Abstract
A portable and simple method was developed for on-site selective determination of As(III) based on the SERS signal of As(III)–O vibration. The method relies on the synergistic effect of nanoparticles aggregation and analyte adsorption. Experimental results demonstrated that phosphate replaced the ligands of HH@Ag NPs, which in turn facilitated the adsorption of As(III) on the surface of HH@Ag NPs. The phosphate was introduced as an agglomerating agent to improve the detection ability of the method for As(III). The method shows good selectivity and linear relationship between 5 × 10−8 and 0.8 × 10−6 M, with the detection limit of 1.8 × 10−9 M. The method was applied to actual water samples and successfully detected As(III), indicating that the method could have application potential in actual detection scenarios.
Graphical abstract

Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Arsenic exists widely in nature in the forms of sulfide and oxide minerals, and to a small extent, in its elemental state. Human activities have exacerbated the migration and transformation of arsenic, resulting in arsenic pollution in the environment [1]. Arsenic contamination in drinking water has been found in many countries, including Vietnam, Bangladesh, India, and parts of China [2,3,4], thus means that numerous people have been exposed to arsenic from water. Arsenic pollutants in solution are mainly present as arsenic acid (H3AsO4, As(V)) and arsenious acid (H3AsO3, As(III)), of which As(III) is more toxic than As(V) or organoarsenic compounds [5, 6]. The study of As(III) is of great significance for the control of water pollution and the study of the migration and transformation of As. Therefore, it is necessary to develop a portable method for the on-site detection of As(III).
Arsenic can be detected by analytical techniques, including UV-vis absorption, ion chromatography, capillary electrophoresis, atomic fluorescence spectroscopy, atomic absorption spectroscopy, inductively coupled plasma-atomic emission spectrometry, and mass spectrometry [7, 8]. These methods rely on laboratory apparatus and can perfectly and accurately measure the arsenic content. However, these methods generally require sample pre-treatment and produce only the total arsenic content, which often causes species transformation. For the speciation analysis of trace elements and field analysis, new method is highly demanded for on-site and rapid detection.
SERS is an ideal technique for speciation analysis and on-site quick analysis due to unique fingerprint signals. Significant developments in micro- and speciation analysis have been achieved with recent advances in nanotechnology [9, 10]. SERS is widely used in environmental pollution and pollutant species research [11,12,13]. Du et al. reported a portable Fe3O4@Ag SERS sensor for the in situ identification of arsenic species (As(III) and As(V)) and applied it to arsenic-contaminated groundwater detection [14]. Magnetic material Fe3O4 is applicable in the fast preconcentration and rapid separation of samples, which are beneficial to on-site SERS analysis. Mulvihill et al. designed dense arrays of Ag nanocrystals fabricated by Langmuir–Blodgett assembly for trace arsenic detection [4]. In these Ag nanocrystals, PVP was introduced for passivation to facilitate the interaction between Ag and arsenic during sensing experiments. Song et al. reported a biosensor based on aptamer and 4-MBA-modified Au@Ag core-shell nanoparticles [15]. In this report, aptamer was effectively absorbed on Au@Ag, avoiding the agglomeration of the nanoparticles, and no SERS signal was generated. As(III) could specifically bind to aptamer to cause the nanoparticles aggregation to generate 4-MBA SERS signal. Then, the SERS signal of 4-MBA indirectly indicates As(III). Significant efforts to detect As(III) have been based on Ag nanoparticles, Ag mirror, or on its indirect detection using Raman signaling molecules [14,15,16,17]. In previous reports, many methods require natural evaporation, magnetic separation, or indirect detection. Therefore, it is significant to develop a convenient method for direct detection of arsenic.
In this study, we aimed to develop a simple and convenient method for detecting ultralow concentrations of As(III)-based HH@Ag NPs colloid substrate. First, the performance of HH@Ag NPs-enhanced As(III) Raman signal was investigated by studying the effect of aggregating agents, ligand and pH. Then, the decisive factors and mechanism of this method are demonstrated according to the SERS spectrum of As(III). Subsequently, the analytical performance is verified from the linear range, selectivity, and anti-interference. Finally, the method was applied to the detection of actual water samples to demonstrate the feasibility on site.
Results and discussion
Since As(III) exists in the form of H3AsO3 in water, the v(As–O) vibrations will appear as characteristic Raman peaks and provide information about As(III) which provides an opportunity for As(III) detection. For trace determination in solution, the detection of As(III) by SERS remains difficult due to the Brownian motion and low concentration of the analyte. Agglomerated nanoparticles with hot spots can enhance the Raman signal of molecules adsorbed in the hot spot area, which provides the possibility for detecting low concentration in solution. In this method, it is critical for agglomeration of Ag nanoparticles and effective analyte adsorption. Therefore, it is necessary to study the influence of various parameters on the experimental results.
pH-mediated sensitivity of the sensor to As(III)
pH is a crucial factor that will greatly affect the detection performance of the analytical method. In this experiment, a phosphate buffer (PB buffer) solution containing As(III) was prepared in advance, and the solution was then mixed with HH@Ag NPs for detection. As shown in Fig. 1, it shows the HH@Ag NPs enhanced As(III) SERS spectra under different pH. The HH@Ag NPs do not enhance the SERS signal of As(III), and no new signals appear in weakly acidic and neutral environments (Fig. 1a). In alkaline environments, it exhibited clear enhanced Raman scattering of As(III), and new Raman peaks appear near 440 cm−1 and 725 cm-1 (Fig. 1a). According to literature reports, the two observed peaks can be attributed to the vibration of As(III)-O [18]. The strong vibrational band at 725 cm−1 results from the ν1 (A1) symmetric As–O stretching mode for arsenite [4, 8, 14]. The minor peak centered at 425 cm−1 is tentatively interpreted as due to the wagging of H–O bonds [8]. As the pH increases, the SERS intensity of As(III) by the HH@Ag NPs increases geometrically and then reaches the strongest at pH 9 and 10 (Fig. 1a, S4). Clearly, it indicated that an alkaline environment is favorable for the detection based on SERS.
HH@Ag NPs enhanced As(III) SERS signal in different pH systems: a PB buffer (0.04 M); b NaOH. In the test, HH@Ag NPs solution (10 μL) and PB buffer or NaOH solution-diluted As(III) (2 × 10−6 M, 10 μL) were mixed and allowed to stand for 5 min; then, an aliquot (1 μL) was dropped and tested on a silicon wafer
To prove the role of pH in the detection process, a control experiment was conducted by using alkali to directly adjust the pH. As shown in Fig. 1b, the expected Raman peaks 440 cm−1 and 725 cm−1 of As(III) did not appear. Under the same pH conditions, the Raman enhancement effect of HH@Ag NPs on As(III) in the PB buffer is much greater than that of the alkali adjustment system (Fig. S4). The same pH shows different conditions, which is due to the different ways of pH adjustment. This shows that phosphate also has a decisive role.
The above analysis shows that the SERS effect is determined by pH and phosphate. The UV-vis spectrum of HH@Ag NPs with PB buffer shows red shifts of the absorption peak and decreases of the intensity of the absorption peak, indicating agglomeration of HH@Ag NPs (Fig. S5a). The Raman peak of 243 cm−1 belongs to Ag-Cl, which also supports the existence of HH@Ag NPs aggregation (Fig. 1a). The UV spectrum of the HH@Ag NPs does not show a considerable change when the pH is adjusted by alkali (Fig. S5b). Meanwhile, there is no a peak at 243 cm−1 appeared when the pH is adjusted by alkali (Fig. 1b). These indicate that HH@Ag NPs are stable in an alkaline environment and do not agglomerate. Therefore, there will be no SERS signal of As(III) in alkali adjustment system. In the PB buffer, pH still has a decisive effect on SERS, although it has no effect on HH@Ag NPs. It should be noted that phosphate has cations (Na+), which can agglomerate HH@Ag NPs [19, 20]. So, the aggregation of HH@Ag NPs is due to phosphate in the PB buffer.
Besides, experimental results show that pH affects the existence of the As(III) and anion of phosphate. According to the literature, H3ASO3 is the predominant As(III) species when the pH of the system is less than 9 [21]. A high pH value condition promotes the transformation of As(III) from a molecular form to an ionic form, which helps the adsorption of As on the surface of hot spots [22]. In general, pH affects the analytes morphology and anion of phosphate, which in turn determines SERS.
The role of aggregating agent in SERS by HH@Ag NPs
In the context of SERS experiments, aggregating agent is also a decisive factor and is needed to induce a large number of “hot spots” that are produced in the gaps between the agglomerated nanoparticles [23]. An experiment is conducted to investigate the aggregating agent in order to achieve better analytical performance. From Fig. 2, it can be seen that the HH@Ag NPs only enhance the SERS signals of As(III) in the PB buffer, and no obvious Raman peak is observed in the others. As(III) SERS peaks are observed at 440 cm−1 and 725 cm−1 in the presence of PB buffer. Thus, it indicated the superiority of phosphate in enhancing the As(III) SERS signal.
SERS spectra of As(III) enhanced by HH@Ag NPs in the presence of different aggregating agents with NaCl (0.08 M, pH 9.08), NaNO3 (0.08 M, pH 9.08), Na2SO4 (0.04 M, pH 9.08), and PB buffer (0.04 M, pH 9.08), respectively. In the test, HH@Ag NPs solution (10 μL) and different aggregating agents solution-diluted As(III) (2 × 10-6 M, 10 μL) were mixed and allowed to stand for 5 min; then, an aliquot (1 μL) was dropped and tested on a silicon wafer
These aggregating agents showed a great difference to the SERS signals of As(III) enhanced by HH@Ag NPs. Both the anion and cation of agglomerating agent will affect the detection effect of SERS [19]. First, it is necessary to investigate the effect of aggregating agents on the agglomeration of HH@Ag NPs. The absorption peak intensity of HH@Ag NPs has been significantly reduced in the presence of the aggregating agents (Fig. S6). There is a peak at 243 cm−1 in the presence of the aggregating agents (Fig. 2). These indicated that agglomerating agent effectively agglomerated HH@Ag NPs. The aggregation of HH@Ag NPs can generate hot spots. If there is adsorption in the hot spot area, SERS signals will be generated; on the contrary, it means that the hot spot area does not effectively adsorb target. In these agglomerating agents, the cation and cation concentrations of the aggregating agent remain the same, and they can promote the agglomeration of HH@Ag NPs. There is no difference in the hot spots generated by cations. In the previous reports, it was pointed out that halogen ions are not suitable for SERS analysis of anions, since halide ions form a strongly bonded surface layer which repels anions [24]. The above analysis shows that the anions affect the adsorption of the target on the surface of HH@Ag NPs.
The role of surface functional groups on the Ag NPs for SERS
Ag NPs synthesized by different synthetic methods and with varied ligands exert different SERS effects. The peak locations differ according to the Ag NPs modifier, appearing at 440 cm−1 and 725 cm−1 on the HH@Ag NPs and at 420 cm−1 and 740 cm−1 on the Cit@Ag NPs (Figs. 1 and 3), respectively. The enhancement of Cit@Ag NPs for the As(III) Raman intensity is weaker than HH@Ag NPs, indicating that Cit@Ag NPs have a weak Raman enhancement effect on As(III) in an alkaline PB buffer (Figs. 1 and 3). This can be attributed to the citrate ligand of Cit@Ag NPs, which makes nanoparticles stable and difficult to agglomerate, and inhibits the adsorption of the analyte on its surface [25,26,27]. Compared with Cit@Ag NPs, the HH@Ag NPs are unstable and easier to form agglomerates, and the ligands are more conducive to the adsorption of analytes, thus forming more hot spots and producing strong SERS signal.
SERS spectra of As(III) enhanced by Cit@Ag NPs in different aggregating agents with NaCl (0.08 M, pH 9.08), NaNO3 (0.08 M, pH 9.08), Na2SO4 (0.04 M, pH 9.08), and PB buffer (0.04 M, pH 6.03, 9.08), respectively. In the test, Cit@Ag NPs solution (10 μL) and different aggregating agents solution-diluted As(III) (2 × 10−6 M, 10 μL) were mixed and allowed to stand for 5 min; then, an aliquot (1 μL) was dropped and tested on a silicon wafer
The model molecule R6G was used as a control to study the effect of Ag NPs ligands. It clearly shows that HH@Ag NPs can generate SERS signal of R6G with the peaks at 1311 cm−1, 1362 cm−1, and 1511 cm−1 in each case (Fig. S7a). It should be noted that HH@Ag NPs can enhance the R6G signal without aggregated agent that can be ascribed to Cl− on the HH@Ag NPs surface which originates from synthesis stage. R6G is a water-soluble cationic dye that can cause nanoparticles to agglomerate through electrostatic action [28,29,30]. Then, it is easy to detect the R6G by HH@Ag NPs. However, unlike the HH@Ag NPs, the Cit@Ag NPs can generate R6G Raman signals only in the presence of NaCl (Fig. S7b). It is difficult to detect the SERS signal under normal circumstances by Cit@Ag NPs. This indicates that different targets and enhancement substrates require different enhancement strategies. The analyte and the agglomerating agent also need to cooperate with each other.
SERS detection mechanism
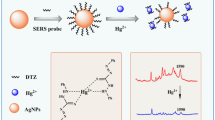
There are two main processes for colloidal SERS: one is the formation of hot spots by cation agglomeration of nanoparticles, and the other is the adsorption of analytes in the hot spots. In this method, HH@Ag NPs can significantly enhance the As(III) Raman signal in alkali PB buffer. The role of PB buffer can be considered from the three aspects of agglomeration nanoparticles, pH, and surface ligand substitution. The cations (Na+) of phosphate disrupt the electrical double layer of HH@Ag NPs, which in turn destroys the stability of the particles in solution [31,32,33]. This promotes aggregation of the HH@Ag NPs and creates more hot spots that make main contribution to the SERS effect [32, 33]. The pH of PB buffer will affect the form of the As(III) and phosphate, which in turn affects the adsorption of the As(III) in the hot spot area. Finally, these impacts are reflected in the SERS signal of As(III).
From the above analysis, the adsorption of the As(III) in the hot spot is affected by anions of phosphate. As the pH increase, HH@Ag NPs showed the Raman peak (925 cm−1, 1097 cm−1) of phosphate, and the Ag-Cl Raman peak (243 cm−1) gradually disappeared (Fig. S8a). The disappearance of Ag-Cl Raman peak (243 cm−1) indicated that the Cl− has separated from the surface of HH@Ag NPs. In the presence of other agglomerating agents, there exists only Raman peak of Ag-Cl (243 cm−1) (Fig. S8b). This shows that phosphate can replace Cl− on the surface of HH@Ag NPs and adsorb on the surface. Other aggregating agents can only agglomerate HH@Ag NPs but cannot replace HH@Ag NPs surface ligands. The SERS signal of As(III) appears accompanied by the phosphate Raman peak, and the Raman peak of Ag-Cl gradually disappears as the pH increases (Fig. 1). This shows that the process of phosphate substitution for the ligand promotes the adsorption of As(III) on the surface of HH@Ag NPs. In general, cations of aggregating agent promote the aggregation of HH@Ag NPs; pH and anions of aggregating agent promote As(III) adsorption on the surface of HH@Ag NPs. These combined action resulted in a strong As(III) SERS signal generated by agglomerated HH@Ag NPs.
As shown in Scheme 1, when the mixed solution of phosphate and As(III) is added to the HH@Ag NPs, cations and anions lead to the agglomeration of HH@Ag NPs and the substitution of Cl− on their surface, respectively, which provides an opportunity for As(III) to adsorb the HH@Ag NPs, thus realizing the SERS detection of As(III). If the anion cannot replace the surface ligand or the target cannot be absorbed in the hot spot, it is difficult to detect the SERS signal of the target.
Analytical performance
To evaluate the detection ability of this method, we performed sensitivity, reliability, and practical application experiments. In Fig. 4a, it shows the SERS spectra of As(III) at varying concentrations. The primary peaks are located at 725 cm−1 and 440 cm−1 that the SERS intensity tends to decrease with declining concentration. It has a linear relationship between the SERS feature signal and the concentration of As(III) in the range 5 × 10−8–0.8 × 10−6 M with LOD 1.8 × 10−9 M (Fig. 4b). It is worth mentioning that the As(III) concentration corresponding to the lowest point of the linear range is lower than the As drinking water standard 1.3× 10−7 (10 ppb), showing its good detection performance. Compared with other reported methods, this method has considerable detection capability and is comparable with the As drinking water standard (Table 1). Due to the fingerprint spectrum of As(III), the method can completely avoid false-positive results while enabling low-concentration analysis.
The effects of different ions on the As(III) SERS signal were studied in order to verify the selectivity of the method, as shown in Fig. 5. Clearly, nitrate and sulfate have no significant effect on the SERS spectrum and minor effect on the intensity of As(III). Carbonate has no significant effect on the SERS spectrum of As(III) and increases the SERS intensity of As(III). Chloride ions do not affect the SERS spectrum of As(III) but reduce the SERS intensity of As(III). Sulfate and nitrate will not be adsorbed and hydrolyzed, so they have minor effect on As(III) SERS signal. Carbonate will not be adsorbed but will be hydrolyzed to increase the pH of the solution, resulting in an increase in SERS intensity of As(III). Compared with other salts, Cl− will inhibit the ligand substitution on the HH@Ag NPs surface by phosphate and will compete with As(III) for adsorption on the HH@Ag NPs surface. These reasons cause the Cl− to reduce the SERS intensity of As(III). Under the interference of coexisting ion, there is no interference bands in the spectrum, indicating that the method has good selectivity to As(III) (Fig. 5a). From Fig. 5b, these effects are tolerable and will not affect the detection of As(III) after exposure to a high concentration of these compounds. The results indicated that the method can selectively detect As(III) with less interference by other ions.
To examine the reliability of the method, parallel experiments were used. The result indicated that the method reliably detects low-concentration As(III) signals (Fig. S9). The SERS intensities under the same concentrations are stable, with RSD of 15.83% and 14.79%, respectively (Fig. S9c). Thus, the method displays good reliability and reproducibility.
For application in actual samples, the method was used to detect As(III) in tap water. As shown in Fig. 6, a weak As(III) SERS signal can be detected at a concentration of 10−7 M, and an obvious As(III) SERS signal can be detected at 10−6 M. Even if there are unfavorable factors such as complex ion interference, the method still responds to As(III) at a concentration of 10−7 M, which illustrates the practical application value of the method in actual sample detection. Due to complex matrices in actual water samples, such as high concentrations of cations and halogen ions, the SERS intensity of As(III) in actual water samples is much lower than that of standard As(III) solutions, which makes the quantitative analysis of As(III) extremely difficult. For on-site analysis, the method detect the SERS signal of As(III) very well, which satisfies the trace detection of arsenic.
Conclusion
This paper reports a method for the rapid on-site selective detection of As(III) based on Ag NPs-enhanced SERS spectra. For this method, it depends on the form of the As(III) and agglomerating agents. The ionic forms of As(III) and phosphate are conductive to detection of low concentrations of As(III).The method exhibited good detection performance, meeting on-site detection requirements. The strong adsorption of As(III) on HH@Ag NPs surface avoids the spectral interference of other ions and improves the selectivity of SERS analysis. The method exhibited good detection performance and was successfully applied to the detection of arsenic in samples. Given the feasibility of this method, it was promising for the on-site detection of As(III).
References
Modheji M, Emadi H, Vojoudi H (2020) Efficient pre-concentration of As(III) in food samples using guanidine-modified magnetic mesoporous silica. J Porous Mater 27:971–978. https://doi.org/10.1007/s10934-020-00873-5
Berg M, Tran HC, Nguyen TC, Pham HV, Schertenleib R, Giger W (2001) Arsenic contamination of groundwater and drinking water in Vietnam: a human health threat. Environ Sci Technol 35:2621–2626. https://doi.org/10.1021/es010027y
Karim MM (2000) Arsenic in groundwater and health problems in Bangladesh. Water Res 34:304–310. https://doi.org/10.1016/S0043-1354(99)00128-1
Mulvihill M, Tao A, Benjauthrit K, Arnold J, Yang P (2008) Surface-enhanced Raman spectroscopy for trace arsenic detection in contaminated water. Angew Chem Int Ed 47:6456–6460. https://doi.org/10.1002/anie.200800776
Kumar Jena B, Retna Raj C (2008) Gold nanoelectrode ensembles for the simultaneous electrochemical detection of ultratrace arsenic, mercury, and copper. Anal Chem 80:4836–4844. https://doi.org/10.1021/ac071064w
Vojoudi H, Badiei A, Bahar S, Mohammadi Ziarani G, Faridbod F, Ganjali MR (2017) Post-modification of nanoporous silica type SBA-15 by bis(3-triethoxysilylpropyl)tetrasulfide as an efficient adsorbent for arsenic removal. Powder Technol 319:271–278. https://doi.org/10.1016/j.powtec.2017.06.028
Han M-J, Hao J, Xu Z, Meng X (2011) Surface-enhanced Raman scattering for arsenate detection on multilayer silver nanofilms. Anal Chim Acta 692:96–102. https://doi.org/10.1016/j.aca.2011.02.054
Yang M, Liamtsau V, Fan C, Sylvers KL, McGoron AJ, Liu G, Fu F, Cai Y (2019) Arsenic speciation on silver nanofilms by surface-enhanced Raman spectroscopy. Anal Chem 91:8280–8288. https://doi.org/10.1021/acs.analchem.9b00999
Lu Z, Wu L, Dai X, Wang Y, Sun M, Zhou C, Du H, Rao H (2021) Novel flexible bifunctional amperometric biosensor based on laser engraved porous graphene array electrodes: highly sensitive electrochemical determination of hydrogen peroxide and glucose. J Hazard Mater 402:123774. https://doi.org/10.1016/j.jhazmat.2020.123774
Lu Z, Li Y, Liu T, Wang G, Sun M, Jiang Y, He H, Wang Y, Zou P, Wang X, Zhao Q, Rao H (2020) A dual-template imprinted polymer electrochemical sensor based on AuNPs and nitrogen-doped graphene oxide quantum dots coated on NiS2/biomass carbon for simultaneous determination of dopamine and chlorpromazine. Chem Eng J 389:124417. https://doi.org/10.1016/j.cej.2020.124417
Chen H, Duan F, Du J, Yin R, Zhu L, Dong J, He K, Sun Z, Wang S (2021) Surface-enhanced Raman scattering for mixing state characterization of individual fine particles during a haze episode in Beijing, China. J Environ Sci 104:216–224. https://doi.org/10.1016/j.jes.2020.12.008
Sun C, Dong W, Peng J, Wan X, Sun Z, Li D, Wang S (2020) Dual-mode fluorescence–SERS sensor for sensitive and selective detection of uranyl ions based on satellite Fe3O4-Au@CdTe nanostructure. Sensors Actuators B Chem 325:128644. https://doi.org/10.1016/j.snb.2020.128644
Li D, Yao D, Li C, Luo Y, Liang A, Wen G, Jiang Z (2020) Nanosol SERS quantitative analytical method: a review. TrAC Trends Anal Chem 127:115885. https://doi.org/10.1016/j.trac.2020.115885
Du J, Cui J, Jing C (2014) Rapid in situ identification of arsenic species using a portable Fe3O4@Ag SERS sensor. Chem Commun 50:347–349. https://doi.org/10.1039/C3CC46920D
Song L, Mao K, Zhou X, Hu J (2016) A novel biosensor based on Au@Ag core–shell nanoparticles for SERS detection of arsenic (III). Talanta 146:285–290. https://doi.org/10.1016/j.talanta.2015.08.052
Xu Z, Hao J, Li F, Meng X (2010) Surface-enhanced Raman spectroscopy of arsenate and arsenite using Ag nanofilm prepared by modified mirror reaction. J Colloid Interface Sci 347:90–95. https://doi.org/10.1016/j.jcis.2010.03.028
Li J, Chen L, Lou T, Wang Y (2011) Highly sensitive SERS detection of As3+ ions in aqueous media using glutathione functionalized silver nanoparticles. ACS Appl Mater Interfaces 3:3936–3941. https://doi.org/10.1021/am200810x
Hao J, Han M-J, Han S, Meng X, Su T-L, Wang QK (2015) SERS detection of arsenic in water: a review. J Environ Sci 36:152–162. https://doi.org/10.1016/j.jes.2015.05.013
Zhou Z-M, Zheng H, Liu T, Xie Z-Z, Luo S-H, Chen G-Y, Tian Z-Q, Liu G-K (2021) Improving SERS sensitivity toward trace sulfonamides: the key role of trade-off interfacial interactions among the target molecules, anions, and cations on the SERS active surface. Anal Chem 93:8603–8612. https://doi.org/10.1021/acs.analchem.1c01530
Xie L, Lu J, Liu T, Chen G, Liu G, Ren B, Tian Z (2020) Key role of direct adsorption on SERS sensitivity: synergistic effect among target, aggregating agent, and surface with Au or Ag colloid as surface-enhanced Raman spectroscopy substrate. J Physical Chem Lett 11:1022–1029. https://doi.org/10.1021/acs.jpclett.9b03724
Lu P, Zhu C (2011) Arsenic Eh–pH diagrams at 25°C and 1 bar. Environ Earth Sci 62:1673–1683. https://doi.org/10.1007/s12665-010-0652-x
Greaves SJ, Griffith WP (1988) Surface-enhanced Raman scattering (SERS) from silver colloids of vanadate, phosphate and arsenate. J Raman Spectrosc 19:503–507. https://doi.org/10.1002/jrs.1250190803
Xu L-J, Zong C, Zheng X-S, Hu P, Feng J-M, Ren B (2014) Label-free detection of native proteins by surface-enhanced Raman spectroscopy using iodide-modified nanoparticles. Anal Chem 86:2238–2245. https://doi.org/10.1021/ac403974n
Bell SEJ, Sirimuthu NMS (2006) Surface-enhanced Raman spectroscopy (SERS) for sub-micromolar detection of DNA/RNA mononucleotides. J Am Chem Soc 128:15580–15581. https://doi.org/10.1021/ja066263w
Sevilla P, García-Blanco F, García-Ramos JV, Sánchez-Cortés S (2009) Aggregation of antitumoral drug emodin on Ag nanoparticles: SEF, SERS and fluorescence lifetime experiments. Phys Chem Chem Phys 11:8342–8348. https://doi.org/10.1039/B903935J
Cañamares MV, Garcia-Ramos JV, Gómez-Varga JD, Domingo C, Sanchez-Cortes S (2005) Comparative study of the morphology, aggregation, adherence to glass, and surface-enhanced Raman scattering activity of silver nanoparticles prepared by chemical reduction of Ag+ using citrate and hydroxylamine. Langmuir 21:8546–8553. https://doi.org/10.1021/la050030l
Cañamares MV, Garcia-Ramos JV, Sanchez-Cortes S, Castillejo M, Oujja M (2008) Comparative SERS effectiveness of silver nanoparticles prepared by different methods: a study of the enhancement factor and the interfacial properties. J Colloid Interface Sci 326:103–109. https://doi.org/10.1016/j.jcis.2008.06.052
Bell SEJ, Sirimuthu NMS (2005) Surface-enhanced Raman spectroscopy as a probe of competitive binding by anions to citrate-reduced silver colloids. J Phys Chem A 109:7405–7410. https://doi.org/10.1021/jp052184f
Dong X, Gu H, Kang J, Yuan X, Wu J (2010) Comparative study of surface-enhanced Raman scattering activities of three kinds of silver colloids when adding anions as aggregating agents. Colloids Surf A Physicochem Eng Asp 368:142–147. https://doi.org/10.1016/j.colsurfa.2010.07.029
Futamata M, Yu Y, Yajima T (2011) Elucidation of electrostatic interaction between cationic dyes and Ag nanoparticles generating enormous SERS enhancement in aqueous solution. J Phys Chem C 115:5271–5279. https://doi.org/10.1021/jp110146y
Kämmer E, Dörfer T, Csáki A, Schumacher W, Da Costa Filho PA, Tarcea N, Fritzsche W, Rösch P, Schmitt M, Popp J (2012) Evaluation of colloids and activation agents for determination of melamine using UV-SERS. J Phys Chem C 116:6083–6091. https://doi.org/10.1021/jp211863y
Bengter H, Tengroth C, Jacobsson SP (2005) New light on Ag-colloid preparation for surface-enhanced FT-Raman spectroscopy: the role of aggregation. J Raman Spectrosc 36:1015–1022. https://doi.org/10.1002/jrs.1399
Yaffe NR, Ingram A, Graham D, Blanch EW (2010) A multi-component optimisation of experimental parameters for maximising SERS enhancements. J Raman Spectrosc 41:618–623. https://doi.org/10.1002/jrs.2495
Li J, Zheng B, Zheng Z, Li Y, Wang J (2020) Highly sensitive and selective colorimetric and SERS dual-mode detection of arsenic (III) based on glutathione functionalized gold nanoparticles. Sensors Actuators Rep 2:100013. https://doi.org/10.1016/j.snr.2020.100013
Acknowledgements
This work was supported by the National Natural Science Foundation of China (21775042), the National Key Research and Development Program of China (2017YFA0207003), Open Fund of Guangdong Provincial Key Laboratory of Petrochemical Pollution Process and Control, Guangdong University of Petrochemical Technology (No. 2018B030322017), and the Fundamental Research Funds for the Central Universities (2020 MS037).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
ESM 1
(DOCX 1548 kb)
Rights and permissions
About this article
Cite this article
Ge, H., Yin, R., Su, P. et al. On-site detection of As(III) based on silver nanoparticles aggregation mediated by phosphates using surface-enhanced Raman scattering (SERS). Microchim Acta 189, 44 (2022). https://doi.org/10.1007/s00604-021-05134-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00604-021-05134-z