.1016/j.saa.2019.117484
Bhardwaj K, Jaiswal A (2022) Plasmonic 3-D wrinkled polymeric shrink film-based SERS substrates for pesticide detection on real-world surfaces. Analyst 562–572. https://doi.org/10.1039/d2an01657e
Xie J, Li L, Khan IM et al (2020) Flexible paper-based SERS substrate strategy for rapid detection of methyl parathion on the surface of fruit. Spectrochim Acta - Part A Mol Biomol Spectrosc 231:118104. https://doi.org/10.1016/j.saa.2020.118104
Jin X, Guo P, Guan P et al (2020) The fabrication of paper separation channel based SERS substrate and its recyclable separation and detection of pesticides. Spectrochim Acta - Part A Mol Biomol Spectrosc 240:118561. https://doi.org/10.1016/j.saa.2020.118561
Zhu Y, Li M, Yu D, Yang L (2014) A novel paper rag as “D-SERS” substrate for detection of pesticide residues at various peels. Talanta 128:117–124. https://doi.org/10.1016/j.talanta.2014.04.066
Luo J, Wang Z, Li Y et al (2021) Durable and flexible Ag-nanowire-embedded PDMS films for the recyclable swabbing detection of malachite green residue in fruits and fingerprints. Sensors Actuators B Chem 347:130602. https://doi.org/10.1016/j.snb.2021.130602
Wu J, Xi J, Chen H et al (2022) Flexible 2D nanocellulose-based SERS substrate for pesticide residue detection. Carbohydr Polym 277:118890. https://doi.org/10.1016/j.carbpol.2021.118890
Yu B, Mao Y, Li J et al (2022) Hydrophobic expanded graphite-covered support to construct flexible and stable SERS substrate for sensitive determination by paste-sampling from irregular surfaces. Spectrochim Acta - Part A Mol Biomol Spectrosc 282:121708. https://doi.org/10.1016/j.saa.2022.121708
Hassan MM, Jiao T, Ahmad W et al (2021) Cellulose paper-based SERS sensor for sensitive detection of 2,4-D residue levels in tea coupled uninformative variable elimination-partial least squares. Spectrochim Acta - Part A Mol Biomol Spectrosc 248. https://doi.org/10.1016/j.saa.2020.119198
Linh VTN, Moon J, Mun CW et al (2019) A facile low-cost paper-based SERS substrate for label-free molecular detection. Sensors Actuators B Chem 291:369–377. https://doi.org/10.1016/j.snb.2019.04.077
Parnsubsakul A, Ngoensawat U, Wutikhun T et al (2020) Silver nanoparticle/bacterial nanocellulose paper composites for paste-and-read SERS detection of pesticides on fruit surfaces. Carbohydr Polym 235:115956. https://doi.org/10.1016/j.carbpol.2020.115956
Li D, Ma Y, Duan H et al (2018) Griess reaction-based paper strip for colorimetric/fluorescent/SERS triple sensing of nitrite. Biosens Bioelectron 99:389–398. https://doi.org/10.1016/j.bios.2017.08.008
Nilghaz A, Mahdi Mousavi S, Amiri A et al (2022) Surface-enhanced Raman spectroscopy substrates for food safety and quality analysis. J Agric Food Chem. https://doi.org/10.1021/acs.jafc.2c00089
Huang CC, Cheng CY, Lai YS (2020) Paper-based flexible surface enhanced Raman scattering platforms and their applications to food safety. Trends Food Sci Technol 100:349–358. https://doi.org/10.1016/j.tifs.2020.04.019
Hu B, Pu H, Sun D-W (2021) Multifunctional cellulose based substrates for SERS smart sensing: principles, applications and emerging trends for food safety detection. Trends Food Sci Technol 110:304–320. https://doi.org/10.1016/j.tifs.2021.02.005
Marques A, Veigas B, Araújo A et al (2019) Paper-based SERS platform for one-step screening of tetracycline in milk. Sci Rep 9:1–8. https://doi.org/10.1038/s41598-019-54380-y
Zhang C, You T, Yang N et al (2019) Hydrophobic paper-based SERS platform for direct-droplet quantitative determination of melamine. Food Chem 287:363–368. https://doi.org/10.1016/j.foodchem.2019.02.094
Chen M, Yang H, Rong L, Chen X (2016) A gas-diffusion microfluidic paper-based analytical device (μPAD) coupled with portable surface-enhanced Raman scattering (SERS): facile determination of sulphite in wines. Analyst 141:5511–5519. https://doi.org/10.1039/c6an00788k
Wu M, Li P, Zhu Q et al (2018) Functional paper-based SERS substrate for rapid and sensitive detection of Sudan dyes in herbal medicine. Spectrochim Acta - Part A Mol Biomol Spectrosc 196:110–116. https://doi.org/10.1016/j.saa.2018.02.014
Lin S, Hasi WLJ, Lin X et al (2015) Rapid and sensitive SERS method for determination of Rhodamine B in chili powder with paper-based substrates. Anal Methods 7:5289–5294. https://doi.org/10.1039/c5ay00028a
Kumar A, Santhanam V (2019) Paper swab based SERS detection of non-permitted colourants from dals and vegetables using a portable spectrometer. Anal Chim Acta 1090:106–113. https://doi.org/10.1016/j.aca.2019.08.073
Song Y, Ma Z, Fang H et al (2020) Au sputtered paper chromatography tandem Raman platform for sensitive detection of heavy metal ions. ACS Sensors 5:1455–1464. https://doi.org/10.1021/acssensors.0c00395
Wu L, Zhang W, Liu C et al (2020) Strawberry-like SiO2/Ag nanocomposites immersed filter paper as SERS substrate for acrylamide detection. Food Chem 328:127106. https://doi.org/10.1016/j.foodchem.2020.127106
Kim H, Trinh BT, Kim KH et al (2021) Au@ZIF-8 SERS paper for food spoilage detection. Biosens Bioelectron 179:113063. https://doi.org/10.1016/j.bios.2021.113063
Wang C, Liu B, Dou X (2016) Silver nanotriangles-loaded filter paper for ultrasensitive SERS detection application benefited by interspacing of sharp edges. Sensors Actuators B Chem 231:357–364. https://doi.org/10.1016/j.snb.2016.03.030
Zhuang J, Zhao Z, Lian K et al (2022) SERS-based CRISPR/Cas assay on microfluidic paper analytical devices for supersensitive detection of pathogenic bacteria in foods. Biosens Bioelectron 207:114167. https://doi.org/10.1016/j.bios.2022.114167
Hou Y, Lv CC, Guo YL et al (2022) Recent advances and applications in paper-based devices for point-of-care testing. J Anal Test 6:247–273. https://doi.org/10.1007/s41664-021-00204-w
Breuch R, Klein D, Siefke E et al (2020) Differentiation of meat-related microorganisms using paper-based surface-enhanced Raman spectroscopy combined with multivariate statistical analysis. Talanta 219:1–7. https://doi.org/10.1016/j.talanta.2020.121315
Villa JEL, Quiñones NR, Fantinatti-Garboggini F, Poppi RJ (2019) Fast discrimination of bacteria using a filter paper–based SERS platform and PLS-DA with uncertainty estimation. Anal Bioanal Chem 411:705–713. https://doi.org/10.1007/s00216-018-1485-9
Lee HG, Choi W, Yang SY et al (2021) PCR-coupled paper-based surface-enhanced Raman scattering (SERS) sensor for rapid and sensitive detection of respiratory bacterial DNA. Sensors Actuators B Chem 326:128802. https://doi.org/10.1016/j.snb.2020.128802
Hilton SH, Hall C, Nguyen HT et al (2020) Phenotypically distinguishing ESBL-producing pathogens using paper-based surface enhanced Raman sensors. Anal Chim Acta 1127:207–216. https://doi.org/10.1016/j.aca.2020.06.068
Xiang S, Ge C, Li S et al (2020) In situ detection of endotoxin in bacteriostatic process by SERS chip integrated array microchambers within bioscaffold nanostructures and SERS tags. ACS Appl Mater Interfaces 12:28985–28992. https://doi.org/10.1021/acsami.0c04897
Kim S, Ansah IB, Park JS et al (2022) Early and direct detection of bacterial signaling molecules through one-pot Au electrodeposition onto paper-based 3D SERS substrates. Sensors Actuators B Chem 358:131504. https://doi.org/10.1016/j.snb.2022.131504
Burr DS, Fatigante WL, Lartey JA et al (2020) Integrating SERS and PSI-MS with dual purpose plasmonic paper substrates for on-site illicit drug confirmation. Anal Chem 92:6676–6683. https://doi.org/10.1021/acs.analchem.0c00562
Ponlamuangdee K, Hornyak GL, Bora T, Bamrungsap S (2020) Graphene oxide/gold nanorod plasmonic paper-a simple and cost-effective SERS substrate for anticancer drug analysis. New J Chem 44:14087–14094. https://doi.org/10.1039/d0nj02448a
Wei W, Huang Q (2017) Rapid fabrication of silver nanoparticle-coated filter paper as SERS substrate for low-abundance molecules detection. Spectrochim Acta - Part A Mol Biomol Spectrosc 179:211–215. https://doi.org/10.1016/j.saa.2017.02.052
Berger AG, Restaino SM, White IM (2017) Vertical-flow paper SERS system for therapeutic drug monitoring of flucytosine in serum. Anal Chim Acta 949:59–66. https://doi.org/10.1016/j.aca.2016.10.035
Bolz A, Panne U, Rurack K, Buurman M (2016) Glass fibre paper-based test strips for sensitive SERS sensing. Anal Methods 8:1313–1318. https://doi.org/10.1039/c5ay03096j
Han S, Zhang C, Sha X et al (2020) Effective SERS method for identification of dexmedetomidine hydrochloride in biological samples. Anal Methods 12:1662–1669. https://doi.org/10.1039/d0ay00019a
Hassanain WA, Izake EL, Sivanesan A, Ayoko GA (2017) Towards interference free HPLC-SERS for the trace analysis of drug metabolites in biological fluids. J Pharm Biomed Anal 136:38–43. https://doi.org/10.1016/j.jpba.2016.12.019
Sallum LF, Soares FLF, Ardila JA, Carneiro RL (2014) Determination of acetylsalicylic acid in commercial tablets by SERS using silver nanoparticle-coated filter paper. Spectrochim Acta - Part A Mol Biomol Spectrosc 133:107–111. https://doi.org/10.1016/j.saa.2014.04.198
Lim WY, Goh CH, Thevarajah TM et al (2020) Using SERS-based microfluidic paper-based device (μPAD) for calibration-free quantitative measurement of AMI cardiac biomarkers. Biosens Bioelectron 147:111792. https://doi.org/10.1016/j.bios.2019.111792
Ngo YH, Then WL, Shen W, Garnier G (2013) Gold nanoparticles paper as a SERS bio-diagnostic platform. J Colloid Interface Sci 409:59–65. https://doi.org/10.1016/j.jcis.2013.07.051
Pan X, Li L, Lin H et al (2019) A graphene oxide-gold nanostar hybrid based-paper biosensor for label-free SERS detection of serum bilirubin for diagnosis of jaundice. Biosens Bioelectron 145:111713. https://doi.org/10.1016/j.bios.2019.111713
Torul H, Çiftçi H, Çetin D et al (2015) Paper membrane-based SERS platform for the determination of glucose in blood samples. Anal Bioanal Chem 407:8243–8251. https://doi.org/10.1007/s00216-015-8966-x
Reokrungruang P, Chatnuntawech I, Dharakul T, Bamrungsap S (2019) A simple paper-based surface enhanced Raman scattering (SERS) platform and magnetic separation for cancer screening. Sensors Actuators B Chem 285:462–469. https://doi.org/10.1016/j.snb.2019.01.090
Lu D, Ran M, Liu Y et al (2020) SERS spectroscopy using Au-Ag nanoshuttles and hydrophobic paper-based Au nanoflower substrate for simultaneous detection of dual cervical cancer–associated serum biomarkers. Anal Bioanal Chem 412:7099–7112. https://doi.org/10.1007/s00216-020-02843-x
Frimpong R, Jang W, Kim JH, Driskell JD (2021) Rapid vertical flow immunoassay on AuNP plasmonic paper for SERS-based point of need diagnostics. Talanta 223:121739. https://doi.org/10.1016/j.talanta.2020.121739
Ma Y, Liu H, Chen Y et al (2020) Improved lateral flow strip based on hydrophilic–hydrophobic SERS substrate for ultra–sensitive and quantitative immunoassay. Appl Surf Sci 529:2–7. https://doi.org/10.1016/j.apsusc.2020.147121
Hu SW, Qiao S, Bin PJ et al (2018) A paper-based SERS test strip for quantitative detection of Mucin-1 in whole blood. Talanta 179:9–14. https://doi.org/10.1016/j.talanta.2017.10.038
Li C, Liu Y, Zhou X, Wang Y (2020) A paper-based SERS assay for sensitive duplex cytokine detection towards the atherosclerosis-associated disease diagnosis. J Mater Chem B 8:3582–3589. https://doi.org/10.1039/c9tb02469g
Villa JEL, Poppi RJ (2016) A portable SERS method for the determination of uric acid using a paper-based substrate and multivariate curve resolution. Analyst 141:1966–1972. https://doi.org/10.1039/c5an02398j
Gumustas A, Caglayan MG, Eryilmaz M et al (2018) Paper based lateral flow immunoassay for the enumeration of: Escherichia coli in urine. Anal Methods 10:1213–1218. https://doi.org/10.1039/c7ay02974h
Ilhan H, Guven B, Dogan U et al (2019) The coupling of immunomagnetic enrichment of bacteria with paper-based platform. Talanta 201:245–252. https://doi.org/10.1016/j.talanta.2019.04.017
Tegegne WA, Su WN, Beyene AB et al (2021) Flexible hydrophobic filter paper-based SERS substrate using silver nanocubes for sensitive and rapid detection of adenine. Microchem J 168:106349. https://doi.org/10.1016/j.microc.2021.106349
Han S, Zhang C, Lin S et al (2021) Sensitive and reliable identification of fentanyl citrate in urine and serum using chloride ion-treated paper-based SERS substrate. Spectrochim Acta - Part A Mol Biomol Spectrosc 251:119463. https://doi.org/10.1016/j.saa.2021.119463
Park M, Jung H, Jeong Y, Jeong KH (2017) Plasmonic Schirmer strip for human tear-based gouty arthritis diagnosis using surface-enhanced Raman scattering. ACS Nano 11:438–443. https://doi.org/10.1021/acsnano.6b06196
Kim WS, Shin JH, Park HK, Choi S (2016) A low-cost, monometallic, surface-enhanced Raman scattering-functionalized paper platform for spot-on bioassays. Sensors Actuators B Chem 222:1112–1118. https://doi.org/10.1016/j.snb.2015.08.030
Kim HS, Kim HJ, Lee J et al (2021) Hand-held Raman spectrometer-based dual detection of creatinine and cortisol in human sweat using silver nanoflakes. Anal Chem 93:14996–15004. https://doi.org/10.1021/acs.analchem.1c02496
Mogera U, Guo H, Namkoong M et al (2022) Wearable plasmonic paper–based microfluidics for continuous sweat analysis. Sci Adv 8:1–12. https://doi.org/10.1126/sciadv.abn1736
Kim W, Lee SH, Ahn YJ et al (2018) A label-free cellulose SERS biosensor chip with improvement of nanoparticle-enhanced LSPR effects for early diagnosis of subarachnoid hemorrhage-induced complications. Biosens Bioelectron 111:59–65. https://doi.org/10.1016/j.bios.2018.04.003
Ahn YJ, Gil YG, Lee YJ et al (2020) A dual-mode colorimetric and SERS detection of hydrogen sulfide in live prostate cancer cells using a silver nanoplate-coated paper assay. Microchem J 155:104724. https://doi.org/10.1016/j.microc.2020.104724
Chen M, Zhang Z, Liu M et al (2017) In situ fabrication of label-free optical sensing paper strips for the rapid surface-enhanced Raman scattering (SERS) detection of brassinosteroids in plant tissues. Talanta 165:313–320. https://doi.org/10.1016/j.talanta.2016.12.072
Xie L, Zeng H, Zhu J et al (2022) State of the art in flexible SERS sensors toward label-free and onsite detection: from design to applications. Nano Res 15:4374–4394. https://doi.org/10.1007/s12274-021-4017-4
Das A, Pant U, Cao C et al (2023) Fabrication of plasmonic nanopyramidal array as flexible SERS substrate for biosensing application. Nano Res 16:1132–1140. https://doi.org/10.1007/s12274-022-4745-0
Zhou Q, Kim T (2016) Review of microfluidic approaches for surface-enhanced Raman scattering. Sensors Actuators B Chem 227:504–514. https://doi.org/10.1016/j.snb.2015.12.069
Guo J, Zeng F, Guo J, Ma X (2020) Preparation and application of microfluidic SERS substrate: challenges and future perspectives. J Mater Sci Technol 37:96–103. https://doi.org/10.1016/j.jmst.2019.06.018
Ding Y, Sun Y, Liu C et al (2023) SERS-based biosensors combined with machine learning for medical application**. ChemistryOpen 12. https://doi.org/10.1002/open.202200192
Mahmoud AYF, Teixeira A, Aranda M et al (2023) Will data analytics revolution finally bring SERS to the clinic? TrAC Trends Anal Chem 117311. https://doi.org/10.1016/j.trac.2023.117311
Funding
This research was supported by the National Natural Science Foundation of China (21904068), the Natural Science Foundation of Jiangsu Province (BK20201351), Jiangsu Specially Appointed Professor program, Introduction of talent research start fund of Nanjing Medical University (NMUR2019007). Shanghai Key Lab of Forensic Science, Ministry of Justice, China (Academy of Forensic Science Open subject) (KF202006). Chunhui Project Foundation of the Education Department of China (HZKY20220178).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Cao, Y., Sun, Y., Yu, RJ. et al. Paper-based substrates for surface-enhanced Raman spectroscopy sensing. Microchim Acta 191, 8 (2024). https://doi.org/10.1007/s00604-023-06086-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00604-023-06086-2