Abstract
A MnO2 nanosheet-assisted ratiometric fluorescence probe based on carbon quantum dots (CQDs) and o-phenylenediamine (OPD) has been developed for the detection of the anticancer drug 6-mercaptopurine (6-MP). CQDs with strong fluorescence are synthesized via the one-step hydrothermal method. MnO2 nanosheets as an oxidase-mimicking nanomaterial directly oxidize OPD into 2,3-diaminophenazine (DAP) which has a fluorescence emission at 570 nm, whereas the fluorescence of CQDs at 445 nm is then reduced by the DAP through the inner filter effect (IFE) under a single excitation wavelength (370 nm). After adding 6-MP, MnO2 nanosheets can be reduced to Mn2+ and lose their oxidase-like property, blocking the IFE with the fluorescence decrease of DAP and fluorescence increase of CQDs. The novel ratiometric fluorescence probe exhibits considerable sensitivity toward 6-MP and linear response is in the 0.46–100.0 μmol L−1 concentration range with the detection limit of 0.14 μmol L−1. Furthermore, the probe shows good selectivity when exposed to a series of interfering other organic and inorganic compounds, and biomolecules and can be applied to the detection for 6-MP in human serum samples and pharmaceutical tablets. Satisfactory recoveries of 6-MP in human serum samples are in the range 96.1–110.9% with the RSD of 1.4 to 3.2%. The amount of 6-MP is successfully estimated as 49.3 mg in pharmaceutical tablet with the RSD of about 2.2%.
Graphical abstract

Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
6-Mercaptopurine (6-MP), a sulfur analogue of adenine, is an anticancer drug with immunosuppressive properties [1]. Clinically, it is mainly used to treat acute lymphocytic leukemia [2], Crohn’s disease [3], and inflammatory bowel disease [4], etc. However, as a cytotoxic antitumor drug, 6-mercaptopurine has serious side effects, which limits its application. Therefore, it is of great significance to develop a simple and effective method to determine the concentration of 6-MP. To date, a series of analytical methods have been applied for the detection of 6-MP, including chemiluminescence [5], electrochemical method [6], colorimetric assays [7], high-performance liquid chromatography [8], and Raman spectroscopy [9]. However, these methods have their drawbacks: chemiluminescence assays have poor selectivity; electrochemical methods have poor repeatability; colorimetric assays involve some organic solvents and environmental toxicity; high-performance liquid chromatography and Raman spectroscopy require expensive equipment and complex sample pretreatment. Consequently, as a better method with comparatively low cost, simple operation, and fast response, fluorescent sensing based on luminescent materials serves as a great alternative to detect 6-MP.
Carbon quantum dots (CQDs) with luminescent properties are quasi-spherical carbon nanomaterials whose dimensions are usually less than 10 nm [10]. Due to these properties of adjustable optical/chemical properties [11], good biocompatibility [12], low toxicity [13], and better water solubility, CQDs have been widely applications in the fields of chemical and biological sensing, photocatalysis, biological imaging, drug delivery, and photodynamic therapy [14,15,16,17,18]. Particularly, chemical sensing based on CQDs usually achieves the determination of analyte through changes in fluorescence signals. Specifically, the fluorescence signal of CQDs is increased or decreased to the analyte due to photoinduced electron transfer (PET) and inner filter effect (IFE), Förster resonance energy transfer (FRET), and other reasons. For example, Li et al. synthesized the N, S/CQDs with citric acid and L-cysteine that could efficiently promote electron transfer and coordination interaction among N, S/CQDs, and Hg2+, achieving the selective and sensitive detection of Hg2+ [19]. Xu et al. used MnO2 nanosheets to reduce the fluorescence of boron nitride quantum dots for sensitive detection of organophosphorus pesticides [20].
However, a single fluorescent signal is susceptible to various analyte-independent disturbances caused by fluorescence probe concentration, light scattering of the sample matrix, excitation source and operation fluctuations, and environmental changes. To solve this limitation, another fluorescence spectral peak is introduced to design dual-emission ratiometric schemes. The obtained signal ratio can reduce the interference with environment/background factors to the greatest extent, so as to realize the reliable detection of specific targets. Han et al. synthesized amino-functionalized mesoporous silica nanoparticle-gold nanocomposites that were integrated with o-phenylenediamine (OPD) to form a ratiometric fluorescence nanoplatform for the detection of alkaline phosphatase activity [21]. The change of ratiometric fluorescence signal is based on the content of oxidized OPD, which can be adjusted by MnO2 nanosheets with oxidizing activity.
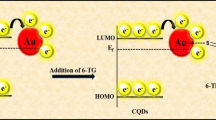
Herein, a ratiometric fluorescence sensing platform based on fluorescent CQDs and non-fluorescent OPD to detect 6-MP was developed. As shown in Scheme 1, MnO2 nanosheets as an oxidase-mimicking nanomaterial directly oxidized OPD into 2,3-diaminophenazine (DAP) with a fluorescence emission at 570 nm, whereas the fluorescence of CQDs at 445 nm would be reduced by the generated DAP through the IFE under 370-nm excitation wavelength. 6-MP reduced MnO2 nanosheets to Mn2+ inhibiting the production of DAP and the effect of IFE with the fluorescence decrease of DAP and fluorescence increase of CQDs. Furthermore, the established ratiometric fluorescence system was successfully employed to the detection for 6-MP in human serum samples and pharmaceutical tablets.
Experimental section
Materials
OPD, 6-MP, morpholine ethyl sulfonic acid (MES), and N-ethylmaleimide (NEM) were purchased from Aladdin Chemistry Co. Ltd. (Shanghai, China). Citric acid monohydrate and KMnO4 were gained from Oriental Reagent Factory (Chongqing, China). 6-Mercaptopurine tablets were obtained from Xinyi Pharmaceutical Factor (Shanghai, China, Batch No. 980206, Labeled content 50 mg/tablet). All reagents were analytical grade and the water used in the work was deionized water.
Instruments
Fluorescence spectra were carried out using F-2500 spectrofluorometer (Hitachi, Tokyo, Japan). UV-vis absorption measurements were performed on UV-2700 spectrophotometer (Hitachi, Tokyo, Japan). Transmission electron microscope (TEM) images of CQDs and MnO2 nanosheets were performed using a Hitachi S-4800 scanning electron microscope (Tokyo, Japan). Fourier transform infrared spectrum (FI-TR) was conducted on FTIR-8400S FTIR spectrometer. The X-ray diffraction (XRD) patterns were given from X-ray diffractometer (Rigaku D/MAX 2550). The characterizations of X-ray photoelectron spectroscopy (XPS) were obtained from Escalab 250Xi (Thermo Fisher Scientific, USA).
Synthesis of CQDs and MnO2 nanosheets
The CQDs and MnO2 nanosheets were prepared according to previously reported study with minor modifications [22, 23]. Specific synthesis steps were described in the Electronic Supporting Material (ESM).
Detection of 6-MP
Typically, 100.0 μL of Britton-Robinson (BR) buffer (4.0 mmol L−1, pH 7.0), 100.0 μL of 6-MP at varying concentrations, and 50.0 μL of 200.0 μg mL−1 MnO2 nanosheets were mixed and incubated for 10 min at room temperature. After that, 100.0 μL of OPD (2.0 mmol L−1) and 600.0 μL of deionized water were added into the mixture and incubated for 90 min at room temperature. Finally, 50.0 μL of CQDs solution was added, and the fluorescence emission spectrum of the whole solution was measured under the excitation wavelength of 370 nm. The fluorescence intensity at the emission wavelengths of 445 nm and 570 nm was recorded.
Real sample assay
The human serum sample was obtained from a healthy volunteer in the Southwest University Hospital. All experimental procedures were performed in compliance with the relevant laws and the institutional guidelines of ethical standards of the institutional committee of Southwest University Hospital The sample analysis experimental protocol was approved by the institutional committee of Southwest University Hospital (approval number 2017-7), and all the guidelines were followed throughout the present study. Briefly, the collected sample was first centrifuged at 5000 rpm for 10 min to obtain serum. 0.20 mL of sample solution was mixed with 0.10 mL of standard 6-MP solution at different concentrations (10.0, 20.0, 40.0 mmol L −1) and then were diluted to 10.0 mL using distilled water. Finally, 100.0 μL of the treated sample was added into 100.0 μL of BR buffer (4.0 mmol L−1, pH 7.0), and was detected by the method described above.
Ten tablets of 6-mercaptopurine were weighed and ground into fine powder. After being homogenized, the powder equivalent of one tablet of 6-mercaptopurine was accurately weighed and transferred into a 100-mL measuring flask, and 50 mL of HCl (0.1 mol L−1) was added. The mixture was heated in a water bath to be dissolved, and then cooled to room temperature. HCl (0.1 mol L −1) was added to the mixture to the mark and filtered. Then, 1.5 mL of sample solution was diluted to 10.0 mL using distilled water. Finally, 100.0 μL of the treated sample was added into 100.0 μL of BR buffer (4.0 mmol L−1, pH 7.0), and was detected by the method described above.
Results and discussion
Characterization
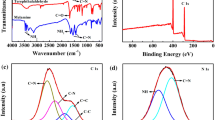
The modality and the average particle size of CQDs were characterized by TEM, as illustrated in Fig. 1a, indicating that as-prepared CQDs were spherical and the average size was estimated to be 2.28 nm. Besides, HR-TEM image disclosed the crystalline nature for CQD samples with a lattice space of 0.21 nm, which corresponded to the (100) plane lattice of graphite. The XRD pattern displayed one broad peak at about 22°, which showed the typical disordered graphitic structure (Fig. 1b). The functional groups of CQDs were characterized by the FT-IR spectrum in Fig. 1c. The strong and broad absorption band at 3448 cm−1 was attributed to the stretching vibrations of O-H and N-H [24]. Furthermore, the band centered at 2080 and 1448 cm−1 was assigned to the asymmetric stretching vibrations of the carboxy group [25]. The absorption band at 1638 cm−1 corresponded to stretching vibrations of C=O. The peaks at 1381 and 1026 cm−1 were the result of the bending of C-N and C-O bonds [26]. XPS was measured to express the composition and superficial element state of CQDs. Figure 1d illustrates that three distinct characteristic peaks located at 284.9, 400.9, and 531.6 eV, which predominated C 1s, N 1s, and O 1s, respectively. The partial XPS spectrum of C 1s could be composed to three peaks with binding energies at 284.7, 285.5, and 287.4 eV, which were generated by to the C-C/C=C, C-O/C-N, and C=O bonds in Fig. S2a [27]. High-resolution spectrum of N 1s (Fig. S2b) contained two peaks, relating to pyridinic N (399.4 eV) and pyrrolic N (401.1 eV) [28]. These results above indicated that CQDs were formed by the sp2 conjugated domain of the aromatic structure and the external groups of containing oxygen and nitrogen.
Optical properties of CQDs
The optical properties of CQDs were further investigated, such as UV-vis absorption spectra, fluorescence spectra, QY, and fluorescent stability. As demonstrated in Fig. 2a, the position of fluorescence emission peak showed slight blue or red shift from 320 to 400 nm and exhibited an excitation-independent optical property for which may be that the surface of CQDs has a single chromophoric structure [29]. Figure 2b shows that CQDs had a strong absorption peak at 268 nm, corresponding to the π−π* transition of the C=C bond, and the absorption peak at 368 nm matched the n−π* transition of the C=O bond [27]. Additionally, the maximum fluorescence emission peak was observed at 445 nm under the excitation wavelength of 370 nm. The QY of CQDs was 62.9% (quinine sulfate as a standard), indicating a good fluorescence performance.
Possible mechanism
Figure 3a displays that MnO2 nanosheets catalytically oxidized OPD into DAP with a strong fluorescence emission at 570 nm, whereas the fluorescence of CQDs at 445 nm would be simultaneously reduced by the generated DAP. When 6-MP was added to the system, the fluorescence of CQDs at 445 nm was restored, while the fluorescence of DAP at 570 nm was reduced under 370-nm excitation wavelength. This was because 6-MP had reducibility and decomposed MnO2 nanosheets to Mn2+. 6-MP with a thiol group achieved several free-radical conjugative reactions to form MP-S· by the oxidization of MnO2 nanosheets. And then the conjugative reactions of MP-S· produced MP-SS-MP in aqueous solutions (Eq. (1)) [30].
a The fluorescence spectra of CQDs, CQDs + OPD, CQDs + 6-MP, CQDs + OPD + MnO2, and CQDs + OPD + MnO2 + 6-MP. b The UV-vis absorption spectra of CQDs, OPD + MnO2, and CQDs + OPD + MnO2. c The UV-vis absorption spectrum of OPD + MnO2 and OPD + MnO2 + 6-MP and the fluorescence spectra of the CQDs. d The fluorescence decay curves of CQDs and CQDs/DAP system
In order to investigate the mechanism, UV-vis absorption spectra of CQDs, OPD + MnO2, and OPD + MnO2 + CQDs were studied. It can be seen from Fig. 3b that the absorption peak at 421 nm of OPD + MnO2 had not shift obviously in the presence of CQDs, indicating that a new complex was not formed. The absorption spectrum of DAP demonstrated large overlap with the fluorescence spectra of the CQDs, which indicated that the inner filter effect (IFE) or Förster resonance energy transfer could be the dominant element of the fluorescence decreasing of CQDs in Fig. 3c [31]. In addition, the change of the UV-vis absorption spectra of OPD + MnO2 system also demonstrated that 6-MP inhibited the process of OPD being oxidated to DAP. Time-resolved fluorescence decay curves in the absence and presence of DAP are shown in Fig. 3d, from which it could be observed that the fluorescence lifetime of CQDs remained constant after the addition of DAP, which implied that there was no electron or energy transfer between DAP and CQDs [32]. The results above implied that the fluorescence decreasing of CQDs triggered via DAP could be primarily owing to IFE.
Optimization of experimental parameters
In order to achieve better experimental result, the incubation time, pH, and concentration of MnO2 nanosheets were optimized. Respective data and figures were given in the ESM (Fig. S5). It was found that the following experimental conditions got the best results: (a) the reaction of OPD and MnO2 nanosheets reached equilibrium after 90 min; (b) optimum pH of 7; (c) optimal concentration of MnO2 nanosheets 10.0 μg mL−1.
Ratiometric detection of 6-MP
A comparison of the analytical performance of this work and other reports is shown in Table 1. Compared with other methods, the ratiometric fluorescence probe based on CQDs and OPD/MnO2 nanosheet system manifested relatively broad linear range and low LOD for the detection of 6-MP. Figure 4a illustrates that as the 6-MP concentration increasing from 0 to 150.0 μmol L−1, the fluorescence intensity of DAP at 570 nm gradually decreased with the increase of the fluorescence intensity of CQDs at 445 nm under 370-nm excitation wavelength. The relationship of ratiometric fluorescence intensity with the 6-MP concentration in the range of 1.0–50.0 μmol L−1 and 50.0–100.0 μmol L−1 is depicted in Fig. 4b, c. The regression equations were as follows: F445/F570 = 0.0231C + 0.322 (R2 = 0.9995) and F445/F570 = 0.0459 C − 0.808 (R2 = 0.9991). The limit of detection (LOD) was acquired to be 0.14 μmol L−1 based on the formula LOD = 3σ/k. A comparison of the analytical performance of this work and other reports is shown in Table 1. Compared with reported fluorescence methods [30, 33], the ratiometric fluorescence probe based on CQDs and OPD/MnO2 nanosheet system manifested a relatively wide detection range and the low LOD. Compared with single-signal chemiluminescence assays [5], electrochemical method [6], and colorimetry assays [7], dual-emission fluorescent probe could decrease or eliminate the errors caused by the fluctuations in environmental/testing conditions. In addition, compared with Raman spectroscopy [9], this method had lower cost, simple operation, and did not need complex sample pretreatment.
Selectivity of 6-MP detection
Selectivity tests were performed to evaluate the potential application of the designed fluorescence probe. In the same condition as detection (4.0 mmol L−1 BR buffer, pH 7.0), a number of interference substances (1000.0 μmol L−1) including Na+, Fe3+, Cu2+, Mn2+, Mg2+, glucose, glycine (Gly), threonine (Thr), alanine (Ala), arginine (Arg), histidine (His), serine (Ser), leucine (Leu), adenosine monophosphate (AMP), uric acid (UA), 6-hydroxypurine (6-HP), kanamycin, vancomycin, adenine, guanine, and other substances (100.0 μmol L−1) such as ascorbic acid (AA) glutathione (GSH) and cysteine (Cys) were added to the CQDs and OPD/MnO2 nanosheets system. The specificity of ratiometric fluorescence response toward 6-MP is illustrated in Fig. 5a, b. As can be seen in Fig. 5a, compared to 6-MP, the ratiometric fluorescence intensity of this system was little affected by other interfering substances. GSH and Cys could interfere with the detection system, for which these thiol-containing species could produce redox reaction with the MnO2 nanosheets. However, they tended to be reacted with the scavenger (N-ethylmaleimide, NEM) [34]. In Fig. 5b, after incubation of GSH and Cys with NEM (0.01 mol L−1), addition of the CQDs and OPD/MnO2 nanosheets system gave a negligible signal changes, whereas 6-MP detection was not disturbed to the introduction of NEM. The reason why NEM could not react with 6-MP to act as a masking agent for GSH and Csy might be related to their structure. The sulfhydryl group of 6-MP was directly connected to the benzene ring, so it had relatively large steric hindrance in binding to NEM. Nevertheless, the sulfhydryl groups of GSH and Cys were connected to the alkyl groups, and the steric hindrance was relatively small, so that they can be more firmly combined with NEM. Accordingly, these results clearly demonstrated that this system still had great potential to be used for reliable 6-MP detection.
Determination of 6-MP in real samples
In order to further evaluate the feasibility and practicality of the present assay based on the ratiometric fluorescent probe, detecting 6-MP in human serum samples and pharmaceutical tablets analysis was investigated. Preparation of real samples was explained in the previous section. All results of these determinations were acquired in the linear range of 1.0–50.0 μmol L−1 and taken from the average consequences of five repeated assessments. The detection of 6-MP in human serum samples and pharmaceutical tablets is carried out in Tables 2, 3. Compared with Chinese pharmacopeia method (UV-vis method) [35], the experimental results showed that there was no significant difference. The satisfactory recoveries of 6-MP in human serum samples were found to be in the range of 96.1–110.9% with the RSD of around 1.4 to 3.2%. The amount of 6-MP was successfully estimated as 49.3 mg in pharmaceutical tablet, which was well accorded with the specification of 50 mg, and the RSD was 2.2%. These results demonstrated the accuracy and reliability of the present method and its great potential for routine analysis.
However, this established method had some limitations. One disadvantage of the method was that the procedure was time consuming, seeing that MnO2 nanosheets and OPD need a long reaction time to generate DAP at room temperature. Additionally, working in the UV made the probe prone to interferences by biomolecules, for which always displayed strong background UV absorption and fluorescence such as serum, cells, and sea water. Therefore, our next steps are to speed up the reaction time by heating and other means and to further purify the serum through the filter membrane to reduce the interference of the serum on working in the UV.
Conclusion
In conclusion, a novel MnO2 nanosheet-assisted ratiometric fluorescent probe based on CQDs and OPD was first prepared and utilized for the detection of 6-MP. Systematically, MnO2 nanosheet oxidized OPD to DAP which emitted yellow fluorescence but reduced the blue fluorescence of CQDs. However, 6-MP reduced MnO2 nanosheets to Mn2+ inhibiting the production of DAP, the fluorescence of CQDs recovered, and the fluorescence of DAP faded, resulting in the color change of fluorescence from yellow to blue. According to the signal change of the ratiometric fluorescence, the quantitative detection of the anticancer drug 6-MP was finally realized. In addition, the fluorescence decreasing of CQDs by DAP was attributed to IFE, proved by the absorption spectrum of DAP demonstrated large overlap with the fluorescence spectra of the CQDs, and the fluorescence lifetime of CQDs remained constant after the addition of DAP. Ultimately, the ratiometric fluorescent probe showed remarkable performance in detecting 6-MP in human serum samples. The above results indicated that the method had certain practicability and application potential in biochemical sensing.
References
Sahasranaman S, Howard D, Roy S (2008) Clinical pharmacology and pharmacogenetics of thiopurines. Eur J Clin Pharmacol 64(8):753–767
Qiu J, Cheng R, Zhang J, Sun H, Deng C, Meng F, Zhong Z (2017) Glutathione-sensitive hyaluronic acid-mercaptopurine prodrug linked via carbonyl vinyl sulfide: a robust and CD44-targeted nanomedicine for leukemia. Biomacromolecules 18(10):3207–3214
Mowat C, Arnott CA, Smith M, Ahmad T et al (2016) Mercaptopurine versus placebo to prevent recurrence of Crohn’s disease after surgical resection (TOPPIC): a multicentre, double-blind, randomised controlled trial. Lancet Gastroenterol Hepatol 1(4):273–282
Mottet C, Schoepfer AM, Juillerat P, Cosnes J, Froehlich F, Kessler-Brondolo V, Seibold F, Rogler G, Vavricka SR, Michetti P (2016) Experts opinion on the practical use of azathioprine and 6-mercaptopurine in inflammatory bowel disease. Inflamm Bowel Dis 22(11):2733–2747
Biparva P, Abedirad SM, Kazemi SY (2015) Silver nanoparticles enhanced a novel TCPO H2O2-safranin O chemiluminescence system for determination of 6-mercaptopurine. Spectrochim Acta A Mol Biomol Spectrosc 145:454–460
Shahrokhian S, Ghorbani-Bidkorbeh F, Mohammadi A, Dinarvand R (2011) Electrochemical determinations of 6-mercaptopurine on the surface of a carbon nanotube-paste electrode modified with a cobalt salophen complex. J Solid State Electrochem 16(4):1643–1650
Rawat KA, Singhal RK, Kailasa SK (2017) One-pot synthesis of silver nanoparticles using folic acid as a reagent for colorimetric and fluorimetric detections of 6-mercaptopurine at nanomolar concentration. Sensors Actuators B Chem 249:30–38
Mawatari H, Kato Y, Nishimura S, Sakura N, Ueda K (1998) Reversed-phase high-performance liquid chromatographic assay method for quantitating 6-mercaptopurine and its methylated and non-methylated metabolites in a single sample. J Chromatogr B 716:392–396
Fu WL, Zhen SJ, Huang CZ (2014) Controllable preparation of graphene oxide/metal nanoparticle hybrids as surface-enhanced Raman scattering substrates for 6-mercaptopurine detection. RSC Adv 4(31):16327–16332
Arcudi F, Dordevic L, Prato M (2019) Design, synthesis, and functionalization strategies of tailored carbon nanodots. Acc Chem Res 52(8):2070–2079
Long YM, Zhou CH, Zhang ZL, Tian ZQ, Bao L, Lin Y, Pang DW (2012) Shifting and non-shifting fluorescence emitted by carbon nanodots. J Mater Chem 22(13):5917–5920
Li Q, Ohulchanskyy TY, Liu RL, Koynov K, Wu DQ, Best A, Kumar R, Bonoiu A, Prasad PN (2010) Photoluminescent carbon dots as biocompatible nanoprobes for targeting cancer cells in vitro. J Phys Chem C 114:12062–12068
HuangYF Zhou R, Zhang H, Kang KB, Zhao M, Peng Y, Wang Q, Zhang HL, Qiu WY (2014) One-potsynthesis of highly luminescent carbon quantum dots and theirnontoxic ingestion by zebrafish for in vivo imaging. Chemistry 20(19):5640–5648
Chen ZB, Wang J, Miao H, Wang L, Wu S, Yang XM (2015) Fluorescent carbon dots derived from lactose for assaying folic acid. Science China Chem 59(4):487–492
Cao L, Sahu S, Anilkumar P, Bunker CE, Xu J, Fernando KA, Wang P, Guliants EA, Tackett KN, Sun YP (2011) Carbon nanoparticles as visible-light photocatalysts for efficient CO2 conversion and beyond. J Am Chem Soc 133(13):4754–4757
Luo PG, Sahu S, Yang ST, Sonkar SK, Wang J, Wang H, LeCroy GE, Cao L, Sun YP (2013) Carbon “quantum” dots for optical bioimaging. J Mater Chem B 1(16):2116–2127
Feng T, Ai X, An G, Yang P, Zhao Y (2016) Charge-convertible carbon dots for imaging-guided drug delivery with enhanced in vivo cancer therapeutic efficiency. ACS Nano 10(4):4410–4420
Pang W, Jiang P, Ding S, Bao Z, Wang N, Wang H, Qu J, Wang D, Gu B, Wei X (2020) Nucleolus-targeted photodynamic anticancer therapy using renal-clearable carbon dots. Adv Healthc Mater 9(16):2000607–2000614
Li L, Yu B, You TY (2015) Nitrogen and sulfur co-doped carbon dots for highly selective and sensitive detection of Hg (II) ions. Biosens Bioelectron 74:263–269
Yan X, Kong D, Jin R, Zhao X, Li HX, Liu FM, Lin YH, Lu GY (2019) Fluorometric and colorimetric analysis of carbamate pesticide via enzyme-triggered decomposition of gold nanoclusters-anchored MnO2 nanocomposite. Sensors Actuators B Chem 290:640–647
Han X, Meng Z, Xia L, Qu F, Kong RM (2020) o-Phenylenediamine/gold nanocluster-based nanoplatform for ratiometric fluorescence detection of alkaline phosphatase activity. Talanta 212:120768–120775
Han Z, Nan D, Yang H, Sun Q, Pan S, Liu H, Hu XL (2019) Carbon quantum dots based ratiometric fluorescence probe for sensitive and selective detection of Cu2+ and glutathione. Sensors Actuators B Chem 298
Peng C, Xing H, Fan X, Xue Y, Li J, Wang E (2019) Glutathione regulated inner filter effect of MnO2 nanosheets on boron nitride quantum dots for sensitive assay. Anal Chem 91(9):5762–5767
Singh R, Kashayap S, Singh V, Kayastha AM, Mishra H, Saxena PS, Srivastava A, Singh RK (2018) QPRTase modified N-doped carbon quantum dots: a fluorescent bioprobe for selective detection of neurotoxin quinolinic acid in human serum. Biosens Bioelectron 101:103–109
Ma Y, Chen AY, Huang YY, He X, Xie XF, He B, Yang JH, Wang XY (2020) Off-on fluorescent switching of boron-doped carbon quantum dots for ultrasensitive sensing of catechol and glutathione. Carbon 162:234–244
Wei S, Yin X, Li H, Du X, Zhang L, Yang Q, Yang R (2020) Multi-color fluorescent carbon dots: graphitized sp2 conjugated domains and surface state energy level co-modulate band gap rather than size effects. Chem Eur J 26(36):8129–8136
Duan L, Du X, Zhao H, Sun Y, Liu W (2020) Sensitive and selective sensing system of metallothioneins based on carbon quantum dots and gold nanoparticles. Anal Chim Acta 1125:177–186
Han M, Wang L, Li S, Bai L, Zhou Y, Sun Y, Huan H, Li H, Liu Y, Kang Z (2017) High-bright fluorescent carbon dot as versatile sensing platform. Talanta 174:265–273
Gude V, Das A, Chatterjee T, Mandal PK (2016) Molecular origin of photoluminescence of carbon dots: aggregation-induced orange-red emission. Phys Chem Chem Phys 18(40):28274–28280
Zhang F, Liu H, Liu Q, Su X (2018) An enzymatic ratiometric fluorescence assay for 6-mercaptopurine by using MoS2 quantum dots. Mikrochim Acta 185(12):540–547
Algar WR, Tavares AJ, Krull UJ (2010) Beyond labels: a review of the application of quantum dots as integrated components of assays, bioprobes, and biosensors utilizing optical transduction. Anal Chim Acta 673(1):1–25
Zu F, Yan F, Bai Z, Xu J, Wang Y, Huang Y, Zhou X (2017) The quenching of the fluorescence of carbon dots: a review on mechanisms and applications. Microchim Acta 184(7):1899–1914
Sun Z, Liu Y, Li Y (2015) Selective recognition of 6-mercaptopurine based on luminescent metal-organic frameworks Fe-MIL-88NH2. Spectrochim Acta A Mol Biomol Spectrosc 139:296–301
Shi M, Cen Y, Xu G, Wei F, Xu X, Cheng X, Chai Y, Sohail M, Hu Q (2019) Ratiometric fluorescence monitoring of alpha-glucosidase activity based on oxidase-like property of MnO2 nanosheet and its application for inhibitor screening. Anal Chim Acta 1077:225–231
The Pharmacopoeia Committee of the People’s Republic of China (2015) The pharmacopoeia of the People’s Republic of China (2nd part). China Medical Science and Technology Press, Beijing, p 1449
Funding
This work was financially supported by the National Natural Science Foundation of China (Grant No. 21705132) and the Fundamental Research Funds for the Central Universities (no. XDJK2019TY003).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
ESM 1
(DOCX 740 kb)
Rights and permissions
About this article
Cite this article
An, X., Chen, R., Chen, Q. et al. A MnO2 nanosheet-assisted ratiometric fluorescence probe based on carbon quantum dots and o-phenylenediamine for determination of 6-mercaptopurine. Microchim Acta 188, 156 (2021). https://doi.org/10.1007/s00604-021-04802-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00604-021-04802-4