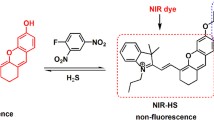
Abstract
The selective detection of hydrogen sulfide in physiological and pathological processes has gained substantial attention in recent years. However, the real-time detection of hydrogen sulfide remains an elusive goal. In this work, a new type of hemicyanidin-based fluorescent “turn-on” probe NTR-HS (Ex = 680 nm, Em = 760 nm) was developed to detected H2S in a very short time (3 min). The fluorescence quantum yield is 0.15 and accompanied with a noticeable color change from violet to blue that can be used to detect H2S in the range 1.04 × 10−7–4 × 10−5 M with a limit of detection of 1.04 x 10-7 M. The NTR-HS probe was also used for imaging of endogenous hydrogen sulfide and mitochondrial localization in HCT116 and HeLa cells. The detection mechanism was studied through fluorescence, UV–Vis, NMR, and mass analysis. Notably, the probe was successfully used to imaging H2S in mice and locating hydrogen sulfide in the large intestine of mice.
Graphical abstract

Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Hydrogen sulfide, as the third gaseous transport agent (after nitric oxide and carbon monoxide), is closely related to physiological diseases and functions in the body [1,2,3,4,5,6], such as take part in the regulation of the function of blood vessels and nervous systems [7,8,9,10]; participating in the process of respiratory diseases and playing a role in preventing oxidative damage [11,12,13,14,15]. Meanwhile, it will cause human poisoning when the concentration of hydrogen sulfide in the air is higher than 10 ppm, and lead to some symptoms such as respiratory irritation, physical weakness, and dyspnea [16,17,18,19,20]. The endogenous H2S is mainly derived from the reaction of thiol-containing compounds with corresponding decomposition by four enzymes: cystathionine beta-synthase (CBS), cystathionine gamma-lyase (CSE), 3-mercaptopyruvate sulfotransferase (MST), and cysteine aminotransferase (CAT) [21,22,23,24,25]. In addition, there is also a cysteine dethiolase in the liver of humans and animals, which can catalyze the decomposition of L-cysteine to generate hydrogen sulfide and ammonia, indirect detection of desulfurase can be achieved according to this process, may be another effective way to monitor liver function. When cancer cells appear in the large intestine, the hydrogen sulfide content will be higher than that expressed by normal colorectal cells, so monitoring the hydrogen sulfide in the large intestine can prevent the occurrence of colorectal cancer in time. Therefore, real-time measurement of H2S is great significance to explore its biological roles and behaviors in vitro and in vivo. Compared with other sophisticated methods for real-time detection of H2S release, such as methylene blue (MB) assay, ion-selective electrode method (ISE) and gas chromatography) [26,27,28,29], fluorescent probe assay with the advantages of high sensitivity, fast response time, strong real-time detection ability, and simple operation is considered as a reliable method [30,31,32,33,34]. Although various types of fluorescent probes for the detection of H2S have been developed based on copper sulfide precipitation, reduction, or addition or ether reaction thiolysis and other methods, there are usually existed problems such as many synthesis steps and slow reaction speed. We have investigated many hydrogen sulfide probes of the same type and compared with our work (Table S1). They have their own outstanding advantages, but there also have some shortcomings in some aspects at the same time. At present, there are few fluorescent probes that have the advantages of simple and quick identification mechanism, fast identification speed, strong anti-interference ability, low detection limit, larger emission wavelength, naked eye colorimetric identification, and application in actual biological samples at same time [35,36,37,38,39], like this work. Integrates superior performance in all aspects, which is the purpose and highlight of this work.
In the present work, we link the hemicyanidin and benzothiazole units through an unsaturated ethylenic bond that has the capable of transfering conjugation effects as the fluorophore, and use easily detached nitrobenzoate units as reactive groups for the detection of hydrogen sulfide (Scheme 1). The probe shows a highly selective response to hydrogen sulfide among various potential competing anions and other biothiol compounds, with strong anti-interference ability. Therefore, it can serve as a potential targeting molecule for 3-MST-rich mitochondrial organelles in cells. In addition, colorectal cancer cells can be effectively distinguished from normal cells and other cancer cells by detecting hydrogen sulfide in living cells.
Materials and methods
Materials and instruments
The drugs used in the experiment were provided by commercial sources and can be used directly without further purification. The anions used in the test were all derived from the sodium, potassium, and calcium salts of their anion. Column chromatography silica gel was 200–300 mesh. Compounds 1 to 3 were synthesized by referring to the reported literatures [16]. The Bruker-AV-400 NMR spectrometer was used to detect 1H NMR (400 Hz) and 13C NMR (101 Hz) at room temperature. The IR spectrum was measured with Nicolet 670 FT-IR spectrophotometer. Mass spectrometry (MS) were measured on a Thermo Scientific LTQ Orbitrap XL mass spectrometer. The UV–Vis absorption spectrum was measured with a Perkin Elmer Lamda-25 UV–Vis spectrophotometer. The fluorescence spectrum was recorded on the F-7000 fluorescence spectrophotometer. Fluorescence imaging of living cells were taken with the 610 × 720 mnm channel under a Nikon confocal fluorescence microscope. IVIS Lumina XRMS Series III was used to perform in vivo imaging of living mice under the 650 × 760 mnm channel. Cell of Hela and HCT116 and C57 mice (male, 7 − 8 weeks old, 25 g) were obtained from Henan University Medical College. All animal experiments were performed in accordance with the guidelines issued by The Ethical Committee of Henan University Medical College.
Synthesis and characterization
Design and synthesis of NTR-HS
The probe displayed a weak fluorescence emission due to the electron withdrawing fluorophore, after hydrogen sulfide splitting the carbonyl–oxygen bond and releasing the NTR-OH, the probe showed a “turn-on” fluorescent response by intramolecular charge transfer (ICT) process. The simple recognition mechanism and fast recognition speed and the turn-on type fluorescence response makes the detection process of the probe in cells and organisms easier, more efficient and faster, and stronger anti-interference ability also help the probe imaging in cells and organisms.
First, methyl benzothiazole was ethylated to form quaternary ammonium salt. Second, the quaternary ammonium salt constructed a conjugate structure with hemianthocyanin derivative through a condensation reaction for improving the fluorophore skeleton. Finally, the phenolic hydroxyl was converted into a recognition unit of probe NTR-HS (Scheme 1). Detailed compound synthesis steps are presented in ESI. The structures of intermediate and probe NTR-HS were confirmed by 1H and 13C nuclear magnetic resonance (NMR) spectroscopy, IR, and electrospray ionization-mass spectrometry (ESI–MS) (Fig. S1-S6).
Spectral analysis
The probe NTR-HS was first dissolved in analytically pure DMSO and its concentration was diluted to 0.01 M, and then prepare a DMSO-PBS solution with a volume ratio of 2:1. Before the detection starts, add 10 μL of NTR-HS with a concentration of 0.01 M to 3 mL of DMSO-PBS (v/v = 2/1) solution, and then add the analytes to start the detection.
Imaging of cells
HeLa cells are a very representative ideal cell model and are widely used in tumor research, biological experiments, and cell culture. HCT116 is a human colon cancer cell with higher levels of hydrogen sulfide than other cancer cells. Therefore, detection and imaging of hydrogen sulfide in these two types of cells can study the association between abnormal levels of hydrogen sulfide in human colon cancer cells and pathology. Cells were incubated in DMEM medium (containing 10% fetal bovine serum; FBS (pH = 7.4), Invitrogen) at 37 °C in an incubator (5% CO2 gas) for 24 h. Before the experiments, add corresponding drugs to different groups of cells and control the incubation time as required. After incubation, the cells were washed with PBS buffer and incubated with metal ions for additional 30 min under 5% CO2. The cells were washed with PBS buffer three times. Cells were seeded on dish for fluorescence microscopic imaging by confocal microscope. The concentration of NTR-HS, HS−, PPG (DL-propargylglycine), AOAA (aminooxyacetic acid) were all 10 μM. Among them, NTR-HS was diluted by 10 μL DMSO-PBS (v/v 2:1) solution, HS−, PPG, AOAA were diluted by PBS buffer (v = 5 μL), the total volume of liquid in each petri dish is 2 mL. Imaging of HeLa cells was used to demonstrate that the fluorescent signal originates from the response of cellular endogenous hydrogen sulfide to the probe NTR-HS. The HCT116 cell imaging experiment was designed to explore the probe NTR-HS to monitor abnormal levels of hydrogen sulfide in the large intestine. Fluorescence imaging of living cells were taken with the 610 × 720 mnm channel under a Nikon confocal fluorescence microscope.
In vivo imaging
C57 mice (25 g) of were used for animal imaging model. Before experiment, the mice were anesthetized with 10% chloral hydrate (0.1 mL, dilute with 0.15 mL PBS). In the in vivo imaging experiment of mice, the concentration of NTR-HS and HS−, were 10 μM. NTR-HS was diluted by the solution of DMSO-PBS (v/v 2:1, v = 20 μL), HS−was diluted by PBS buffer (pH = 7.4, v = 30 μL), the total injection volume is 50 μL each time. Since the fur of the mouse does not interfere with the fluorescent signal of the probe, after anesthetizing the mouse, intraperitoneal injection available as needed. The position circled in blue was the needle hole for injection, and the position circled in green was the location of the mouse's large intestine. Imaging of live mice was taken in the 650 × 760 m nm channel of the IVIS Lumina XRMS Series III.
Results and discussion
Spectra response
The effect of pH for the fluorescence of probe NTR-HS was investigated in the absence or presence of HS−. As shown in Fig. S7, a remarkable fluorescence enhancement was observed when the probe was treated with HS− within the pH range from 4 to 12. This result indicated that the probe NTR-HS had a relatively wide range of pH values for the detection of HS− including the best microenvironment pH 7.3 of the biological system.
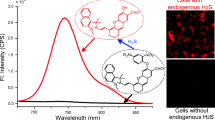
Record the fluorescence spectra of the probe NTR-HS (30 μM) with a series of analytes (300 μM.). As shown in Fig. 1A, no significant fluorescence was observed at 760 nm (when excited at 680 nm) in the free probe solution (quantum yield Φ = 0.10), while a significant enhancement was observed after treatment with HS− (quantum yield Φ = 0.15). UV–Vis analysis results were consistent with fluorescence results (Fig. 1B). Add some common cation, anions, and amino acids (such as Na+, K+, Ca2+, HCO3−, H2PO4−, HPO42−, SO42−, Cl−, HSO3−, SCN−, S2O82−, CA, F−, Cys, and Arg) to the probe solution as interferences of HS− and record the fluorescence strength to test the selectivity of NTR-HS. As shown in Fig. 1A, these analytes did not triggered significant fluorescence changes, which demonstrated probe NTR-HS had a high selectivity for HS− over aforementioned analytes. The time-dependent changes of the fluorescence intensity of the probe NTR-HS at 760 nm after the addition of HS− (30 μM) were investigated (Fig. 1C). The solution color changed from purple to blue very quickly, which could be easily realized the naked eye (Fig. 1D). Compared with other probes investigated, NTR-HS has the advantages of large emission wavelength and fast response (Table S1), so the probe NTR-HS may be an excellent candidate for the detection of H2S. To explore the response details of probe NTR-HS for HS−, the fluorescence emission and UV absorption spectra titration experiments were performed.
A Fluorescence changes of NTR-HS (30 μM) in response to analytes (such as Na+, K+, Ca2+, HCO3−, H2PO4−, HPO42−, SO42−, Cl−, HSO3−, SCN−, S2O82−, CA, F−, Cys, and Arg; 300 μM), (x = 680, m = 760, absorption, and emission slit widths are 5 μm). B Absorbance changes of NTR-HS (30 μM) in response to analytes (300 μM). C Time-dependent fluorescence of the NTR-HS (30 μM) and HS− (30 μM), (x = 680, m = 760, absorption and emission slit widths are 5 μm). D Color change diagram of NTR-HS and HS.− (30 μM) recognition process under natural light in 3 min
As shown in Fig. 2A, the fluorescence emission at 760 nm showed remarkable “turn-on” response with the gradual addition of HS− (0–4.00 × 10−5 M). The free NTR-HS showed obvious absorption peak at 550 (Fig. 2B), after addition with HS− (0–5.00 × 10−5 M), the absorption band at 550 nm gradually decreased and three new absorption peaks appeared at 400, 680, and 750 nm, which indicated that the NTR-HS changed to a novel compound. The fluorescence intensity calibration curve and the figure of linear relation of fluorescence intensities between NTR-HS (30 μM) and the low concentration of HS− (3.33–20.00 μM) were listed in Fig. 2C, the linear equation is y = 85.83 + 1.49 × 107x, R2 = 0.98. And the limit of detection for HS− was 1.04 × 10−7 M based on 3σ/k [40,41,42] (insert of Fig. 2C), the RSD of the fluorescence intensity of the titration curve was shown in Fig. S9. The results indicated that probe displayed a high sensitivity for the detection of HS−. Then, we evaluated the anti-interference ability of probe to hydrogen sulfide from aforementioned various analytes. As shown in Fig. 2D, the fluorescence changes little in the presence of other species, which demonstrated the probe had high selectivity for detecting endogenous hydrogen sulfide in biological systems. And according to the reports, the normal concentration of HS− in the blood plasma and brain are about 50–160 μM and 10–100 μM, respectively [43], combined with the better anti-interference ability of the probe, so probe NTR-HS could be used in vivo for the detection HS− in endogenous HS− in cells, plasma, and tissues.
A Fluorescence changes of NTR-HS (3.33 × 10−5 M) titration with HS− (0–1.20 equiv.). (x = 680, m = 760, absorption and emission slit widths are 5 μm). B Absorbance spectral changes of NTR-HS (30 μM) titration with HS− (0–1.50 equiv.). C Fluorescence intensity calibration curve for NTR-HS titration with HS− (3.33 × 10−6–4.00 × 10−5 M); Inser: The linear relationship between the fluorescence intensity of NTR-HS and the concentration of HS− at low concentrations (3.33 × 10−6–2.00 × 10−5 M), And the detection limit for HS− was 1.04 × 10−7 M. (x = 680, m = 760, absorption and emission slit widths are 5 μm). D Competitive experiments between other analytes and HS− (4.00 × 10−5 M) (blue bars, numbered 1–14 from left to right include that F−, Cl−, HCO3−, HSO3−, H2PO4−, SCN−, NO3−, HPO42−, Cr2O72−, SO42−, S2O82−, Arg, CA, Cys, 3.33 × 10−.4 M). (x = 680, m = 760, absorption and emission slit widths are 5 μm)
Response mechanism
The detection mechanism of NTR-HS for H2S is accomplished through the pathway of ester hydrolysis. The ester hydrolysis process involves the strong nucleophile HS− attacks p-nitrobenzoyl esters and generates the fluorophores NTR-OH and nitrobenzoyl-SH, as shown in Scheme 2. This sensing mechanism study was investigated by the 1H NMR, HRMS, and 1H-1H-COSY spectroscopy. Comparing the 1H NMR spectra of the probe NTR-HS and the compound NTR-OH, it was found that the 1H NMR spectrum changes significantly when the p-nitrobenzoyl group was introduced into the structure of the compound NTR-OH. Two new peaks appeared at 8.35 ppm, which belonged to the four aromatic hydrogens on the p-nitrobenzoyl group. The introduction of nitrobenzoyl group reduced the electron cloud density of the compound NTR-OH, leading to the chemical shift of peak of f, g, i, and j occurred in varying degree’s moving to the low field. When HS− was added to the probe, the chemical shifts of the two new peaks at 8.35 ppm moved to 8.09 ppm, and the peaks f, g, i, and j also moved to the high field in different units (Fig. S10). And in 1H-1H-COSY, the corresponding characteristic peaks of p-nitrobenzoyl also show great changes before and after HS− was added (Fig. S9). We attribute this result to the fact that the addition of HS− destroys the ester group and releases the compound NTR-OH. The ESI–MS spectroscopy of the probe NTR-HS treated with HS− were performed under the condition of an anion source (Fig. S10D, S10E). According to the results, the characteristic peaks of m/z 387.1303 (calcd for NTR-O−, 387.1293) and 181.9907 (calcd for nitrobenzothioic S-acid anion, 181.9917) were observed, which were correspond to the fluorophore (NTR-OH) and p-nitrothiobenzoic acid generated after ester decomposition, respectively.
Fluorescence imaging in living cells and mice
To further explore the biological applications of the probes, the ability of the probes to image endogenous H2S in living cells was examined. Firstly, we tested the biocompatibility of probe and the viability of the living cells was also evaluated by the MTT assay (Fig. S11) and find that the cell survival rate was above 86% when the probe concentration reached 10 μM, which indicated that the probe had low cytotoxicity and could be used for potential applications in living cell culture models. PPG was DL-propargylglycine known as a commercial inhibitor for CBS and CSE. Aminooxyacetic acid (AOAA), a well-known inhibitor for endogenous H2S producing enzymes CBS and CSE, was selected as an inhibitor of hydrogen sulfide production in colorectal cancer cells.
The imaging results of HeLa cells showed that the cells only incubated with the probe showed weak fluorescence intensity (Fig. 3A, B, C), while the cells incubated with PPG and then with the probe can hardly find fluorescence signal (Fig. 3D, E, F), and the cells incubated with the probe and H2S exhibited strong red fluorescence (Fig. 3G, H, I), which indicated that the fluorescent signal of HeLa cells came from the reaction of the probe with endogenous hydrogen sulfide (Fig. 3A to I). The imaging of HCT116 cells showed that the cells incubated with the probe and H2S showed strong red fluorescence (Fig. 3J), the cells only incubated with the probe showed moderate fluorescence (Fig. 3K), and the cells treated with AOAA first and then with the probe showed almost no fluorescence (Fig. 3L). This result also indicated that the fluorescent signal in HCT116 cells was derived from the reaction of its endogenous H2S with the probe (Fig. 3J to L). The quantitative analysis of cell fluorescence intensity of B, E, H and J, K, L is shown in Fig. 3M and N. In addition, we also compared normal cells and cancer cells. NTR-HS (10 μM) was added to Hela and BEAS-2B cells under the same conditions and incubated for 30 min. As shown in Fig. S12, both Hela and BEAS-2B cells showed weak fluorescent signal. Combining the above cell imaging results, we found that the fluorescence intensity of the probe in human colon cancer cells was about two times higher than that in HeLa cells, and was also significantly higher than normal cells, that is, the levels of H2S in rectal cancer cells were higher than in normal cells and other cancer cells, which suggested that the probe could be used to detect the presence of colorectal cancer cells and monitor abnormal levels of hydrogen sulfide in colorectal cancer cells.
A, B, C Imaging of Hela cells added NTR-HS (10 μM) and incubated 30 min. D, E, F Imaging of Hela cells that incubated with PPG (10 μM) 1 h and then added NTR-HS (10 μM) and incubated for 30 min. G, H, I Imaging of Hela cells after added NTR-HS (10 μM) and HS− (10 μM) and incubated 30 min. J Imaging of HCT116 cells incubated with NTR-HS (10 μM) and HS.− (10 μM) 30 min. K Imaging of HCT116 cells incubated with NTR-HS (10 μM) 30 min. L Imaging of HCT116 cells that incubated with AOAA (10 μM) 6 h and then added NTR-HS (10 μM) and incubated 30 min. M Fluorescence intensity of cells in groups B, E, and H. N Fluorescence intensity of cells in groups J, K, and L
3MST in mitochondria can be used as a mediator to metabolize cysteine to produce hydrogen sulfide. Based on this, the co-localization imaging experiment of mitochondrial organelles was carried out and realized the localization of H2S signal at the subcellular level. In this experiment, the commercially available mitochondrial organelle localization dye Mito-Tracker Green was used as a reference (λx = 450–490 nm, λm = 500–540 nm). Firstly, incubated SHSY5Y cells for 24 h, then added probe NTR-HS and Mito-Tracker Green continue incubated for 30 min. Then selected two channels (λx1 = 488 nm, λx2 = 610 nm) to recording the fluorescence intensity of the two probes in the cells. As shown in Fig. 4, Mito-Tracker Green shown bright green fluorescence, NTR-HS shown bright red fluorescence (Fig. 4A–D), and the merged image of the two images was appears as a uniform bright yellow fluorescence (the Pearson coefficient was high to 0.96, Fig. 4E, F), which indicated that the two probes had very similar positioning effects on mitochondria, and the probe had the great potential to be used in the location of mitochondria.
At last, NTR-HS was used for imaging in living animals. Injected the probe NTR-HS (10 μM, dissolved in PBS-DMSO, v/v = 2/1) into the cavity of one mouse (Fig. 5, left), meanwhile, the same amount of HS− and probe were mixed (10 μM) and then injected into intra-abdominal of another mouse (Fig. 5, right). Then recorded the fluorescence distribution and fluorescence intensity at 1, 3, 5, 7, and 9 min, respectively. The results showed that when probe was injected into mice, obvious fluorescent signals appeared quickly, and reached a peak in about 3 min (Fig. S13), then the signal intensity gradually decreased due to metabolism or decomposition (Fig. 5), but this speculation has not been confirmed by research. It was worth noting that the intensity of the signal above the large intestine of the mice was consistently high, which was attributed to the high content of hydrogen sulfide in the large intestine. However, the fluorescence intensity of mice injected with the probe and HS− mixture showed a continuous decrease, this is due to the fact that the reaction of the probe with H2S has reached its peak. The experimental results showed that the probe can detect the endogenous hydrogen sulfide level in mice very quickly, and has a certain ability to express the hydrogen sulfide level in the large intestine.
In vivo imaging of the probe NTR-HS in living mice. Injected the probe NTR-HS (10 μM, dissolved in PBS-DMSO, v/v = 2/1) into the cavity of one mouse (Fig. 5, left); meanwhile, the same amount of HS − and probe were mixed (10 μM) and then injected into intra-abdominal of another mouse (Fig. 5, right), then recorded the fluorescence distribution and fluorescence intensity at 1, 3, 5, 7, and 9 min, respectively
Conclusions
We designed and synthesized a novel near-infrared probe, NTR-HS, which overcomes the incompatibility problems of most reactive probes in terms of reaction speed and selectivity, which is shown in that it can complete the detection in 3 min, and not affected by other sulfhydryl compounds. The lower LOD (1.04 × 10−7 M), larger emission wavelength (760 nm), and visible color change to the naked eye make NTR-HS can easily cope with the detection of H2S in vitro. Combined with its LOD, which is much lower than the H2S level in blood, strong anti-interference ability and good biocompatibility, the probe has been successfully used for live-cell imaging and imaging and monitoring endogenous H2S in vivo in mice, and the results suggest that the probe may be able to detect colorectal cancer cells. Furthermore, in mitochondrial localization experiments, the Pearson coefficient for mitochondrial localization can be as high as 0.96 compared to commercial dyes. Therefore, the probe NTR-HS has great potential in the mitochondrial localization and the study of physiopathological processes related to H2S and 3MST.
Data availability
All requests with regard to data and materials can be addressed to Xuejun Liu, liuxuejun@henu.edu.cn; Kuoxi Xu, xukx@henu.edu.cn.
Code availability
Not applicable.
References
Li HN, Fang YX, Yan JJ, Ren XY, Zheng C, Wu B, Wang SY, Li ZL, Hua HM, Wang P, Li DH (2021) Hydrogen sulfide capture: from absorption in polar liquids to oxide, zeolite, and metal–organic framework adsorbents and membranes. Trac-Trend Anal Chem 134:116117
Yang MW, Fan JL, Du JJ, Peng XJ (2020) Small-molecule fluorescent probes for imaging gaseous signaling molecules: current progress and future implications. Chem Sci 11:5127–5141
Butwong N, Srijaranai S, Luong JHT (2016) Lighting up ATP in cells and tissues using a simple aptamer-based fluorescent probe. Microchim Acta 183:1243–1249
Wang R, Gu X, Li Q, Gao J, Shi B, Xu G, Zhu T, Tian H, Zhao C (2020) Aggregation enhanced responsiveness of rationally designed probes to hydrogen sulfide for targeted cancer imaging. J Am Chem Soc 142:15084–15090
Wu L, Sun Y, Sugimoto K, Luo Z, Ishigaki Y, Pu K, Suzuki T, Chen HY, Ye D (2018) Engineering of electrochromic materials as activatable probes for molecular imaging and photodynamic therapy. J Am Chem Soc 140:16340–16352
Zou XJ, Ma YC, Guo LE, Liu WX, Liu MJ, Zou CG, Zhou Y, Zhang JF (2014) A lysosome-targeted fluorescent chemodosimeter for monitoring endogenous and exogenous hydrogen sulfide by in vivo imaging. Chem Commun 50:13833–13836
Wang J, Wen Y, Huo F, Yin C (2019) A highly sensitive fluorescent probe for hydrogen sulfide based on dicyanoisophorone and its imaging in living cells. Sens Actuators B 294:141–147
Gong DY, Zhu XT, Tian YJ, Han SC, Deng M, Iqbal A, Liu WS, Qin WW, Guo HC (2017) A phenylselenium-substituted BODIPY fluorescent turn-off probe for fluorescence imaging of hydrogen sulfide in living cells. Anal Chem 89:1801–1807
Mao GJ, Wei TT, Wang XX, Huan SY, Lu DQ, Zhang J, Zhang XB, Tan WH, She GL, Yu RQ (2013) High-sensitivity naphthalene-based two-photon fluorescent probe suitable for direct bioimaging of H2S in living cells. Anal Chem 85:7875–7881
Wang H, Li YN, Yang SX, Tian HY, Liang S, Sun BG (2019) Dual-function fluorescent probe for detection of hydrogen sulfide and water content in dimethyl sulfoxide. ACS Omega 4:10695–10701
Pak YL, Li J, Ko KC, Kim G, Lee JY, Yoon JY (2016) Mitochondria-targeted reaction based fluorescent probe for hydrogen sulfide. Anal Chem 88:5476–5481
Kang J, Huo F, Ning P, Meng X, Chao J, Yin C (2017) Two red-emission single and double ‘arms’ fluorescent materials stemed from ‘one-pot’ reaction for hydrogen sulfide vivo imaging. Sens Actuators B 250:342–350
Zhu HC, Liang CX, Cai XY, Zhang HM, Liu CY, Jia P, Li ZL, Yu YM, Zhang X, Sheng WL, Zhu BC (2020) Rational design of a targetable fluorescent probe for visualizing H2S production under Golgi stress response elicited by Monensin. Anal Chem 92:1883–1889
Yang L, Su YN, Geng YN, Zhang Y, Ren XJ, He L, Song XZ (2018) A triple-emission fluorescent probe for discriminatory detection of cysteine/homocysteine, glutathione/hydrogen sulfide and thiophenol in living cells. ACS Sens 3:1863–1869
Yang G, Wu L, Jiang B, Yang W, Qi J, Cao K, Meng Q, Mustafa AK, Mu W, Zhang S, Snyder SH, Wang R (2008) H2S as a physiologic vasorelaxant: hypertension in mice with deletion of cystathionine γ-lyase. Science 322:587–590
Li B, Mei H, Wang M, Gu X, Hao J, Xie X, Xu K (2021) A near-infrared fluorescent probe for imaging of endogenous hydrogen sulfide in living cells and mice. Dye Pigment 189:109231
Zhang WJ, Huo FJ, Yin CX (2019) Photocontrolled single-/dual-site alternative fluorescence probes distinguishing detection of H2S/SO2 in vivo. Org Lett 21:5277–5280
Zhou KY, Yang YT, Zhou TT, Jin M, Yin CX (2021) Design strategy of multifunctional and high efficient hydrogen sulfide NIR fluorescent probe and its application in vivo. Dyes Pigments 185:108901
Zhong KL, Chen L, Pan YX, Yan XM, Hou SH, Tang YW, Xue G, Li JR, Tang LJ (2019) A colorimetric and near-infrared fluorescent probe for detection of hydrogen sulfide and its real multiple applications. Spectrochim Acta A 221:117135
Wang JP, Wen Y, Huo FJ, Yin CX (2019) Based ‘successive’ nucleophilic substitution mitochondrial-targeted H2S red light emissive fluorescent probe and its imaging in mice. Sens Actuators B 297:126773
Zhao Q, Huo F, Kang J, Zhang Y, Yin C (2018) A novel FRET-based fluorescent probe for the selective detection of hydrogen sulfide (H2S) and its application for bioimaging. J Mater Chem B 6:4903–4908
Tang YH, Kong XQ, Liu ZR, Xu A, Lin WY (2016) Lysosome-targeted turn-on fluorescent probe for endogenous formaldehyde in living cells. Anal Chem 88:9359–9363
Wang YY, Yang CT, Xu S, Chen W, Xian M (2019) Hydrogen sulfide mediated tandem reaction of selenenyl sulfides and its application in fluorescent probe development. Org Lett 21:7573–7576
Li T, Yin CX, Chao JB, Zhang WJ, Huo FJ (2020) An ultra-fast, NIR, mitochondria-targeted fluorescent probe for sulfur dioxide based on benzopyrylium and its imaging of in living cells. Sens Actuators B 305:127336
Zhang Y, Yang H, Wei D, Zhang X, Wang J, Wu X, Chang J (2021) Mitochondria-targeted nanoparticles in treatment of neurodegenerative diseases. Exploration 1:20210115
Bae SK, Heo CH, Choi DJ, Sen D, Joe EH, Cho BR, Kim HM (2013) A ratiometric two-photon fluorescent probe reveals reduction in mitochondrial H2S production in parkinson’s disease gene knockout astrocytes. J Am Chem Soc 135:9915–9923
Ashton TD, Katrina A, Jolliffe B, Pfeffer FM (2015) Luminescent probes for the bioimaging of small anionic species in vitro and in vivo. Chem Soc Rev 14:4547–4595
Jin XL, Wu SP, She MY, Jia YF, Hao LK, Yin B, Wang LY, Obst M, Shen YH, Zhang YM, Li JL (2016) A novel fluorescein-based fluorescent probe for detecting H2S and its real applications in blood plasma and biological imaging. Anal Chem 88:11253–11260
Wang X, Sun J, Zhang WH, Ma XX, Lv JZ, Tang B (2013) A near-infrared ratiometric fluorescent probe for rapid and highly sensitive imaging of endogenous hydrogen sulfide in living cells. Chem Sci 4:2551–2556
Wu Z, Liang D, Tang X (2016) Visualizing hydrogen sulfide in mitochondria and lysosome of living cells and in tumors of living mice with positively charged fluorescent chemosensors. Anal Chem 88:9213–9218
Wu ZS, Li Z, Yang L, Han JH, Han SF (2012) Fluorogenic detection of hydrogen sulfide via reductive unmasking of o-azidomethylbenzoyl-coumarin conjugate. Chem Commun 48:10120–10122
Roberta P, Alessandro P, Enrico D (2018) Biochemical sensing with macrocyclic receptors. Chem Soc Rev 47:7006–7026
Tang YH, Ma YY, Yin JL, Lin WY (2019) Strategies for designing organic fluorescent probes for biological imaging of reactive carbonyl species. Chem Soc Rev 48:4036–4048
Wanderi K, Cui Z (2022) Organic fluorescent nanoprobes with NIR-IIb characteristics for deep learning. Exploration 2:20210097
Qian MS, Zhang LW, Pu ZJ, Xia J, Chen LL, Xia Y, Cui HY, Wang JY, Peng XJ (2018) A NIR fluorescent probe for the detection and visualization of hydrogen sulfide using the aldehyde group assisted thiolysis of dinitrophenyl ether strategy. J Mater Chem B 6:7916–7925
Yang Y, Zhao Q, Feng W, Li F (2013) Luminescent chemodosimeters for bioimaging. Chem Rev 113(1):192–270
Hammers MD, Taormina MJ, Cerda MM, Montoya LA, Seidenkranz DT, Parthasarathy R, Pluth MD (2015) A bright fluorescent probe for H2S enables analyte-responsive, 3D imaging in live zebrafish using light sheet fluorescence microscopy. J Am Chem Soc 137:10216–10223
Palanisamy R, Vignesh KK, Antony PB, Anna BC, Maciej M, Cheol SK, Chan HP, Princy MJ, Byung-Hyun P, Myung-Kwan H, Ae RK, Dong JY (2021) Simple colorimetric and fluorescence chemosensing probe for selective detection of Sn2+ ions in an aqueous solution: evaluation of the novel sensing mechanism and its bioimaging applications. Anal Chem 93:801–811
Zheng JR, Xu Y, Fan LX, Qin SH, Li H, Sang MM, Li RX, Chen HY, Yuan ZW, Li BW (2020) A Bioresponsive near-infrared fluorescent probe for facile and persistent live-cell tracking. Small 16:2002211
Li B, Gu X, Wang M, Liu X, Xu K (2021) A novel “off-on-off” fluorescent probe for sensing of Fe3+ and F− successively in aqueous solution and its application in cells. Dye Pigment 194:109637
Chang Y, Qin H, Wang X, Li X, Li M, Yang H, Xu K, Qing G (2020) Visible and reversible restrict of molecular configuration by copper ion and pyrophosphate. ACS Sens 5:2438–2447
Wang P, Fu J, Yao K, Chang Y, Xu K, Xu Y (2018) A novel quinoline-derived fluorescent “turn-on” probe for Cu2+ with highly selectivity and sensitivity and its application in cell imaging. Sens Actuators B 273:1070–1076
Wang XD, An L, Tian QW, Cui KL (2019) Recent progress in H2S activated diagnosis and treatment agents. RSC Adv 9:33578–33588
Funding
This work was supported by the Teacher Education Curriculum Reform Research Project of Henan Province (2020-JSJYZD-019).
Author information
Authors and Affiliations
Contributions
Bai Li: resources, writing, supervision, data curation. Minghui Wang: conceptualization, methodology, project administration, software. Xin Gu: data curation, visualization. Jiajia Chen: conceptualization, visualization. Xiaoyan Yang: review and editing. Xindi Yang: methodology, software. Xuejin Liu: review and editing, supervision, funding acquisition. Kuoxi Xu: Resources, writing—review and editing, supervision, data curation.
Corresponding authors
Ethics declarations
Ethics approval
All applicable international, national, and/or institutional guidelines for the care and use of animals were followed.
Consent to participate
Not applicable.
Consent for publication
Not applicable.
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Li, B., Wang, M., Gu, X. et al. A hemicyanidin-based NIR fluorescent probe for detection of H2S and imaging study in cells and mice. Microchim Acta 189, 291 (2022). https://doi.org/10.1007/s00604-022-05374-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00604-022-05374-7