Abstract
When thiolactic acid–capped gold nanoclusters (AuNCs@TLA) with strong near-infrared (NIR, 800 nm) emission were applied to detect metal ions, only Ag+ induced the generation of two new emission peaks at 610 and 670 nm in sequence and quenching the original NIR emission. The new peak at 670 nm generated after the 800-nm emission disappeared utterly. The ratiometric and turn-on responses showed different linear concentration ranges (0.10–4.0 μmol·L−1 and 10–50 μmol·L−1) toward Ag+, and the limit of detection (LOD) was 40 nmol·L−1. Especially, the probe exhibited extremely high selectivity and strong anti-interference from other metal ions. Mechanism studies showed that the novel responses were attributed to the anti-galvanic reaction of AuNCs to Ag+ and formation of bimetallic nanoclusters. The two new emission peaks were due to the composition change and size growth of the metal core. Besides, bovine serum albumin (BSA) has been employed as a signal amplifier based on the assembly-induced emission enhancement properties of AuNCs, which improved the LOD to 10 nmol·L−1. Moreover, the ratiometric method is feasible for Ag+ detection in diluted serum with high recovery rates, showing large application potential in the biological system. The present study supplies a novel ratiometric probe for Ag+ with a two-stage response and provides a novel signal amplifier of BSA, which will facilitate and promote the application of NIR-emitted metal nanoclusters in biological system.
Graphical Abstract

Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Silver is widely used in a variety of fields, such as additive, antibacterial materials, pharmaceuticals, conductive materials, electronics, and scientific research [1, 2], resulting in seriously polluting water resources and soil [3, 4]. Ag+ can combine with the free thiol group to inhibit sulfhydryl enzymes and lead to serious threats to human health after entering the body, such as damaging the cardiovascular, immune, and nervous system [5, 6]. And the Guidelines for Drinking-water Quality-World Health Organization bans silver ions above 0.10 mg∙L−1 (927 nmol·L−1) in drinking water to protect public health [7]. Thus, the Ag+ determination has gained more and more attention [8,9,10]. Compared with traditional Ag+ detection methods, such as atomic absorption spectroscopy [11], high-performance liquid chromatography [12], and inductively coupled plasma mass spectrometry [13], luminescence probe presented obvious advantages such as simple operation, low cost, real time, and in situ [14, 15]. Therefore, it is an urgent need to develop a highly selective and sensitive luminescence probe for Ag+ determination in environments and biological systems.
Gold nanoclusters (AuNCs) have attracted considerable interest in the fields of fluorescent sensing, and imaging as their excellent photophysical properties [16, 17]. Most of all, their emission wavelength could be varied from visible to near-infrared (NIR) window by changing the ligands and/or the number of metal atoms within the core, which is conducive to broadening their functions and applications [16, 18, 19]. Since NIR emission can eliminate the interference of auto-fluorescence and reduce the background noise of tissue, NIR-emitted AuNCs have better promise for biological applications [20,21,22]. He et al. have reported that NIR-emitted gold nanoclusters (AuNCs, 810 nm) were in situ fabricated by using an inherent cyclopeptide nanofiber as a template, which was applied in cellular endocytosis pathways and co-localization analysis [23]. Jia’s group has prepared glutathione-capped gold nanoclusters (Au25(SG)18) with excellent NIR emissions around 1050 nm, which could efficiently bind to hydroxyapatite of the bone matrix and enable application in luminescence imaging in vivo in high resolution and contrast [24]. Despite they have made some research progress through a lot of effort [25], the types, fluorescence quantum yields (QYs), and applications of NIR-emitted AuNCs which are still very limited, they need to be expanded and enriched.
In the previous work, we prepared thiolactic acid–capped gold nanoclusters (AuNCs@TLA) through a hydrothermal method, which showed a strong NIR emission (around 800 nm) [26]. Because of their good biological application potential, the NIR-emitted AuNCs@TLA were applied to detect metal ions in biological systems. Interestingly, only Ag+ induced the two new emission peaks at 610 and 670 nm in sequence generation and quenched the original NIR emission of AuNCs@TLA. As the two-stage responses, it showed two linear ranges. Especially, the novel ratiometric response had very high sensitivity and selectivity toward Ag+, and the limit of detection (LOD) was calculated to be 40 nmol·L−1. The mechanism study indicated that the novel responses were attributed to the anti-galvanic reaction (AGR) of AuNCs to Ag+ and bimetallic nanoclusters generation, and the two new emission peaks were due to the size growth and composition change of the metalcore. As the previous results showed that bovine serum albumin (BSA) could improve the NIR emission of AuNCs@TLA via assembly-induced emission enhancement (AIEE) [26], it was employed as a signal amplifier to improve the determination performance. The result showed that it can increase the emission intensity and improve the limit of detection to reach 10 nmol·L−1. Then the ratiometric method was applied to detect Ag+ in diluted serum, which showed good recovery rates, indicating very high biological application potential. The present study supplies a novel ratiometric probe of Ag+ based on NIR-emitted AuNCs, which will facilitate and promote the application of NIR-emitted metal nanoclusters.
Experimental
Titrating Ag+ into AuNCs@TLA in the MES-NaOH buffer
All fluorescence spectra were recorded on a Shimadzu (Japan) RF-5301PC fluorescence spectrophotometer. Samples were dissolved in the MES-NaOH buffer (20.0 mmol·L−1, pH = 6.5) and contained in 1 cm × 1 cm quartz cuvettes (1 mL volume). And the concentration of AuNCs@TLA in the titration experiment was fixed at 50 μg∙mL−1. The titration was performed by adding different amounts of Ag+ to AuNCs in the buffer and monitored by the fluorescence spectrophotometer. The selectivity and competition experiments were also carried out by titrating other metal ions and common anions into the systems.
Determination of Ag+ by AuNCs@TLA in serum
The sample was analyzed as follows: AuNCs@TLA (100 μg⋅mL−1) in 20 mmol·L−1 MES-NaOH buffer (pH = 6.5) was prepared firstly. A sample was first diluted with different volumes of MES-NaOH buffer, then added to the AuNC solution (Vsample:Vbuffer:VAuNCs = 1:49:50, 5:45:50, 10:40:50) and monitored by fluorescence spectrometer. The involved Ag+ was quantitatively determined based on the calibration plot, and the concentration was obtained by calculating the average value. The serum was diluted by MES-NaOH buffer (20.0 mmol·L−1, pH = 6.5) to a concentration of 10% for the next use. And the spiked sample was analyzed by following the above method with minor modification, where the diluted serum was used as a buffer and AuNCs@TLA (100 μg⋅mL−1) were dissolved into the serum buffer.
Results and discussion
Determination of Ag+ based on the AuNCs@TLA
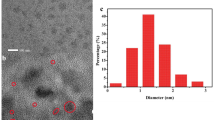
Thiolactic acid–capped gold nanoclusters (AuNCs@TLA) were prepared by following the reported hydrothermal method [26]. As shown in Fig. 1A, the AuNCs showed a NIR emission around 800 nm under 450-nm excitation, which is consistent well with the previous results [26]. And the QY of AuNCs@TLA was measured to be 0.45% by employing a fluorescence spectrophotometer with an integrating sphere. Transmission electron microscopy (TEM) was employed to observe the size and morphology. It shows good monodisperse nanospheres with an average diameter of 1.78 nm by statistical calculation of 200 particles (Fig. S1), indicating that gold nanoclusters have been successfully prepared. Compared to other prepared methods [27, 28], it has the advantages of simple operation, short reaction time, and no additional reductant.
A Fluorescence spectra of AuNCs@TLA (50 μg∙mL−1) in the presence of different amounts of AgNO3 (0, 1.0, 2.0, 3.0, 4.0, 6.0, 8.0, 10, 13, 16, 20, 25, 30, 35, 40, 45, 50, 60, 70, 90, 100, 120 μmol·L−1); B photographs of AuNCs@TLA (50 μg∙mL−1) in the presence of different amounts of Ag+ (0–10 μmol·L−1) under ultraviolet light; C the plot of the corresponding emission intensity ratio (I610/I800) in depending on the concentration of AgNO3 (0–10 μmol·L−1); D the linear response of I610/I800 to Ag+ in the range of 0–4.0 μmol·L−1; E the plot of corresponding emission intensity ratio (I670/I610) in depending on the concentration of AgNO3 (10–120 μmol·L−1); F the linear response of I670/I610 to Ag+ in the range of 10–50 μmol·L−.1
When titrating Ag+ to AuNCs@TLA in MES-NaOH buffer, a new emission peak centered at 610 nm appears and increases, and the original NIR emission of AuNCs@TLA quenches gradually and completely disappears at 10 μmol·L−1 Ag+ (Fig. 1A). After that, another emission peak around 670 nm generates and enhances gradually, while the emission at 610 nm remains unchanged except for the effect of the emission enhancement at 670 nm. At the same time, the color of the solution under UV light (365 nm) gradually brightens from colorless to red (Fig. 1B). As the two-stage response, the concentration-dependent effect was also investigated in two different concentration ranges. Firstly, the intensity ratio between two emission bands at 610 to 800 nm was plotted with the Ag+ concentration in the range from 0 to 10 μmol·L−1 (Fig. 1C), which showed a linear range of 0.10–4.0 μmol·L−1 (Fig. 1D). The results indicate that AuNCs can be used to quantitatively detect trace amounts of Ag+. And the LOD was calculated to be 40 nmol·L−1 through a threefold standard deviation of the blank intensity corresponding to the Ag+ concentration at the linear response plot (3σ/m, where σ is the standard deviation of the blank and m is the slope of the calibration plot). The LOD was much lower than the maximum permissible concentration of 0.10 mg∙L−1 (927 nmol·L−1) recommended by WHO for Ag+ in drinking water, which showed high practical application potential. Secondly, the intensity ratio between two new emission bands at 670 to 610 nm was also plotted with the Ag+ concentration in the range from 10 to 120 μmol·L−1 (Fig. 1E), which exhibited a good linear response in the range from 10 to 50 μmol·L−1 (Fig. 1F). The results show that the probe can also quantitatively detect large amounts of Ag+. By combining the two-stage response to Ag+, the NIR probe can linearly detect Ag+ in both low (0.10–4.0 μmol·L−1) and high (10–50 μmol·L−1) concentration ranges. The lower range can improve the detection sensitivity, and the higher range is conducive to the detection of Ag+ in a broad range. Particularly, the present method is a NIR probe, which has better biological application potential.
To improve the detection performance toward Ag+, several conditions such as pH, concentration of AuNCs, and ionic strength were further optimized. As shown in Fig. S2, the effect of pH is investigated by regulating the pH range from 4.55 to 9.97. The emission intensity of I800 for AuNCs decreases gradually with the pH increase until pH at 6.50, which suggests that acidic condition is conducive to the fluorescence emission of the AuNCs. While in the presence of Ag+ (100 μmol∙L−1), the emission intensity at 670 nm also decreases with the pH increase. Considering the fluorescence intensity ratio and practical application, the weakly acidic condition is favorable for the highly sensitive detection of Ag+ by the AuNCs. For the accuracy of the test results, the pH is finally fixed at 6.5 in the MES-NaOH buffer.
Then the concentration of AuNCs was optimized by monitoring the fluorescence spectra. As depicted in Fig. S3, the intensity ratio of I610/I800 decreases with an increase in the AuNCs concentration up to 125 μg∙mL−1. The concentration and detection sensitivity of AuNCs are considered comprehensively, and 50 μg∙mL−1 is selected as the optimal concentration. In addition, the effect of ionic strength was also investigated by adding NaCl to the system. It shows that NaCl has little effect on the detection of Ag+ (Fig. S4). Therefore, 50 μg∙mL−1 AuNCs@TLA in MES-NaOH buffer (pH = 6.5) is selected as the optimal condition for the detection of Ag+.
The AuNCs@TLA was also applied to detect other common metal ions and common anions in surface water. As shown in Fig. S5A, these metal ions (such as Ca2+, Cd2+, Ce3+, Co2+, Cr3+, Cu2+, Fe2+, Hg2+, K+, Mg2+, Mn2+, Na+, NH4+, Ni2+, Pb2+, and Zn2+) and common anions (such as Br−, Cl−, CO32−, I−, NO3−, S2−, SO32−, and SO42−) can not induce any new emission peaks as Ag+, except for a few of them quenching the NIR emission (800 nm). When calculating the changes in the emission intensity ratio of I610/I800, it exhibited very high selectivity toward Ag+ over other metal ions and common anions (Fig. S5B). In addition, when introducing organic species (cysteine and glutathione) to the system, they did not induce any new emission peaks as well (Fig. S5B). The titration experiments were also conducted by mixing Ag+ and other metal ions and common anions to evaluate the anti-interference property of AuNCs. The results show that they have little effect on the quantitative detection of Ag+ (Fig. S6). However, the red emission of AuNCs and Ag+ could be quenched by some metal ions (including Cr3+ and Cu2+) weakly, which will decrease the intensity ratio and interference with the detection result, being limit the application of this method. The AuNCs were a ratiometric probe for Ag+ detection, which improved the anti-interference and eliminated the effects of photobleaching significantly. And the stabilities of the AuNCs@TLA and the detection method were also studied by long-term storage (1 month) at 4 ℃, which showed that the luminescence emission of the nanoclusters almost remained constant, and the response to Ag+ also remained unchanged. Therefore, the proposed method can be used to accurately detect trace amounts of Ag+ in the environment.
Employing bovine serum albumin as a signal amplifier
In the previous work, we observed that BSA can directly induce emission enhancement of AuNCs@TLA based on AIEE [26]. Thus, BSA was employed as a signal amplifier, and Ag+ was directly titrated to the assembly of AuNCs@TLA and BSA (AuNCs-BSA) in MES-NaOH buffer and monitored by fluorescence spectra. As shown in Fig. 2A, it can also induce the generation and enhancement of new peaks at 610 nm and 670 nm in turn and quenching the NIR emission at 800 nm. Besides, in the presence of 4.0 μmol·L−1 BSA, the emission intensities at 610, 670, and 800 nm increase about 3.5-, 3.9-, and 2.7-fold, respectively (Fig. S7). As a comparison, when BSA was joined to the system after Ag+ addition, it can also enhance the emission at 610 nm and 670 nm (Fig. S8). Especially, there is little effect on the amplification factor in either addition order, which indicates that the addition order does not affect the final luminescence emission, being attributed to the completely different response mechanism. Therefore, BSA can significantly improve Ag+ determination as a signal amplifier. Then the concentration-dependent effect of Ag+ was also investigated. The intensity ratio between two emission bands at 610 to 800 nm was plotted with the Ag+ concentration in the range from 0 to 10 μmol·L−1 (Fig. 2C). It shows a linear range from 0.10 to 1.0 μmol·L−1 (Fig. 2D), and a LOD was calculated to be 10 nmol·L−1. The intensity ratio between two new emission bands at 670 to 610 nm was plotted with the Ag+ concentration in the range from 10 to 120 μmol·L−1 (Fig. 2E), which exhibits a good linear response in the range from 10 to 50 μmol·L−1 (Fig. 2F). Compared with the results without BSA, the determination parameters are greatly improved. The present method has a ratiometric response, which has many advantages, such as strong anti-interference ability, anti-photobleaching, very high selectivity, and few false positive results. Compared with the previous reports [29,30,31,32], though the detection performance of the proposed method is not as good as those reported (Table S1), the anti-interference ability is more outstanding, especially, since the present method is a NIR and ratiometric probe, it has better biological application potential.
A Fluorescence spectra of AuNCs-BSA (50 μg∙mL−1 and 6 μmol·L−1) in the presence of different amounts of Ag+ (0, 0.3, 0.5, 0.8, 1.0, 2.0, 3.0, 4.0, 6.0, 8.0, 10, 13, 20, 25, 30, 40, 50, 60, 80, 90, 100, 120 μmol·L−1); B photographs of AuNCs@TLA in the presence of different amounts of Ag+ (0–100 μmol·L−1) under ultraviolet light; C the plot of the corresponding emission intensity ratio (I610/I800) in depending on the concentration of AgNO3 (0–10 μmol·L−1); D the linear response of I610/I800 to Ag+ in the range of 0–1.0 μmol·L−1; E the plot of the corresponding emission intensity ratio (I670/I610) in depending on the concentration of AgNO3 (10–120 μmol·L−1); F the linear response of I670/I610 to Ag+ in the range of 10–50 μmol·L−1
Intrinsic mechanism of AuNCs@TLA responses to Ag+
UV–Vis absorption spectra were employed to monitor the changes of AuNCs@TLA in the presence of Ag+. As shown in Fig. S9, the absorption peak around 300 nm increases weakly along with the addition of Ag+, which suggests that the surface electron energy of AuNCs@TLA is changed with the incorporation of Ag+, being consistent well with the luminescence changes. The result indicates that the luminescence species induced by Ag+ are distinct from the original AuNCs@TLA, and the appearance and enhancement of new emissions could be attributed to the structure or composition changes of the nanoclusters.
Recently, AGR has been revealed for constructing bimetallic nanoclusters and improving the optical properties, where noble metals in nanoclusters could reduce active metal ions, such as AuNCs to Ag+ [33, 34]. In consequence, XPS was employed to determine the content and oxidation states of AuNCs@TLA in the presence of Ag+. The binding energy of Au is observed at 85.1 and 88.8 eV (Fig. 3A). After splitting the Au 4f7/2 peak into two components at 84.2 and 85.1 eV, it can be calculated that the final product contains 13.8% Au(0) and 86.2% Au(I). Compared to the results of AuNCs@TLA (40.3% Au(0) and 59.7% Au(I) [26]), it suggests that the metal core changes from Au(0) to Au(I) in the presence of Ag+, while the binding energy of silver shows extensive peaks at 368.4 and 374.4 eV (Fig. 3B), being assigned for the Ag 3d5/2 and Ag 3d3/2, respectively. Accordingly [32, 35, 35], the Ag 3d5/2 peak can be assigned to the appearance of silver in its zero-valent in the system. The results indicate a redox reaction occurs between Au(0) in the core and Ag+, which induces the conversion of Au(0) to Au(I) and the generation of Ag(0). Therefore, the luminescence response is mainly attributed to the AGR of AuNCs@TLA to Ag+ and forming bimetallic nanoclusters (Au-AgNCs). XPS spectrum of S 2p and O 1 s were then investigated, respectively. The binding energy of S 2p at 163.4 eV (Fig. S10A) can be assigned to the S atom bound to the core in both AuNCs and Au-AgNCs [36, 37]. And no typical oxidized sulfur with a peak around 168.2 eV is observed, indicating the complete formation of Au(I) − TLA complex on the surface of the nanoclusters. In addition, the binding energy of O 1 s at 532.2 eV (Fig. S10B) corresponds to the C − O − and C = O species, which does not show any change in the presence of Ag+ [38, 39], suggesting no Ag+-carboxylate shell forms on the surface of Au-AgNCs. Therefore, the Ag+-induced emission changes are attributed to the Ag+ reduction by the core of AuNCs and deposition on the surface, which induces the formation of Au-AgNCs with a strong red emission.
Then TEM was employed to reveal the size and morphology changes. In the presence of 8.0 and 100 μmol·L−1 Ag+, the average diameters of AuNCs increase to 2.13 and 2.38 nm through statistics and calculations from the typical TEM images (Fig. 4). A large number of studies have shown that the luminescence properties of metal nanoclusters are closely related to the size and composition of the metal core. For example, Zheng et al. have prepared PAMAM encapsulated AuNCs with different sizes and emissions, including Au5 (385 nm), Au8 (455 nm), Au13 (510 nm), Au23 (760 nm), and Au31 (866 nm) [40, 41]. Roy et al. have also obtained a series of glutathione-capped AuNCs with tunable emissions from blue to NIR windows by increasing the core size [42]. These size-dependent emission changes suggest that the two-stage luminescence changes in the present work could be attributed to the size growth and composition changes of the metal core.
To further reveal the interaction between AuNCs@TLA and Ag+, a small thiolate ligand of cysteine was employed in the system, which could strongly bind to Ag+ via the formation of cysteine − Ag+ complexes. In the presence of cysteine, the 610-nm emission of Au-AgNCs1@TLA increases significantly rather than returns to NIR emission of AuNCs@TLA, while that at 670 nm of Au-AgNCs2@TLA also enhances rather than recovers (Fig. S11), indicating the irreversible emission changes. The Ag+ addition increased the particle size and the ligands number remained unchanged, which induced the surface coverage to decrease considerably. Therefore, the cysteine-induced emission enhancement of Au-AgNCs can be attributed to the increased density of the surface ligand. The result further demonstrates that Ag atoms are deposited on the surface of the gold core resulting in size growth and low surface coverage. Besides, ethylenediaminetetraacetic acid (EDTA) was also employed to bind free Ag+ in the system. As depicted, EDTA cannot cause any emission change of Au-AgNCs@TLA (Fig. S12), which suggests that no Ag+-carboxylate shell forms on the surface of Au-AgNCs. Therefore, the luminescence enhancement induced by BSA is attributed to the AIEE property, while the Ag+-induced emission change is ascribed to the AGR and size growth (Scheme 1). In the present method for the determination of Ag+, BSA acts as a signal amplifier, which can greatly increase the detection performance and improve the biological application potential.
Determintion of Ag+ in diluted serum
Since cysteine can increase the emission intensity of AuNCs@TLA and Ag+ greatly, which will interfere with the accurate detection of Ag+, it needs to be optimized to eliminate these interferences. Thus, BSA had been employed as a shielding agent, and the interference effects of cysteine and glutathione were investigated by introducing BSA to the system. As shown in Fig. S13, the fluorescence response toward Ag+ did not show much difference in the presence of cysteine and glutathione (0.50 mmol·L−1), which may be due to the shielding effect or protective effect of BSA. Therefore, in the presence of BSA, the system can achieve accurate quantitative detection of Ag+ in biological systems.
The proposed method was further employed to detect Ag+ in diluted serum. Firstly, the original concentration of Ag+ in the serum was assayed by employing inductively coupled plasma-mass spectrometry, which showed no Ag+ was present in the serum. Then titrating Ag+ to the AuNCs@TLA in diluted serum, as shown in Fig. S14, it could also induce new emissions centered at ~ 610 and 670 nm in order. And the response shows good linear with the Ag+ concentration in the range of 0.10–4.0 μmol·L−1 and 10–50 μmol·L−1, which indicates that the proposed method can be used to detect trace amounts of Ag+ in serum. In addition, different amounts of Ag+ were spiked to diluted serum, respectively, to get a final concentration between 0–1.0 and 25–40 μmol·L−1. The spiked samples were quantitatively measured through mixing with NIR-emitted AuNCs and calculated by comparing them with the standard curve equation. The recovery rates were calculated to be between 95.26% and 103.67% (Table 1), being accurate enough and satisfactory for practical purposes. Therefore, the proposed method has significant application potential for Ag+ determination in complex environments.
Conclusions
Briefly, the NIR emitted AuNCs@TLA were applied to detect Ag+, which induced new emission peaks at ~ 610 and 670 nm in sequence and quenched the initial emission gradually. The novel response shows very high selectivity toward Ag+ and strong anti-interference among other common metal ions. Further study showed that the novel luminescence responses were attributed to the anti-galvanic reaction (AGR) of AuNCs to Ag+ and forming bimetallic nanoclusters, while the two new emission peaks were due to the composition change and size growth of the metal core. When employing BSA as a signal amplifier based on the assembly-induced emission enhancement (AIEE) properties of AuNCs, it improved the detection performance significantly. In addition, the method can be used to detect Ag+ in serum with very good recovery rates, showing high potential for practical application. However, the emission of AuNCs could be quenched by some metal ions (including Cr3+ and Cu2+) weakly, which will decrease the intensity ratio and limit the application of this method. Therefore, the present study provides a novel ratiometric probe of Ag+ with a two-stage response and employs BSA as a signal amplifier to improve the detection performance, which will promote more studies focusing on the applications of NIR-emitted MNCs in biological systems.
Data Availability
All data generated or analysed during this study are included in this published article and its supplementary information files.
References
Lalley J, Dionysiou DD, Varma RS, Shankara S, Yang DJ, Nadagouda MN (2014) Silver-based antibacterial surfaces for drinking water disinfection - an overview. Curr Opin Chem Eng 3:25–29
Dai F, Xie M, Wang Y, Zhang L, Zhang Z, Lu X (2022) Synergistic effect improves the response of active sites to target variations for picomolar detection of silver ions. Anal Chem 94:10462–10469
Rasheed T, Bilal M, Nabeel F, Iqbal HMN, Li C, Zhou Y (2018) Fluorescent sensor based models for the detection of environmentally-related toxic heavy metals. Sci Total Environ 615:476–485
Liu T, Fu L, Yin C, Wu M, Chen L, Niu N (2022) Design of smartphone platform by ratiometric fluorescent for visual detection of silver ions. Microchem J 174:107016
Choi S, Lee G, Park IS, Son M, Kim W, Lee H, Lee SY, Na S, Yoon DS, Bashir R, Park J, Lee SW (2016) Detection of silver ions using dielectrophoretic tweezers-based force spectroscopy. Anal Chem 88:10867–10875
Gao Z, Liu GG, Ye H, Rauschendorfer R, Tang D, Xia X (2017) Facile colorimetric detection of silver ions with picomolar sensitivity. Anal Chem 89:3622–3629
Wang YW, Wang M, Wang L, Xu H, Tang S, Yang HH, Zhang L, Song HA (2017) Simple assay for ultrasensitive colorimetric detection of Ag+ at picomolar levels using platinum nanoparticles. Sensors 17:2521
Benton EN, Marpu SB, Omary MA (2019) Ratiometric phosphorescent silver sensor: detection and quantification of free silver ions within silver nanoparticles. ACS Appl Mater Interfaces 11:15038–15043
Song Y, Wang X, Liu H, Wang X, Li D, Zhu HL, Qian Y (2022) A high selective colorimetric fluorescent probe for detection of silver ions in vitro and in vivo and its application on test strips. Talanta 246:123366
Chun KY, Oh Y, Rho J, Ahn JH, Kim YJ, Choi HR, Baik S (2010) Highly conductive, printable and stretchable composite films of carbon nanotubes and silver. Nat Nanotechnol 5:853–857
Musil S, Kratzer J, Vobecký M, Benada O, Matoušek T (2010) Silver chemical vapor generation for atomic absorption spectrometry: minimization of transport losses, interferences and application to water analysis. J Anal At Spectrom 25:1618–1626
Hanley TA, Saadawi R, Zhang P, Caruso JA, Landero-Figueroa J (2014) Separation of silver ions and starch modified silver nanoparticles using high performance liquid chromatography with ultraviolet and inductively coupled mass spectrometric detection. Spectrochim Acta B 100:173–179
Krachler M, Mohl C, Emons H, Shotyk W (2002) Analytical procedures for the determination of selected trace elements in peat and plant samples by inductively coupled plasma mass spectrometry. Spectrochim Acta B 57:1277–1289
Săcărescu L, Chibac-Scutaru AL, Roman G, Săcărescu G, Simionescu M (2023) Selective detection of metal ions, sulfites and glutathione with fluorescent pyrazolines: a review. Environ Chem Lett 21:561–596
Hu T, Lai Q, Fan W, Zhang Y, Liu Z (2023) Advances in portable heavy metal ion sensors. Sensors 23:4125
Kang X, Zhu M (2019) Tailoring the photoluminescence of atomically precise nanoclusters. Chem Soc Rev 48:2422–2457
Chakraborty I, Pradeep T (2017) Atomically precise clusters of noble metals: emerging link between atoms and nanoparticles. Chem Rev 117:8208–8271
Song X, Zhu W, Ge X, Li R, Li S, Chen X, Song J, Xie J, Chen X, Yang H (2021) A new class of NIR-II gold nanocluster-based protein biolabels for in vivo tumor-targeted imaging. Angew Chem Int Edit 60:1306–1312
Xiao Y, Wu Z, Yao Q, Xie J (2021) Luminescent metal nanoclusters: biosensing strategies and bioimaging applications. Aggregate 2:114–132
Yamazaki S, Oh E, Susumu K, Medintz IL, Scott AM (2021) Excited-state dynamics of photoluminescent gold nanoclusters and their assemblies with quantum dot donors. J Phys Chem C 125:12073–12085
Kim J, Piao Y, Hyeon T (2009) Multifunctional nanostructured materials for multimodal imaging, and simultaneous imaging and therapy. Chem Soc Rev 38:372–390
Crawford SE, Hartmann MJ, Millstone JE (2019) Surface chemistry-mediated near-infrared emission of small coinage metal nanoparticles. Acc Chem Res 52:695–703
He K, Zhu J, Gong L, Tan Y, Chen H, Liang H, Huang B, Liu J (2021) In situ self-assembly of near-infrared-emitting gold nanoparticles into body-clearable 1D nanostructures with rapid lysosome escape and fast cellular excretion. Nano Res 14:1087–1094
Li D, Liu Q, Qi Q, Shi H, Hsu EC, Chen W, Yuan W, Wu Y, Lin S, Zeng Y, Xiao Z, Xu L, Zhang Y, Stoyanova T, Jia W, Cheng Z (2020) Gold nanoclusters for NIR-II fluorescence imaging of bones. Small 16:2003851
Li D, Chen Z, Mei X (2017) Fluorescence enhancement for noble metal nanoclusters. Adv Colloid Interfac 250:25–39
Liang QY, Wang C, Li HW, Qi DY, Wu Y (2023) Controlled-fabrication and assembly-induced emission enhancement (AIEE) of near-infrared emitted gold nanoclusters capped by thiolactic acid. J Mol Liq 377:121516
Fu L, Gao X, Dong S, Hsu HY, Zou G (2021) Surface-defect-induced and synergetic-effect-enhanced NIR-II electrochemiluminescence of Au−Ag bimetallic nanoclusters and its spectral sensing. Anal Chem 93:4909–4915
Wan XK, Xu WW, Yuan SF, Gao Y, Zeng XC, Wang QM (2015) A near-infrared-emissive alkynyl-protected Au24 nanocluster. Angew Chem Int Ed 54:9683–9686
Lee J, Park J, Lee HH, Park H, Kim HI, Kim WJ (2015) Fluorescence switch for silver ion detection utilizing dimerization of DNA-Ag nanoclusters. Biosens Bioelectron 68:642–647
Zhao XE, Lei C, Gao Y, Gao H, Zhu S, Yang X, You J, Wang H (2017) A ratiometric fluorescent nanosensor for the detection of silver ions using graphene quantum dots. Sens Actuators B-Chem 253:239–246
Hao C, Wei J, Zong S, Wang Z, Wang H, Cui Y (2023) Highly sensitive and specific detection of silver ions using a dual-color fluorescence co-localization strategy. Analyst 148:675–682
Zhou Z, Cen J, Jiang N, Sun Y, Li Z, Yang L (2023) A ratiometric fluorescent nanoprobe based on CdSe quantum dots for the detection of Ag+ in environmental samples and living cells. Spectrochim Acta A 290:122302
Gan Z, Xia N, Woo Z (2018) Discovery, mechanism, and application of antigalvanic reaction. Acc Chem Res 51:2774–2783
Liu X, Astruc D (2017) From galvanic to anti-galvanic synthesis of bimetallic nanoparticles and applications in catalysis, sensing, and materials science. Adv Mater 29:1605305
Liu J, Yuan XX, Li HW, Wu Y (2017) Hydrothermal synthesis of novel photosensitive gold and silver bimetallic nanoclusters protected by adenosine monophosphate (AMP). J Mater Chem C 5:9979–9985
Jiang J, Conroy CV, Kyetny MM, Lake GJ, Padelford JW, Ahuja T, Wang G (2014) Oxidation at the core-ligand interface of au lipoic acid nanoclusters that enhances the near-IR luminescence. J Phys Chem C 118:20680–20687
Sun J, Wu H, Jin Y (2014) Synthesis of thiolated Ag/Au bimetallic nanoclusters exhibiting an anti-galvanic reduction mechanism and composition-dependent fluorescence. Nanoscale 6:5449–5457
Chen Y, Yang T, Pan H, Yuan Y, Chen L, Liu M, Zhang K, Zhang S, Wu P, Xu J (2014) Photoemission mechanism of water-soluble silver nanoclusters: ligand-to-metal-metal charge transfer vs strong coupling between surface plasmon and emitters. J Am Chem Soc 136:1686–1689
Wang Y, Liu L, Gong L, Chen Y, Liu J (2018) Reactivity toward Ag+: a general strategy to generate a new emissive center from NIR-emitting gold nanoparticles. J Phys Chem Lett 9:557–562
Zheng J, Zhang CW, Dickson RM (2004) Highly fluorescent, water-soluble, size-tunable gold quantum dots. Phys Rev Lett 93:077402
Zheng J, Zhou C, Yu M, Liu J (2012) Different sized luminescent gold nanoparticles. Nanoscale 4:4073–4083
Roy S, Baral A, Bhattacharjee R, Jana B, Datta A, Ghosh S, Banerjee A (2015) Preparation of multi-coloured different sized fluorescent gold clusters from blue to NIR, structural analysis of the blue emitting Au-7 cluster, and cell-imaging by the NIR gold cluster. Nanoscale 7:1912–1920
Funding
This study received financial supports from the National Natural Science Foundation of China (Nos. 21875085) and the Jilin Provincial Science and Technology Development Plan Project (No. 20230204040YY).
Author information
Authors and Affiliations
Contributions
Qi-Yu Liang: data curation, investigation, software, methodology. Chong Wang: methodology, validation. Hong-Wei Li: data curation, supervision, writing—review and editing. Yuqing Wu: methodology, supervision, funding acquisition, writing—review and editing. All authors have approved and read the final manuscript.
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Liang, QY., Wang, C., Li, HW. et al. A ratiometric luminescence probe for selective detection of Ag+ based on thiolactic acid–capped gold nanoclusters with near-infrared emission and employing bovine serum albumin as a signal amplifier. Microchim Acta 190, 374 (2023). https://doi.org/10.1007/s00604-023-05955-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00604-023-05955-0