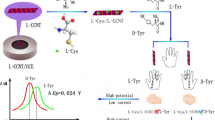
Abstract
A chiral sensor for the electrochemical identification of tryptophan (Trp) isomers is described. The electrochemical sensor was prepared based on the combination of (a) carbon black (CB-COOH) as conductive material, (b) Cu2+-modified β-cyclodextrin (Cu-β-CD), and (c) β-CD-based metal–organic frameworks (β-CD-MOF) as chiral selectors. The Cu-β-CD can be self-assembled into the CB-COOH and β-CD-MOF through electrostatic interactions, which was characterized by zeta potential analysis. UV–vis spectroscopy proved that Cu-β-CD displays a higher combination for d-Trp than l-Trp, and the β-CD-MOF at the surface of the GCE has a higher affinity for l-Trp than d-Trp, which endow an easier permeation of l-Trp to the surface of the electrode, thus leading to a larger electrochemical signal of differential pulse voltammetry (DPV). The enantioselectivity for l-Trp over d-Trp (IL/ID) is 2.13, with a low detection limit for d-Trp (11.18 μM) and l-Trp (5.48 μM). In addition, the proposed chiral sensor can be chosen to determine the percentage of d-Trp in enantiomer mixture solutions and real sample detection with a recovery from 98.2 to 102.8% for l-Trp and 97.9 to 101.1% for d-Trp.
Graphical Abstract

Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Chirality, a significant characteristic in the world, plays an important role in life processes [1]. Chiral substances with different configurations display the same physical and chemical features, but show different or even opposite influences on the operating mechanism of biological behavior. Therefore, the enantioselective analysis of chiral compounds is of vital importance not only for medical science and biochemistry, but also for a deep understanding of the living world [2,3,4].
As chiral molecules, l-amino acids act as key elements for building blocks of living substances, while d-amino acids can cause adverse reactions to life entities [5, 6]. Therefore, research focusing on the chiral recognition of amino acids is of great value and can help us obtain useful information for a deep understanding of the living world. Among them, l-tryptophan (l-Trp) plays a vital role in the clinic and the synthesis of protein and is widely used in food and medical industries. d-Tryptophan (d-Trp) can be used as an important intermediate to synthesize anticancer drugs and immunological agents [7,8,9]. Therefore, it is essential and fascinating to design facile and useful techniques for identifying Trp enantiomers in the field of medical and biological systems. Various conventional strategies, including chromatographic or spectrographic analytical methods, have been developed for chiral identification of Trp enantiomers [10,11,12,13,14,15,16]. However, most of the above methods still possess several shortcomings such as requiring complicated sample pretreatments, expensive instruments, and professional laboratories. Compared with the above-reported methods, electrochemical methods have received increasing attention because of their attractive characteristics, such as facile operation, environmental friendliness, fast response, accurate detection, and high sensitivity.
To construct electrochemical chiral sensors, the fabrication of a super-enantioselective chiral element is of vital importance. To date, various chiral selector materials, such as metal–organic frameworks (MOFs), amino acids, and polysaccharides, have been widely used to chiral detect Trp due to their adjustable structures and three-dimensional pore properties [17,18,19,20,21,22,23,24,25,26,27,28,29,30,31]. Among them, β-cyclodextrins (β-CD) and their derivatives have been widely used as chiral detection materials because of their nontoxicity, low price, extensive sources, and biocompatible properties. Meanwhile, the seven glucose units that exist in β-CD can form a truncated-cone-shape with a hydrophobic inner cavity and hydrophilic shell, and it can also offer a suitable chiral microenvironment, thus enabling the facile chiral combination of enantiomers into its cavities. Fu et al. designed an electrochemical chiral sensor (β-CD-PtNPs/GNs) for the chiral recognition of Trps, and the complexation between the –NH2 of l-Trp and β-CD was easier than that of d-Trp, thus leading to a stronger binding ability of l-Trp with a larger oxidation peak [19]. Meanwhile, MWCNTs modified with ferrocene and β-CD (MWCNTs-Fc/β-CD) were designed and utilized as electrochemical chiral sensors for chiral identification of Trp isomers by Niu et al. The stronger binding between l-Trp and β-CD, together with the chiral microenvironment and improvement of the electrochemical signal, allows the above sensor to detect Trp enantiomers with a higher peak current ratio [20]. By using phosphorus nanosheets as the conducting material, a 6-O-α-maltosyl-β-cyclodextrin-based electrochemical chiral sensor was fabricated by Yu et al., and l-Trp can easily arrive at the surface of the as-prepared chiral sensor (BPNSs-G2-β-CD) with a higher oxidation peak [21]. The above results indicate that composite materials based on β-CD and its derivatives show great potential for the recognition of Trp enantiomers.
With the aim of developing an efficient electrochemical chiral detection method for Trp isomers, a sensitive sensor was fabricated by combining the excellent characteristics of Cu2+-functionalized β-CD (Cu-β-CD) and β-CD-based metal–organic frame (β-CD-MOF). Herein, the Cu-β-CD and β-CD-MOF acted as chiral selectors, and carboxylated carbon black (CB-COOH) was introduced to improve the electrical signal. The above electrochemical sensing platform (GCE/Cu-β-CD + β-CD-MOF + CB-COOH) displayed sensitive, selective, and effective chiral recognition toward Trp, and can also be applied to accurately predict the detect the concentration of l-Trp and d-Trp in real samples and the percentage of d-Trp in racemic mixtures.
Experimental section
Materials
β-Cyclodextrin (β-CD, 98%), CuSO4·5H2O, NaOH, KOH, KCl, NaH2PO4·2H2O, Na2HPO4·2H2O, K3Fe(CN)6, K4Fe(CN)6, l-tryptophan (l-Trp, 99%), d-tryptophan (d-Trp, 98%), urea (UA), glucose (GLU), ascorbic acid (AA), and other amino acids were obtained from Sigma Aldrich. The chemicals were of analytical level and deionized water was used. All experiments were performed more than three times.
Characterization
A Perkin Elmer 400 spectrometer was used to record FT-IR data. A Thermo Scientific K-Alpha X-ray photoelectron spectrometer was chosen to record the XPS. XRD was obtained by an XRD-6100 (Shimadzu) and UV–vis absorption spectra were measured based on a UV-2550 PC spectrophotometer. SEM images were obtained using an S-4200 instrument (Hitachi). Cyclic voltammetry (CV) and differential pulse voltammetry (DPV) were measured in a CHI 660E electrochemical workstation. A typical three-electrode method was used: a glassy carbon electrode (GCE, Φ = 3 mm) was selected as the working electrode, a Ag/AgCl electrode acted as the reference electrode, and a platinum sheet was utilized as the auxiliary electrode. Electrochemical impedance spectroscopy (EIS) results were recorded by a VERSASTAT4 electrochemical workstation.
Preparation of Cu-β-CD
First, a total of 150 mg CuSO4·5H2O (15 mL, 0.04 M) was added to 200 mg NaOH solution (10 mL, 0.5 M) containing 227 mg β-CD, and blue precipitate (Cu(OH)2) was formed immediately. After agitation for 12 h, the Cu(OH)2 precipitate was removed via filtration, and 210 mL of ethanol was added to the filtrate to produce a blue suspension, which was left standing for 24 h and filtered to obtain Cu-β-CD. Finally, the products were washed with ethanol and deionized water, and dried at 25 °C for further use [32].
Synthesis of CB-COOH
The preparation of carboxyl derivatives of carbon black (CB-COOH) was similar to reported work [28]. Briefly, 200 mg of highly conductive carbon black and 15 mL of HNO3 (2.2 M) were added to the reactor, and reacted for 24 h at 150 °C. After the temperature was cooled to 25 °C, the reaction products were diluted, filtered, washed with deionized water, and finally dried under vacuum at 80 °C. The relevant FT-IR spectra of CB and CB-COOH are shown in Fig. S1.
Synthesis of β-CD-MOF
β-CD (0.586 g) and KOH (0.224 g) were dissolved in H2O (20 mL) by ultrasound treatment. After filtration with a 0.45-μm filter, the above solution was transferred to a closed glass bottle, 25 mL methanol was added, and the bottle was placed in a drying oven at 50 °C for 3 days so that the methanol steam slowly diffused in the solution. Transparent and colorless crystals were observed on the bottom and wall of the glass bottle. The products were filtered, washed with methanol three times, and dried at 50 °C to collect β-CD-MOF [29].
Fabrication of the chiral electrodes
The GCEs (diameter: 3 mm) were polished with 1.0, 0.3, and 0.05 μm Al2O3 and washed with double-distilled water, ethanol, and Milli-Q water for 1 min. For GCE/Cu-β-CD + β-CD-MOF + CB-COOH, 1 mg CB-COOH, 3 mg Cu-β-CD, and 5 mg β-CD-MOF were dispersed in 1 mL Milli-Q water and ultrasonicated for 2 min. Then, the above hybrid dispersion (5 μL) was added dropwise to the GCE through a physisorption process and dried with infrared radiation heating. The other electrodes were also prepared through a similar process.
Electrochemical chiral detection of Trp isomers
Differential pulse voltammetry (DPV) characterization analysis was used for chiral identification of Trp. The electrodes were immersed in PBS solution (25 mL, 0.1 M, pH: 4 to 8) containing l- or d-Trp (5 mM) for 60 s and then measured by DPV with a potential varying from 0.4 to 1.2 V. The detailed parameters were as follows: pulse amplitude, 0.05 V; pulse width, 0.06 s; sample width, 0.02 s; pulse period, 0.5 s; quiet time, 2 s; scan rate, 0.004 V s−1.
Results and discussion
Materials characterization
The as-prepared Cu-β-CD and β-CD-MOF were investigated by FT-IR. Typical peaks centered at 3400 cm−1, 2927 cm−1, 1080 cm−1, and 1028 cm−1 stand for the stretching vibrations of –OH, –CH2, C–O–C, and C–O of β-CD, and the above characteristic peaks are still retained in Cu-β-CD and β-CD-MOF (Fig. 1). Moreover, the structure of Cu-β-CD was investigated by FT-IR, UV–vis, and XPS. In comparison with β-CD, a characteristic peak at 877 cm−1 can be observed (Fig. S2), referring to the harmonized vibration of HOH and Cu2+. The peak at 581 cm−1 obviously deteriorated, indicating the formation of Cu–O, and the peak at 1449 cm−1 stands for the bending vibration of O–H linked to Cu2+ [32]. Meanwhile, the UV–vis spectrum in Fig. S3 shows a typical absorbance peak of Cu2+ located at 240 nm [32]. XPS analysis can be utilized for further research on the composition of Cu-β-CD. As illustrated in Fig. 2a, three typical peaks, C1s at 286.83 eV, O1s at 533.07 eV, and Cu2p at 933.6 eV, can be observed with atomic percentages of 58.05%, 40.62%, and 1.33%, respectively [33]. The C1s spectra in Fig. 2b were divided into three peaks, implying that three different valence C states exist, including C–C (284.8 eV), C–O (286.0 eV), and C = O (287.2 eV). The O1s spectra for Cu-β-CD in Fig. 2c exhibited four distinct peaks that were 533.2 eV of C–O–C, 532.4.0 eV of –C–OH, 531.5 eV of C = O, and 530.4 eV of Cu–O. Figure 2d shows the Cu2p spectra, and characteristic peaks for Cu2p3/2 was at 933.5 eV, and Cu2p1/2 was at 953.3 eV. The XPS analysis is in agreement with the FT-IR results and further indicates that β-CD was successfully coordinated with Cu.
SEM images were chosen to characterize the morphologies of the synthesized electrode materials. As shown in Fig. 3a, CB-COOH presented a loose particle aggregation state. Meanwhile, β-CD-MOF displays a rectangular shape with different sizes ranging from millimeters to microns, and a fragile property with several cracks can be observed (Fig. 3b), which may offer abundant suitable chiral recognition sites for Trp isomers [32]. The XRD pattern of β-CD-MOF in Fig. S4 displays four typical sharp peaks at 6.4°, 9.1°, 12.6°, and 18.3°, indicating the crystallization of β-CD-MOF [32, 34]. As presented in Fig. 3c, Cu-β-CD is a polyporous cluster nanostructure that can expand active areas and provide abundant identification sites. The above results demonstrated the successful preparation of Cu-β-CD and β-CD-MOF, when Cu-β-CD and β-CD-MOF were self-assembled on CB-COOH, the nanocomposites of CB-COOH + Cu-β-CD + β-CD-MOF retained the polyporous cluster nanostructure but with smooth and wrinkled film structures (Fig. 3d). Meanwhile, as shown in the FT-IR spectra in Fig. S5, the characteristic peaks still exist for CB-COOH + Cu-β-CD + β-CD-MOF, indicating the successful preparation of the composite material without destroying the functional groups.
Electrochemical properties
The CV and EIS experiments were carried out to further study the electrochemical behaviors of different working electrodes. The CVs, which were performed in [Fe(CN)6]4−/3− solution (5 mM) with 0.1 M KCl (scan rate: 50 mV s−1), showed a pair of clear oxidation and redox peaks in the range of − 0.2 to 0.6 V for all the measured working electrodes, including GCE, GCE/CB-COOH, GCE/Cu-β-CD, GCE/β-CD-MOF, GCE/Cu-β-CD + CB-COOH, GCE/β-CD-MOF + CB-COOH, GCE/Cu-β-CD + β-CD-MOF, and GCE/Cu-β-CD + β-CD-MOF + CB-COOH (Fig. 4a). It is obvious that the peak current of GCE/CB-COOH is much higher than that of the other electrodes due to its excellent electron transport capacity, and the peak currents of GCE/β-CD-MOF, GCE/Cu-β-CD, and GCE/Cu-β-CD + β-CD-MOF were weaker than those of the other working electrodes owing to their poor conductivity.
Meanwhile, the electroactive areas of the above electrodes were calculated using the following equation [15, 18]:
Ip stands for the peak current of the anode, n is the number of transferred electrons, A is the working electrode area, D stands for the diffusion coefficient of 7.6 × 10−6 cm2 s−1, and C and ν stand for the amount of [Fe(CN)6]4−/3− and scan rate, respectively. The calculated electroactive areas of GCE, GCE/CB-COOH, GCE/Cu-β-CD, GCE/β-CD-MOF, GCE/Cu-β-CD + CB-COOH, GCE/β-CD-MOF + CB-COOH, GCE/Cu-β-CD + β-CD-MOF, and GCE/Cu-β-CD + β-CD-MOF + CB-COOH were 4.92 mm2, 8.58 mm2, 5.31 mm2, 5.89 mm2, 6.69 mm2, 7.71 mm2, 3.45 mm2, and 7.13 mm2, respectively. The electroactive area of GCE/Cu-β-CD + β-CD-MOF + CB-COOH was approximately 1.45 times of the bare GCE, which indicating that the Cu-β-CD + β-CD-MOF + CB-COOH can introduce more active area with the compatibility of Trp isomers.
EIS spectra were selected to deeply investigate the charge transfer ability for the above working electrodes. As shown in the Nyquist diagrams of Fig. 4b, the straight line at low frequencies corresponds to the diffusion-limiting electrochemical process, whereas the diameter of the semicircle at high frequencies corresponds to the electron transfer resistance (Rct). We should note here that although electrostatic repulsions between COO− groups on the CB and Fe(CN)63−/4− might cause the conductive property to decrease, the Rct of GCE/CB-COOH still displayed the smallest diameter of the semicircle, which was ascribed to the large electroactive surface area of CB and it could facilitate electron transfer and accelerate during the whole redox process [35]. The Rct can be increased with the addition of Cu-β-CD or β-CD-MOF, indicating that electron exchange was inhibited by Cu-β-CD or β-CD-MOF which possesses poor conductivity. Moreover, the calculated Rct values of GCE, GCE/CB-COOH, GCE/Cu-β-CD, GCE/β-CD-MOF, GCE/Cu-β-CD + CB-COOH, GCE/β-CD-MOF + CB-COOH, GCE/Cu-β-CD + β-CD-MOF, and GCE/Cu-β-CD + β-CD-MOF + CB-COOH were 304, 2.73, 200, 184, 170, 103, 502, and 132 Ω, respectively. The order of the above Rct values was illustrated as GCE/Cu-β-CD + β-CD-MOF > GCE > GCE/Cu-β-CD > GCE/β-CD-MOF > GCE/Cu-β-CD + CB-COOH > GCE/Cu-β-CD + β-CD-MOF + CB-COOH > GCE/β-CD-MOF + CB-COOH > GCE/CB-COOH, which agreed closely with the CV results.
Meanwhile, the CVs of GCE/Cu-β-CD + β-CD-MOF + CB-COOH at different sweep speeds were recorded. The anodic peak and the cathodic peak moved toward positive and negative potential as the scan rates increased from 10 to 90 mV s−1, which was ascribed to the increase in the uncompensated resistance (Ru) between the working electrode (WE) and the reference electrode (RE) (Fig. 4c). Moreover, the redox peak current of GCE/Cu-β-CD + β-CD-MOF + CB-COOH presents a linear relationship to the square root of the scan rate, which indicates a diffusion-controlled electrochemical process. The equations were expressed as Ipa = 11.59 ν1/2 − 9.74 (R2 = 0.986) and Ipc = − 7.87 ν1/2 − 21.87 (R2 = 0.998) (Fig. 4d) [18, 20].
Electrochemical chiral identification of Trp enantiomers
The chiral distinction of the Trp isomer with the above electrodes was carried out by DPV. The signal of the electrochemical peak current was located at 0.8 V for all the prepared electrodes, and the oxidation peak currents almost overlapped for l-Trp and d-Trp without any enantiorecognition ability due to the absence of chiral sites for the GCE (Fig. 5a). However, once CB-COOH was modified onto the GCE, the peak current was significantly increased, demonstrating that the electron transfer ability can be improved via CB-COOH, but the Trp enantiomers could still not be recognized (Fig. 5b). Similar to the result of GCE, the oxidation peak currents also overlapped for GCE/β-CD-MOF because of the poor electrical conductivity (Fig. 5c). Compared with the GCE, the recognition efficiency (IL/ID) can be increased for GCE/Cu-β-CD (Fig. 5d–g), but the recognition results were not satisfactory. It was interesting to note that with the modification of Cu-β-CD, β-CD-MOF, and CB-COOH on the GCE, the recognition efficiency (IL/ID) can be further amplified up to 2.13, which may be attributed to the fact that the electroactivity was increased by CB-COOH, and β-CD-MOF + Cu-β-CD not only provides sufficient chiral microenvironment, but also acts as chiral selection centers, easily capturing d-Trp and leading to a higher permeation of l-Trp to the electrode surface with higher oxidation peak currents of l-Trp (Fig. 5h).
Optimization of experimental conditions for chiral identification
To obtain better recognition efficiency for the designed electrochemical sensor, selecting the optimal conditions for Trp isomers detection is of great significance. Firstly, the concentration of Trp isomers for DPV peak current responses was studied. As shown in Fig. S6a, the recognition efficiency (IL/ID) increased gradually with increasing the concentrations of Trp isomers, and reached a maximum value at a concentration of Trp isomers of 5 mM; it was apt to be stable with continued increases in the amounts of l-Trp and d-Trp. Therefore, the optimal concentration for l-Trp and d-Trp was 5 mM. Then, the incubation time between Trp isomers and GCE/Cu-β-CD + β-CD-MOF + CB-COOH was researched. As shown in Fig. S6b, with increasing incubation time from 0 to 60 s, the recognition efficiency (IL/ID) increased obviously. However, with a prolonged incubation time over 60 s, the peak current ratio decreased obviously, which might be ascribed to the Cu-β-CD + β-CD-MOF + CB-COOH falling off from the bare GCE. Hence, the optimal incubation time was selected to be 60 s. In addition, the electrochemical chiral detection of Trp isomers by β-CD-MOF and Cu-β-CD was realized via H-bond formation between chiral centers and Trp, so the temperature and pH should have significant effects on the recognition ability. As shown in Fig. S6c, the highest value of IL/ID for GCE/Cu-β-CD + β-CD-MOF + CB-COOH was obtained at 25 °C. When the temperature was low, the orientation and motion of GCE/Cu-β-CD + β-CD-MOF + CB-COOH and d-Trp were restricted, which can inhibit the formation of favorable H-bonds, thus leading to lower recognition efficiency. Moreover, the H-bonds could be destroyed more frequently with a higher temperature system due to increasing orientation disorder, resulting in reduced recognition efficiency [15, 33, 34]. Additionally, the recognition efficiency changed with pH values varying from 4.0 to 8.0 and reached the maximum recognition efficiency at pH = 6.0, which is approximate to the isoelectric point of Trp (5.89) (Fig. S6d). When the pH values were lower than the isoelectric point, Trp was in a positively charged state, and the high-energy water that existed in Cu-β-CD was in the H+ (H2O)n state (n = 6 or 7). The stronger electrostatic repulsion force could inhibit Trp from entering the cavity of Cu-β-CD and decrease the chiral identification ability [32]. When the pH value was higher than the isoelectric point, Trp was in a state of negative charge, and Cu2+ at the wider opening of Cu-β-CD could bind with Trp based on electrostatic attraction instead of incorporating Trp into the cavity, which also decreased the chiral recognition efficiency [30, 32, 36].
Calibration plots of Trp isomers
The standard calibration curves of Trp isomers with different concentrations under the above-optimized conditions were measured via the DPV method based on GCE/Cu-β-CD + β-CD-MOF + CB-COOH, and the results are depicted in Fig. S7. The peak currents of DPV increased as the concentrations of Trp enantiomers increased, and it presented two linear relationships with the concentrations of Trp isomers changing from 0.3 to 9 mM. Above two linear correlation equations for l-Trp (Fig. S7a) and d-Trp (Fig. S7b) were Ip (μA) = 5.956 CL-Trp (mM) + 20.91 (R2 = 0.984) and Ip (μA) = 2.917 CD-Trp (mM) + 13.10 (R2 = 0.979). Moreover, the limits of detection (LOD) of GCE/Cu-β-CD + β-CD-MOF + CB-COOH for l-Trp and d-Trp were calculated to be 5.48 μM and 11.18 μM (S/N = 3), respectively. In addition, compared with other electrochemical sensors for Trp enantiomers summarized in Table S1, this work presented satisfactory chiral identification efficiency, a wider linear range, and a comparable lower detection limit toward the chiral identification of Trp isomers.
Reproducibility, stability, and selectivity
The repeatability and stability of GCE/Cu-β-CD + β-CD-MOF + CB-COOH were characterized by using five independent and continuous DPV measurements. The results showed that the relative standard deviations (RSDs) of l- and d-Trp were 2.10% and 1.20%, respectively (Fig. S8a). After storing at 4 °C for 7 days, the peak current remained at 95.46% of the initial value by the DPV test. All of the above results indicated the good reproducibility and satisfactory long-term stability of the GCE/Cu-β-CD + β-CD-MOF + CB-COOH-based electrochemical sensor (Fig. S8b).
Furthermore, it was also essential to research the detection ability of sensors with other interfering substances. As shown in Fig. 6a, potentially interfering ions were used to record the ability to resist disturbance. The results showed that Na+ and K+ displayed negligible effects on the current ratio of IL/ID, but the presence of Mg2+, Fe2+, or Ca2+ had a great influence on the value of IL/ID, which may be ascribed to the strong coordination ability that occupied the active site and resulted in poor chiral identification ability [18]. Meanwhile, in the presence of other interferents at 5 mM, such as UA, AA, GLU, and other amino acids, the above chemicals had little effect on the identification efficiency (Fig. 6b), indicating that the as-prepared electrochemical sensor shows excellent anti-interference and satisfying selectivity toward Trp chiral detection.
a The current ratio (IL/ID) of GCE/Cu-β-CD + β-CD-MOF + CB-COOH without interferes, and with the presence of Ca2+, Fe2+, Mg2+, K+, and Na.+. b The current ratio (IL/ID) of GCE/Cu-β-CD + β-CD-MOF + CB-COOH with the presence of 5 mM urea (UA), ascorbic acid (AA), glucose (GLU), l-lysine (l-Lys), l-cysteine (l-Cys), and l-glutamic (l-Glu)
The mechanism of electrochemical identification
To obtain a deeper understanding of the mechanism for the chiral recognition of Trp isomers, different components that exist in GCE/Cu-β-CD+β-CD-MOF+CB-COOH were studied in detail. Usually, the combination with amino acids can enhance the wettability of materials [18, 26]. As illustrated in Fig. S9a, after incubation with d-Trp solution for Cu-β-CD, the contact angle of water was 52.3°, much smaller than that with l-Trp solution treatment (Fig. S9b, 55.6°). Meanwhile, the UV-vis spectroscopy indicated that the typical absorption peak at 286 nm for d-Trp (Fig. S10a) was much larger than that of l-Trp (Fig. S10b). The above results indicated a higher affinity for d-Trp compared with l-Trp isomers for Cu-β-CD. Furthermore, the contact angle of water of β-CD-MOF for incubation with d-Trp solutions (Fig. S11a, 60.1°) was slightly larger than that of l-Trp solutions (Fig. S11b, 51.2°), and the typical absorption peak at 286 nm in Fig. S12 for d-Trp was much smaller than that of l-Trp, demonstrating the higher affinity for l-Trp compared with d-Trp isomers for β-CD-MOF. Based on the above results, it could be reasonable that the as-prepared electrode materials can effectively separate the Trp isomers. Moreover, as illustrated in Fig. S13a, the bare GCE displayed the largest water contact angle of 62.3°, which decreased to 60.7° after the modification of Cu-β-CD+β-CD-MOF+CB-COOH on its surface due to the introduction of hydroxyl and carboxyl groups (Fig. S13b). After treatment with Trp solutions, the contact angle of water decreased to 45.2° for L-Trp and 32.5° for D-Trp (Fig. S13c and S13d), indicating a higher affinity for d-Trp compared with l-Trp isomers. In addition, UV-vis spectroscopy was recorded to further study the mechanism of electrochemical identification. After incubation of chiral materials with Trp isomers, the typical absorption peak at 286 nm for d-Trp was much larger than that of l-Trp (Fig. S14a and S14b), which was in accordance with the results shown in Fig. S9-12 and indicated that the as-prepared chiral materials exhibited stronger affinity for d-Trp than for l-Trp.
To obtain a better understanding of the combination process for the electrode material that self-assembles on the GCE, zeta potential characterization was carried out. As shown in Fig. S15, the zeta potential values for CB-COOH, Cu-β-CD, and β-CD-MOF were − 55.4, 76.7, and − 132.7 mV, respectively, thus indicating that Cu-β-CD can be assemble with the CB-COOH and β-CD-MOF through electrostatic interactions. Herein, the possible chiral recognition mechanism for Trp is illustrated in Scheme 1. Firstly, the CB-COOH can not only improve the electrical signal, but also lead to a self-assembled site for coordination with Cu-β-CD through the interaction between the carboxyl group of CB and Cu2+ at the wide opening of Cu-β-CD [15]. Owing to the formation of a cap-like binuclear hydroxyl bridge by Cu2+ at the wide opening of Cu-β-CD (chiral selector 1) that is fixed on the surface of CB-COOH and β-CD-MOF, it can prevent high-energy water escape from its cavity and force Trp enantiomers to enter through a narrow entrance. The high-energy water is more likely to form H-bonds with the –NH2 of d-Trp due to steric reasons [32, 36], which endows the Cu-β-CD (chiral selector 1) with a higher affinity for d-Trp and leads to a higher permeation of l-Trp to the surface of the working electrode. Meanwhile, the overall specific surface area can be increased by β-CD-MOF (chiral selector 2) upon binding several Cu-β-CD (chiral selector 1) to its surface by electrostatic interaction. The natural β-CD in chiral selector 2 can drive the indole groups of both l- and d-Trp to enter its cavity from the wider opening side, but with easier complexation and a higher affinity for l-Trp. Overall, the above two chiral selectors finally caused more d-Trp to form H-bonds in the cavity of Cu-β-CD, and more l-Trp accumulated on the β-CD-MOF at the electrode surface, thus leading to a higher oxidation peak current for l-Trp than for d-Trp.
Measurement of the binding constant
The binding thermodynamics were evaluated by UV–vis analysis. As shown in Fig. 7, different concentrations of Cu-β-CD + β-CD-MOF (0.1–0.5 mM) were added to the 0.2 mM l- or d-Trp solution and the corresponding UV–vis spectra were recorded. With increasing concentrations of Cu-β-CD + β-CD-MOF, the absorbance intensity at 286 nm increased gradually, which was ascribed to the improvement in the solubility of Trp [22, 26]. The stoichiometry ratio and binding constant of GCE/Cu-β-CD + β-CD-MOF + CB-COOH and Trp isomers could be determined via the classical equation as follows:
where A0 and A stand for the absorbance of Trp at 286 nm in the absence and presence of Cu-β-CD + β-CD-MOF. K represents the binding constant for the Cu-β-CD + β-CD-MOF and Trp system. The differential molar extinction coefficient of Trp in the presence and absence of Cu-β-CD + β-CD-MOF is represented by Δε. [CTrp] and [Cu-β-CD + β-CD-MOF] represent the concentrations of Trp isomers and Cu-β-CD + β-CD-MOF, respectively. K and n were obtained by plotting 1/A0 and 1/[Cu-β-CD + β-CD-MOF], and it can present a straight line only when n = 1, which indicates the formation of a 1:1 complex between Cu-β-CD + β-CD-MOF and Trp isomers. The binding constant K was calculated to be 1.32 for d-Trp, much higher than that of l-Trp (0.689) (Fig. 7c, d). The results demonstrated the favorable binding ability toward d-Trp for the designed sensor, which is in accordance with the results of the DPV and contact angle of water experiments.
Chiral recognition in Trp solutions with different enantiomeric excesses
The ability to predict the ratio of l- and d-Trp isomers with different enantiomeric excesses is of great significance for its practical application. Herein, the designed sensor was chosen to detect the current responses in different ratios of racemic mixture solutions at a fixed concentration of Trp of 5 mM. It was obvious that the peak currents of DPV decreased with increasing the percentages of d-Trp (Fig. 8a), and it presented an excellent linear relationship with R2 = 0.995 (Fig. 8b). The satisfactory results further demonstrated that GCE/Cu-β-CD + β-CD-MOF + CB-COOH can accurately predict the percentage of d-Trp isomers in racemic solutions.
Actual sample analysis
To verify the feasibility and practical application of GCE/Cu-β-CD + β-CD-MOF + CB-COOH, the standard addition method was selected to detect l-Trp and d-Trp in human urine. As shown in Table S2 and S3, the RSD of l-Trp ranged from 3.0 to 4.5%, with a recovery change from 98.2 to 102.8%. The RSD of d-Trp ranged from 3.1 to 4.3%, with a recovery change from 97.9 to 101.1%. The results showed that the designed sensor displayed acceptable application ability in the stereoselective detection of Trp enantiomers.
Conclusion
In summary, a novel and feasible electrochemical chiral sensor based on GCE/Cu-β-CD + β-CD-MOF + CB-COOH was fabricated for the recognition of Trp isomers by DPV. The designed sensor showed a stronger enantioselective affinity toward d-Trp, thus resulting in a higher permeation of l-Trp to the electrode surface and a larger oxidation peak current for l-Trp. The as-prepared electrochemical chiral sensor displayed good sensitivity and selectivity toward the identification of Trp enantiomers with a satisfactory recognition efficiency (IL/ID = 2.13). Due to its good stability, sensitivity, and repeatability, the described method can be utilized to predict the percentages of d-Trp in enantiomer mixture solutions and the amount of Trp in real samples. This work may provide some useful references and open up a new way to fabricate effective electrochemical chiral platforms for detecting chiral molecules.
References
Zhao Y, Askarpour AN, Sun L, Shi J, Li X, Alu A (2017) Chirality detection of enantiomers using twisted optical metamaterials. Nat Commun 8:14180. https://doi.org/10.1038/ncomms14180
Zhang Z, Chen K, Tang K, Chen K, Li R, Sun X, Hu Y, Liu Q, Chen M, Yang H, Chen X (2023) Quinine-fabricated surface-enhanced raman spectroscopy chiral sensing platform enables simultaneous enantioselective discrimination and identification of aliphatic amino acids. Anal Chem 95(11):4923–4931. https://doi.org/10.1021/acs.analchem.2c04839
Wang Z, Ji X, Zhao J, Ji J, Li G, Yang G, Xia H, Hou J (2022) Preparation of fluorescein-modified polymer dots and their application in chiral discrimination of lysine enantiomers. Mikrochim Acta 190(1):29. https://doi.org/10.1007/s00604-022-05608-8
Han C, Hou X, Zhang H, Guo W, Li H, Jiang L (2011) Enantioselective recognition in biomimetic single artificial nanochannels. J Am Chem Soc 133(20):7644–1647. https://doi.org/10.1021/ja2004939
Liang W, Rong Y, Fan L, Dong W, Dong Q, Yang C, Zhong Z, Dong C, Shuang S, Wong W-Y (2018) 3D graphene/hydroxypropyl-β-cyclodextrin nanocomposite as an electrochemical chiral sensor for the recognition of tryptophan enantiomers. J Mater Chem C 6(47):12822–12829. https://doi.org/10.1039/c8tc04448a
Gopal R, Seo CH, Song PI, Park Y (2013) Effect of repetitive lysine-tryptophan motifs on the bactericidal activity of antimicrobial peptides. Amino Acids 44(2):645–660. https://doi.org/10.1007/s00726-012-1388-6
Wu S, Ye Q, Wu D, Tao Y, Kong Y (2020) Enantioselective recognition of chiral tryptophan with achiral glycine through the strategy of chirality transfer. Anal Chem 92(17):11927–11934. https://doi.org/10.1021/acs.analchem.0c02335
Budisa N, Rubini M, Bae JH, Weyher E, Wenger W, Golbik R, Huber R, Moroder L (2002) Global replacement of tryptophan with aminotryptophans generates non-invasive protein-based optical pH sensors. Angew Chem Int Ed 41(21):4121–4066. https://doi.org/10.1002/1521-3773(20021104)41:21
Niu X, Yang X, Li H, Liu J, Liu Z, Wang K (2020) Application of chiral materials in electrochemical sensors. Mikrochim Acta 187(12):676. https://doi.org/10.1007/s00604-020-04646-4
Liu C, Li B, Xu C (2014) Colorimetric chiral discrimination and determination of enantiometric excess of D/L-tryptophan using silver nanoparticles. Microchimica Acta 181(11–12):1407–1413. https://doi.org/10.1007/s00604-014-1281-y
Wu D, Yu Y, Zhang J, Guo L, Kong Y (2018) Chiral Poly(ionic liquid) with nonconjugated backbone as a fluorescent enantioselective sensor for phenylalaninol and tryptophan. ACS Appl Mater Interfaces 10(27):23362–23368. https://doi.org/10.1021/acsami.8b04869
Du Y, Mo Z, Shuai C, Pei H, Wang J, Chen Y, Yue R, He S (2021) Construction of a novel highly electroactive nano-composite film modified with cellulose gum for the electrochemical recognition of tryptophan isomers. J Electroanal Chem 898:115636. https://doi.org/10.1016/j.jelechem.2021.115636
Zhang JD, Kabir KMM, Donald WA (2018) Metal-ion free chiral analysis of amino acids as small as proline using high-definition differential ion mobility mass spectrometry. Anal Chim Acta 1036:172–178. https://doi.org/10.1016/j.aca.2018.06.026
Tayade K, Sonawane M, Torawane P, Singh A, Singh N, Kuwar A (2017) A chemosensor selection for the fluorescence identification of tryptophan (Trp) amino acids in aqueous solutions with nanomolar detection. Sensors Actuat B-Chem 246:563–569. https://doi.org/10.1016/j.snb.2017.02.121
Niu X, Yang X, Mo Z, Liu N, Guo R, Pan Z, Liu Z (2019) Electrochemical chiral sensing of tryptophan enantiomers by using 3D nitrogen-doped reduced graphene oxide and self-assembled polysaccharides. Mikrochim Acta 186(8):557. https://doi.org/10.1007/s00604-019-3682-4
Yang X, Niu X, Mo Z, Guo R, Liu N, Zhao P, Liu Z (2019) Perylene-functionalized graphene sheets modified with chitosan for voltammetric discrimination of tryptophan enantiomers. Mikrochim Acta 186(6):333. https://doi.org/10.1007/s00604-019-3442-5
Mo Z, Niu X, Gao H, Li Z, Meng S, Guo R (2018) Electrochemical recognition for tryptophan enantiomers based on 3, 4, 9, 10-perylenetetracarboxylic acid–chitosan composite film. J Solid State Electrochem 22(8):2405–2412. https://doi.org/10.1007/s10008-018-3960-9
Ji J, Qu L, Wang Z, Li G, Feng W, Yang G (2022) A facile electrochemical chiral sensor for tryptophan enantiomers based on multiwalled carbon nanotube/hydroxypropyl-β-cyclodextrin functionalized carboxymethyl cellulose. Microchem J 175:107133. https://doi.org/10.1016/j.microc.2021.107133
Xu J, Wang Q, Xuan C, Xia Q, Lin X, Fu Y (2016) Chiral recognition of tryptophan enantiomers based on β-cyclodextrin-platinum nanoparticles/graphene nanohybrids modified electrode. Electroanalysis 28(4):868–873. https://doi.org/10.1002/elan.201500548
Niu X, Yang X, Mo Z, Guo R, Liu N, Zhao P, Liu Z, Ouyang M (2019) Voltammetric enantiomeric differentiation of tryptophan by using multiwalled carbon nanotubes functionalized with ferrocene and β-cyclodextrin. Electrochimica Acta 297:650–659. https://doi.org/10.1016/j.electacta.2018.12.041
Zou J, Yu JG (2020) Nafion-stabilized black phosphorus nanosheets-maltosyl-beta-cyclodextrin as a chiral sensor for tryptophan enantiomers. Mater Sci Eng C 112:110910. https://doi.org/10.1016/j.msec.2020.110910
Tao Y, Dai J, Kong Y, Sha Y (2014) Temperature-sensitive electrochemical recognition of tryptophan enantiomers based on beta-cyclodextrin self-assembled on poly(L-glutamic acid). Anal Chem 86(5):2633–2639. https://doi.org/10.1021/ac403935s
Zou J, Chen XQ, Zhao GQ, Jiang XY, Jiao FP, Yu JG (2019) A novel electrochemical chiral interface based on the synergistic effect of polysaccharides for the recognition of tyrosine enantiomers. Talanta 195:628–637. https://doi.org/10.1016/j.talanta.2018.11.107
Li H, Wang L, Yan S, Chen J, Zhang M, Zhao R, Niu X, Wang K (2022) Fusiform-like metal-organic framework for enantioselective discrimination of tryptophan enantiomers. Electrochim Acta 419:140409. https://doi.org/10.1016/j.electacta.2022.140409
Hou Y, Liang J, Kuang X, Kuang R (2022) Simultaneous electrochemical recognition of tryptophan and penicillamine enantiomers based on MOF-modified beta-CD. Carbohydr Polym 290:119474. https://doi.org/10.1016/j.carbpol.2022.119474
Pei H, Wang J, Jin X, Zhang X, Liu W, Guo R, Liu N, Mo Z (2022) An electrochemical chiral sensor based on glutamic acid functionalized graphene-gold nanocomposites for chiral recognition of tryptophan enantiomers. J Electroanal Chem 913:116283. https://doi.org/10.1016/j.jelechem.2022.116283
Huang Y, Wang YY, An R, Gao EQ, Yue Q (2023) Highly efficient versus null electrochemical enantioselective recognition controlled by achiral colinkers in homochiral metal-organic frameworks. ACS Sensors 8(2):774–783. https://doi.org/10.1021/acssensors.2c02320
Zang Z, Hu Z, Li Z, He Q, Chang X (2009) Synthesis, characterization and application of ethylenediamine-modified multiwalled carbon nanotubes for selective solid-phase extraction and preconcentration of metal ions. J Haz Mat 172(2–3):958–963. https://doi.org/10.1016/j.jhazmat.2009.07.078
Smaldone RA, Forgan RS, Furukawa H, Gassensmith JJ, Slawin AM, Yaghi OM, Stoddart JF (2010) Metal-organic frameworks from edible natural products. Angew Chem Int Ed 49(46):8630–8634. https://doi.org/10.1002/anie.201002343
Gong T, Zhu S, Huang S, Gu P, Xiong Y, Zhang J, Jiang X (2022) A renewable electrochemical sensor based on a self-assembled framework of chiral molecules for efficient identification of tryptophan isomers. Anal Chim Acta 1191:339276. https://doi.org/10.1016/j.aca.2021.339276
Liu C, Wang P, Liu X, Yi X, Zhou Z, Liu D (2019) Multifunctional β-cyclodextrin MOF-derived porous carbon as efficient herbicides adsorbent and potassium fertilizer. ACS Sustain Chem Eng 7(17):14479–14489. https://doi.org/10.1021/acssuschemeng.9b01911
Tao Y, Gu X, Deng L, Qin Y, Xue H, Kong Y (2015) Chiral recognition of d-tryptophan by confining high-energy water molecules inside the cavity of copper-modified β-cyclodextrin. J Phys Chem C 119(15):8183–8190. https://doi.org/10.1021/acs.jpcc.5b00927
Li T, Wang Y, Kan X (2021) Electrochemical chiral recognition of tryptophan enantiomers based on copper-modified β-cyclodextrin. J Electroanal Chem 902:115817. https://doi.org/10.1016/j.jelechem.2021.115817
Xu L, Xing CY, Ke D, Chen L, Qiu ZJ, Zeng SL, Li BJ, Zhang S (2020) Amino-functionalized beta-cyclodextrin to construct green metal-organic framework materials for CO(2) capture. ACS Appl Mater Interfaces 12(2):3032–3041. https://doi.org/10.1021/acsami.9b20003
Zhang Y, Liu G, Yao X, Gao S, Xie J, Xu H, Lin N (2018) Electrochemical chiral sensor based on cellulose nanocrystals and multiwall carbon nanotubes for discrimination of tryptophan enantiomers. Cellulose 25(7):3861–3871. https://doi.org/10.1007/s10570-018-1816-1
Tao Y, Gu X, Yang B, Deng L, Bao L, Kong Y, Chu F, Qin Y (2017) Electrochemical enantioselective recognition in a highly ordered self-assembly framework. Anal. Chem. 89(3):1900–1906. https://doi.org/10.1021/acs.analchem.6b04377
Funding
This study received financial support by the Fundamental Research Funds for the Central Universities (2572021BU05), the National Natural Science Foundation of China (NSFC, No. 51803021), and the Natural Science Foundation of Heilongjiang Province (LH2020E007).
Author information
Authors and Affiliations
Contributions
Jiamin Liang: investigation, methodology, writing—original draft; Yuxin Song, Yanan Zhao, and Yue Gao: investigation, methodology, validation. Guang Yang: project administration, investigation, funding acquisition, writing—original draft.
Corresponding author
Ethics declarations
Consent to participate
Consent to participate was obtained from the authors.
Consent for publication
Consent to publication was obtained from the authors.
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Liang, J., Song, Y., Zhao, Y. et al. A sensitive electrochemical sensor for chiral detection of tryptophan enantiomers by using carbon black and β‑cyclodextrin. Microchim Acta 190, 433 (2023). https://doi.org/10.1007/s00604-023-06011-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00604-023-06011-7