Abstract
DNA aptamers that bind to bovine pregnancy-associated glycoproteins (bPAGs) were selected by the systematic evolution of ligands by exponential enrichment (SELEX) procedure coupled to surface plasmon resonance (SPR) and high-throughput sequencing (HTS) technology. After seven rounds of selection using carboxylated magnetic beads (MB) coated with bovine pregnancy-associated glycoproteins 9 (bPAG9) and bovine serum albumin (BSA) as target and counter targets, respectively, two aptamers designated as A1 and A24 showed high affinities to bPAG9 (Kd = 1.04 and 2.5 nM). Moreover, the specificity was determined by testing the non-targets bPAG4, bPAG6, bPAG16, BSA, and ovalbumin (OVA). Results showed that two aptamers demonstrated broad group specificity to bPAG family. Subsequently, a colorimetric sandwich enzyme-linked aptamer assay was developed for ultrasensitive detection of bPAG9 based on hybridization chain reaction (HCR) amplification strategy. The method exhibited a broad determination from 0.134 to 134 ng/mL with a detection limit of 0.037 ng/mL. The method has been successfully applied to determine bPAGs in real samples. The results demonstrate that the developed aptamers could be used as promising molecular probes for the development of pregnancy diagnostic tools.

In this study, we first selected aptamers against bovine pregnancy-associated glycoproteins (bPAGs) as molecular recognition elements and then developed a colorimetric enzyme-linked aptamer assay utilizing hybridization chain reaction to detect bPAGs in the serum.The GA can't be deleted, the modified GA can not upload. So themodified GA and figures will be send to CorrAdmin3@spi-global.com
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Accurate and timely detection of early pregnancy in dairy cows is a critical tool for shortening the calving interval, thereby improving the reproductive efficiency and profitability [1, 2]. Thus, simple, accurate, and inexpensive early detection of pregnancy is an essential component of modern dairy farming. Transrectal palpation is widely used for the diagnosis of pregnancy in cows; however, it can be difficult to diagnose the pregnancy status accurately earlier than 40 days after insemination [3]. Ultrasonography is an accurate method for the early diagnosis of pregnancy from day 29 after insemination of dairy cows [4] but requires expensive instruments and an experienced operator. Antibody-based immunoassays for detecting bPAGs in blood or milk have been developed and commercialized to determine the pregnancy status in cows [5,6,7]. This method could supplement or replace rectal palpation and ultrasonography for the early diagnosis of pregnancy. Nonetheless, antibody instability, high cost, and complex production procedures limit the application of these methods. Thus, it is imperative to develop new recognition molecules for bPAGs that can be produced easily and stably.
Aptamers are single-stranded DNA or RNA oligonucleotides obtained by the SELEX, which can bind the target analytes with high affinity and specificity [8]. Compared with antibodies, aptamers have not only high affinity and specificity but also many advantages such as chemical synthesis, ease of modification, low cost, and high stability. Therefore, aptamers have been widely utilized as molecular recognition probes in the field of analysis [9,10,11,12,13]. To date, numerous high-affinity DNA aptamers have been successfully selected against various target analytes [14,15,16,17,18]. However, the identification and characterization of aptamers against the bPAGs have not yet been reported.
In the SELEX or biological detection, magnetic bead has been widely used as the solid-phase carrier for target immobilization due to their unique properties, such as large surface area, good stability, easy surface modification, less sample demand, and simple operation. For example, antithrombin aptamer with high affinity and specificity was successfully isolated after four rounds of selection by novel magnetic cross-linking precipitation (MCP)-SELEX approach [19]. Wang et al. [14] utilized MB-SELEX to obtain six aptamers against brain natriuretic peptide (BNP) after 14 rounds of selection. Furthermore, magnetic nanoparticles also have been utilized to construct aptasensors for detection of various target analytes [9, 13].
In the present report, MB-SELEX was employed to select the highly specific aptamers for bPAG9 which is expressed early in pregnancy (by day 25) [20]. After seven rounds of selection, two aptamers against bPAG9 were successfully obtained with high binding capacity and broad group specificity, which are optimal for the future detection of the bPAG family. The selected aptamers could be used as promising affinity probes for the development of cost-effective, sensitive, and innovative pregnancy diagnosis technology for the detection of bPAGs.
Materials and methods
Reagents and materials
Dynabeads® MyOne™ carboxylic acid was purchased from Invitrogen (Carlsbad, CA, USA, https://www.thermofisher.com/order/catalog/product/65011). EvaGreen® Dye, 20X in water was purchased from Biotium (Hayward, CA, USA, https://biotium.com/product/evagreen-dye-20x-in-water/). N-hydroxysuccinimide (NHS), 1-ethyl-3-(3-dimethyl aminopropyl) carbodiimide hydrochloride (EDC), sodium dodecyl sulfate (SDS), streptavidin (SA), BSA, OVA, horseradish peroxidase-labeled streptavidin (HRP-SA), 3,3′,5,5′-tetramethylbenzidine dihydrochloride (TMB), Pfu DNA polymerase, 2×TBE-urea sample buffer, dNTP, TBE buffer, Gelred™ nucleic acid gel stain, and UNIQ-10 spin column oligo DNA purification kit were purchased from Sangon Biotech (Shanghai, China, https://www.sangon.com/). Protein screening kit V1.0 and fluorescent ssDNA ladder were purchased from Anhui Aptamy Biotechnology Co., Ltd. (Hefei, China, https://www.aptamy.com/). All other chemicals of analytical grade were purchased from Sinopharm Chemical Reagent Co., Ltd. (Shanghai, China, https://www.instrument.com.cn/netshow/SH101458/). Streptavidin-coated plates were purchased from Thermo Fisher Scientific Inc. (MA, USA, https://www.thermofisher.com/cn/zh/home.html).
Recombinant proteins
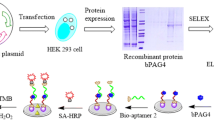
The recombinant proteins, bPAG9 (gene ID NM_176620.2), bPAG4 (gene ID NM_176615.2), bPAG6 (gene ID NM_176617.2), and bPAG16 (gene ID NM_176625.1), were prepared in our laboratory [21], expressed in eukaryotic expression systems (human embryonic kidney 293 cells), and purified by affinity chromatography with > 90% purity. The experimental methods are described in detailed in the Supporting information.
Random library and primers
A single-stranded DNA (ssDNA) library consisted of a central random region of 40 nt flanked by two constant primer regions that were used as the initial pool. Two polymerase chain reaction (PCR) primers “S25-FAM” and “A25-polyA” were used for the amplification of the oligonucleotides during the SELEX process and for the separation of ssDNA from the double-stranded DNA (dsDNA). Two quantitative real-time PCR (qPCR) primers “S25” and “A25” were used for the quantification of the oligonucleotides in the aptamer selection process. The sequences of library and primer are listed in Table S1. The ssDNA library was synthesized and purified by Sangon Biotech (Shanghai, China). The rest of the oligonucleotides were synthesized by GenScript Co., Ltd. (Nanjing, China).
Immobilization of bPAG9 on magnetic beads
The carboxylic group–functionalized MBs were used for the immobilization of bPAG9 by covalent binding. Firstly, 50 μL of carboxylic-functionalized MBs (10 mg/mL) was washed five times with 100 μL binding buffer (BB; 150 mM NaCl, 20 mM HEPES, 1 mM KCl, 1 mM CaCl2, 1 mM MgCl2, pH 7.4). Then, the beads were incubated with 50 μL of 0.4 M EDC and 50 μL of 0.1 M NHS with slow stirring at room temperature for 20 min. Then, the MBs were separated using a permanent magnet and immediately washed with 200 μL BB. Next, 70 μL of NaAc (10 mM, pH 5.0) and 30 μL bPAG9 (0.5 mg/mL) were added to the activated MB and mixed for 60 min at room temperature by agitation. After incubation followed by magnetic separation and three washes with 100 μL of BB, the particles were blocked with 100 μL ethanolamine (1.0 M, pH 8.5) for 15 min with mild shaking. Finally, the bPAG9-MB conjugates were washed and re-suspended in 50 μL BB and stored at 4 °C until use. The BSA-MB conjugates were also prepared simultaneously following the same procedure.
SELEX process
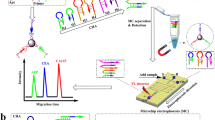
The SELEX procedures were based on previous work with several modifications [14], as illustrated in Scheme 1. The experimental methods are described in detailed in the Supplement section.
High-throughput sequencing
After aptamer selection was complete, the ssDNA pools obtained from each round were diluted with BB to a final concentration of 0.5 μM and amplified by PCR using different index-barcode primers (Table S2). PCR was performed under the same conditions as described in the SELEX process. The PCR products were imaged by 8% PAGE to observe the desired product at 76 bp and confirm the absence of contaminants or byproducts. The amplified dsDNA was pooled for purification using the UNIQ-10 spin column oligo DNA purification kit and quantitated using the NanoDrop 2000c spectrophotometer. Finally, the purified dsDNA was sequenced at the Novogene Technology Co., Ltd. (Beijing, China). The secondary structure of the aptamers was predicted by free-energy minimization algorithm using Mfold (http://unafold.rna.albany.edu/?q=mfold) at 150 mM NaCl, 0.5 mM MgCl2 at 25 °C.
Affinity assays by SPR
The affinity of the enriched pool and selected aptamer for the bPAG9 was assessed by SPR analysis on a Biacore T200 SPR instrument (GE Healthcare, USA) using a CM5 chip (Biacore) at room temperature (25 °C). Briefly, the chip was washed two times with 50 mM NaOH containing 1% SDS at a flow rate of 10 μL/min. Then, it was activated by injecting 50 μL of 0.4 M EDC and 0.1 M NHS (1:1, v/v) at a flow rate of 10 μL/min for 10 min, followed by injecting protein with certain concentrations for 180 s (10 μL/min), resulting in amine coupling of the protein to the activated surface. The remaining unreacted functional groups were blocked by injecting 1 M ethanolamine-HCl (pH 8.5) for 10 min at the same flow rate. The resulting ssDNA was diluted with BB and injected into the flow cell at 30 μL/min for 180 s, and the unbound ssDNA was washed with BB for 30 min at a flow rate of 10 μL/min. To ensure that no residual ssDNA was retained on the surface before the injection of the next concentration, the ligand was regenerated by injecting 1 M NaCl for 1 min at a flow rate of 30 μL/min. The channel of unbound bPAG9 served as a negative control. The negative control flow cell was subtracted as a baseline, and the data analyzed using the BIAevaluation 3.0. The dissociation constant (Kd) was calculated based on a Langmuir 1:1 curve fit using the BIA evaluation 4.0 software (Biacore).
Specificity analysis of selected aptamers
In order to investigate the specificity of the selected aptamers for targeting bPAG9, bPAG4, bPAG6, bPAG16, BSA, and OVA were tested at 30 μg/mL via SPR using the protocol described in the above method. The BB without any target protein was used as a blank control.
HCR-based sandwich enzyme-linked aptamer assay
To demonstrate the potential application of the aptamers in the quantitative determination of bPAGs, a HCR-based sandwich enzyme-linked aptamer assay (HCR-SELAA) was developed. All the oligonucleotides used in this assay are listed in Scheme 2. Prior to use, biotin-hairpin DNA 1 (bio-H1) and biotin-hairpin DNA 2 (bio-H2) were separately diluted to 1 μM, heated at 95 °C for 5 min, and then cooled to room temperature for 1 h. The assay was performed using SA-coated white microplates at room temperature. Before biotinylated capture probe (bio-CP) immobilization, the microplates were washed three times with 200 μL of BB, after which 100 μL of bio-CP (100 nM) was added to each well and incubated for 30 min with mild shaking. Following washing, 100 μL of various concentrations of bPAG9 solution was added and incubated for another 20 min. The plate was washed again, and 100 μL of detection probe (DP, 150 nM) was added to each well and incubated for 20 min to construct the sandwich-type complexes of CP-bPAG9-DP. The unbound DNA was removed by washing with BB for three times. Subsequently, the plate was incubated with a mixed liquid containing 50 μL bio-H1 and 50 μL bio-H2 for 60 min. After washing, 100 μL of SA-HRP (0.025 U/mL) was added to the well and incubated for 10 min. The plate was washed with BB three times and 100 μL of TMB solution was added to each well. After incubation for 10 min, the reaction was stopped by addition of 100 μL of 2.0 M H2SO4, and the absorbance was detected at 450 nm with a microtiter plate reader (Mk3; Thermo, USA).
Analytical application in real samples
The blood samples used for the HCR-SELAA were obtained from Holstein Friesian cows at 30 days after insemination. Pregnancy status was confirmed by transrectal ultrasonography using a portable B-mode ultrasound scanner equipped with a 6.0/8.0 MHz linear-array transducer (Tringa Linear VET, Esaote/Pie Medical, Italy). After confirmation, the blood sample was collected from the tail vein of the pregnant and non-pregnant cows using vacutainers. The blood was centrifuged at 2000×g for 10 min at room temperature. The collected serum was transferred to fresh tubes and subjected to analysis as described above. The 5 ng/mL of bPAG9 standard was used as positive controls.
Results and discussion
Selection of aptamers
The efficient monitoring of the progression of SELEX is essential for the successful selection of aptamers with optimal binding affinities. qPCR amplification curve (AC), pool recovery, and bonding analyses were used to monitor the convergence of the aptamer species during the selection process [22, 23]. As shown in Fig. 1a, after the amplification curve reached the plateau, the decline of fluorescence was weakening gradually with increase in the selection round number, and then gradually become smooth at R7, which indicates that the diversity of DNA libraries decreased and the degree of enrichment increased with continual SELEX [22]. Simultaneously, the amount of ssDNA bound to bPAG9-coated MBs would increase with progress in the number of the selection round. The results in Fig. 1b demonstrated that the retention rate of the ssDNA pool increased gradually with an increase in the selection from R1–R4, and reached a maximum at R7. Conversely, the binding rate of R7 for the counter selection reached the minimum (Fig. 1b), indicating a high affinity and specificity of the candidates to bPAG9.
a Amplification curve and b retention rate of ssDNA pool after each SELEX round. c Affinity analysis of ssDNA pool after every round by SPR. The concentration of the pool was 0.5 μM, and the concentration of the proteins was 50 μg/mL. d The PAGE of ssDNA of different selection rounds of asymmetric PCR. M, marker; lanes 1–7, PCR products from enrichment rounds 1–7; 2 μL of ssDNA pool at a concentration of 0.5 μM
The affinities of the enriched pool toward bPAG9 after every selection cycle were further evaluated by SPR. As shown in Fig. 1c, the initial ssDNA library (R0) did not have an apparent affinity toward bPAG9 even at the highest bPAG9 concentration. The response signals (RUs) increased slightly with the selection round number increasing from R1 to R5, and a substantial increase was observed at R6. No further evolution was observed between R6 and R7. These results suggested that the candidates in the pool for the target were enriched and displayed an increased affinity for the target. Thus, pool 7 can be considered the end of productive selection. After each SELEX round, aptamer pools with the expected size were also analyzed by 8% PAGE; the length of all the selected aptamers was about 76 bp (Fig. 1d), which was in accordance with the designed length of the sequence of the aptamer.
High-throughput sequencing
After R7, the SELEX process was complete, and the selected aptamer pool was high-throughput sequencing. After discarding the sequences with mismatches in the constant regions and with random regions that differed in length from 36 nts, 5.2 × 105 sequences were analyzed for the frequency distribution of the random N36 part. A total of 43 sequences were represented > 500 times, 26 sequences presented over 1000 times, and 2 sequences were represented over 5000 times. Figure 2 summarizes the composition of the sequencing results over the seven selection rounds. Also, the abundance of the top 10, 100, and 500 sequences increased with increased rounds of selection. After the seven-round selection, the top 10 sequences were enriched to 6.72% in the total of 523,000 sequences, indicating that the complexity of the cycle libraries decreases with the progression of the SELEX, which was in agreement with the AC.
Affinity and specificity of selected aptamers
The top 300 sequences were classified into four families based on the similarity of DNA sequences. The high abundant sequences from each family were synthesized, and their affinity for bPAG9 was assessed by SPR. According to the results shown in Fig. 3, of the 96 candidates, aptamer A24 showed the strongest binding affinity to bPAG9 as compared with the other aptamers, followed by aptamer A6 (RU = 22.2), aptamer A18 (RU = 21.3), aptamer A9 (RU = 20.8), and aptamer A10 (RU = 20.5). However, these aptamers share remarkable homology with A24, which makes them unsuitable for use in sandwich analysis methods due to the same binding site. Therefore, aptamer A1 sharing low homology with aptamer A24 was selected. Then, the Kd of aptamers A1 and A24 to the target bPAG9 was testified using a concentration series (0–500 nM) of each ssDNA aptamer and a constant concentration of bPAG9 (30 μg/mL). Figure 4a and b revealed that the Kd values of the two aptamers were at the nanomolar level, and the lowest Kd of 1.04 nM was obtained for aptamer A1. This suggested that the selected aptamers with high affinity for the target bPAG9 are essential for the highly sensitive detection of bPAG9.
To characterize the specificity of the selected aptamer, A1 and A24 were tested against a variety of other proteins, including bPAG family, BSA, and OVA. As shown in Fig. 4c and d, two aptamers strongly bound not only to bPAG9 but also to the homologous proteins (bPAG4, bPAG6, and bPAG16) in similar rank order of binding affinity for each bPAG. The bPAG9 is a member of a family of PAGs characterized by a highly conserved bilobed structure. Therefore, aptamers selected against bPAG9 may cross-react with the other member of the bPAG family. Furthermore, none of the aptamers bounds to a structurally distinct chemical, BSA, and OVA, indicating that aptamers selected in this study are specific to bPAGs.
Prediction of aptamer structure
To further characterize the selected aptamers, the secondary structures of two aptamers were predicted using Mfold based on the criterion of minimum free energy. As shown in Fig. S1, stem-loop structure constitutes the major part of the aptamers, which was reported previously as one of the most widespread structural elements for target recognition [14, 17, 24, 25]. In addition, the high percentage of G/C was found in the selected two aptamer sequences, which was considered to increase the stability of the aptamer-target complex [26]. A1 contains 11G, 20C, 11A, and 14T, while A24 contains 25G, 9C, 9A, and 13T.
HCR-based sandwich enzyme-linked aptamer assay
To demonstrate the potential application of the aptamers in the quantitative determination of bPAGs, a highly sensitive and simple HCR-SELAA colorimetric assay was conducted. As illustrated in Scheme 2, this assay system consists of four main components: a bio-CP (bio-aptamer A1), two hairpin DNAs (bio-H1 and bio-H2), and a detection probe (DP). The DP consists of the aptamer A24 sequence and the initiation sequence (in italics) to trigger the HCR. The fragment at the 3′ end of bio-H1 (in italics) is complementary to the 3′ end of bio-H2 (in italics), and the 5′ end of bio-H2 (in bold) is complementary to the 5′ end of bio-H1 (in bold). For the detection, in the presence of the target protein, bPAG9 is captured by bio-CP immobilized on the microplate. Following the addition of DP, a sandwich CP-bPAG9-DP complex is formed due to the affinity of aptamer toward target protein at different epitopes. Then, the initiation sequence at the 3′ end of DP is complementary to the 3′ end of bio-H1 (in italics), and 24-bp at the 5′ end of bio-H1 (in bold) hybridizes with 24-bp at the end of bio-H2 (in bold); the newly exposed 3′ end of bio-H2 (in italics) hybridized with 3′ end of bio-H1 (in italics). Thus, each initiation sequence on the sandwiched probes triggered a chain reaction of hybridization events with the bio-H1and bio-H2 to yield nicked double-helices DNA polymer. When the SA-HRP is added, it can specifically bind with biotin in DNA polymer, thus obtains a big UV-vis absorption signal by the HRP catalyzing H2O2 + TMB system. By this means we could determine the concentration of bPAGs according to the intensities of UV-vis absorbance or change of color.
To achieve a high detection sensitivity, several experimental parameters were optimized including the aptamer concentration, bio-H1/bio-H2 concentration, SA-HRP concentration, and incubation time for HCR. The corresponding results are shown in Fig. S2. The following experimental conditions were found to give best results: (A) capture probe concentration of 100 nM, and detection probe concentration of 150 nM; (B) SA-HRP concentration of 0.025 U/mL; (C) bio-H1/bio-H2 concentrations of 600 nM; (D) interaction time of 20 min between bPAGs and aptamer; (E) HCR hybridization time of 60 min.
Under the optimal conditions, the linear range and detection limit of colorimetric assay were evaluated by using a series of bPAG9 standard solution within the concentration range of 0–2680 ng/mL. As shown in Fig. 5, the color intensity of the solution increased with increasing the concentration of bPAG9 from 0.0134 to 2680 ng/mL. A qualitative detection for bPAG9 can be achieved by visual observation, with the visual limit of 0.134 ng/mL. Furthermore, the variation of the UV-vis absorbance at 450 nm with different concentrations of the target protein is shown in Fig. 5. A good linear correlation was obtained between the absorbance and the concentration of bPAG9 from 0.134 to 134 ng/mL, with the correlation coefficient (R) of 0.990. The limit of detection (LOD) is estimated to be 0.037 ng/mL based on 3σ/slope (σ, standard deviation of the blank samples, n = 10).
Analytical application in real samples
To validate the actual effectiveness of the developed visual HCR-SELAA, 6 serum samples from pregnant cows were analyzed by the proposed method. As shown in Fig. S3, all serum samples from pregnant cows were shown to be positive, which was in accordance with ultrasonography. This suggested that the HCR-SELAA had good accuracy and reliability in real samples.
Conclusion
In this study, aptamers against target bPAG9 were screened successfully after seven rounds of selection by MB-SELEX technology. The selected aptamers have high affinity and specificity to the bPAG family. Furthermore, a signal-amplified colorimetric bioassay with the selected aptamer was developed for ultrasensitive detection of bPAGs. The developed method provides a detection limit of 0.037 ng/mL for instrument detection and 0.134 ng/mL for visual detection. Moreover, our assay had been successfully applied in real samples, which indicate it holds promising potential for broad applications in early pregnancy diagnosis.
References
LeBlanc S (2015) Assessing the association of the level of milk production with reproductive performance in dairy cattle. J Reprod Dev 56:1–7
Kossaibati MA, Esslemont RJ (1997) The costs of production diseases in dairy herds in England. Vet J 154:41–51
Youngquist RS (2007) Pregnany diagnosis. In: Youngquist RS, Threlfall WR (eds) Current therapy in large animal theriogenology, 2nd edn. Saunder, Missouri, pp 294–303
Romano JE, Thompson JA, Forrest DW, Westhusin ME, Tomaszewski MA, Kraemer DC (2006) Early pregnancy diagnosis by transrectal ultrasonography in dairy cattle. Theriogenology 66:1034–1041
Karen A, Sousa NMD, Beckers JF, Bajcsy ÁC, Tibold J, Mádl I, Szenci O (2015) Comparison of a commercial bovine pregnancy associated glycoprotein ELISA test and a pregnancy associated glycoprotein radiomimmunoassay test for early pregnancy diagnosis in dairy cattle. Anim Reprod Sci 159:31–37
Friedrich M, Holtz W (2010) Establishment of an ELISA for measuring bovine pregnancy-associated glycoprotein in serum or milk and its application for early pregnancy detection. Reprod Domest Anim 45:142–146
Dufour S, Durocher J, Dubuc J, Dendukuri N, Hassan S, Buczinski S (2017) Bayesian estimation of sensitivity and specificity of a milk pregnancy-associated glycoprotein-based ELISA and of transrectal ultrasonographic exam for diagnosis of pregnancy at 28 to 45 days following breeding in dairy cows. Prev Vet Med 140:122–133
Yuan Q, Lu DQ, Zhang XB, Chen Z, Tan WH (2012) Aptamer-conjugated optical nanomaterials for bioanalysis. Trac-Trend Anal Chem 39:72–86
Lu CX, Tang ZG, Liu CB, Kang LC, Sun FX (2015) Magnetic-nanobead-based competitive enzyme-linked aptamer assay for the analysis of oxytetracycline in food. Anal Bioanal Chem 407:4155–4163
Wang S, Dong YY, Liang XG (2018) Development of a SPR aptasensor containing oriented aptamer for direct capture and detection of tetracycline in multiple honey samples. Biosens Bioelectron 109:1–7
Liu C, Guo YJ, Luo F, Rao PF, Fu CL, Wang SY (2017) Homogeneous electrochemical method for Ochratoxin A determination based on target triggered aptamer hairpin switch and exonuclease III-assisted recycling amplification. Food Anal Methods 10(6):1982–1990
Wu SJ, Liu LH, Duan N, Li Q, Zhou Y, Wang ZP (2018) An aptamer-based lateral flow test strip for rapid detection of zearalenone in corn samples. J Agric Food Chem 66(8):1949–1954
Liu CB, Lu CX, Tang ZG, Chen X, Sun FX (2015) Aptamer-functionalized magnetic nanoparticles for simultaneous fluorometric determination of oxytetracycline and kanamycin. Microchim Acta 182(15):2567–2575
Wang Y, Wu JJ, Chen YJ, Xue F, Teng J, Cao JX, Lu CX, Chen W (2015) Magnetic microparticle-based SELEX process for the identification of highly specific aptamers of heart marker–brain natriuretic peptide. Microchim Acta 182:331–339
Chen XJ, Huang YK, Duan N, Wu SJ, Xia Y, Ma XY, Zhu CQ, Jiang Y, Ding ZS, Wang ZP (2014) Selection and characterization of single stranded DNA aptamers recognizing fumonisin B1. Microchim Acta 181:1317–1324
Yu XF, Chen F, Wang RH, Li YB (2018) Whole-bacterium SELEX of DNA aptamers for rapid detection of E. coli O157:H7 using a QCM sensor. J Biotechnol 266:39–49
Niazi JH, Lee SJ, Kim YS, Gu MB (2008) ssDNA aptamers that selectively bind oxytetracycline. Bioorg Med Chem 16:1254–1261
Wu YG, Zhan SS, Wang LM, Zhou P (2014) Selection of a DNA aptamer for cadmium detection based on cationic polymer mediated aggregation of gold nanoparticles. Analyst 139(6):1550–1561
Qiao N, Li J, Wu X, Diao DL, Zhao JX, Li JY, Ren XJ, Ding XF, Shangguan DH, Lou XH (2019) Speeding up in vitro discovery of structure-switching aptamers via magnetic cross-linking precipitation. Anal Chem 91:13383–13389
Green JA, Xie S, Quan X, Bao B, Gan X, Mathialagan N, Beckers JF, Roberts RM (2000) Pregnancy-associated bovine and ovine glycoproteins exhibit spatially and temporally distinct expression patterns during pregnancy. Biol Reprod 62:1624–1631
Liu CB, Shi GQ, Lu CX (2019) Eukaryotic expression and purification of recombinant bovine pregnancy associated glycoprotein-9. Xinjiang Agric Sci 56(8):1552–1559 (in chinese)
Luo ZF, He L, Wang JJ, Fang XN, Zhang LY (2017) Developing a combined strategy for monitoring the progress of aptamer selection. Analyst 142:3136–3139
Gao SX, Hu W, Zheng X, Cai S, Wu JH (2019) Functionalized aptamer with an antiparallel G-quadruplex: structural remodeling, recognition mechanism, and diagnostic applications targeting CTGF. Biosens Bioelectron 142:111475
Niazi JH, Lee SJ, Gu MB (2008) Single-stranded DNA aptamers specific for antibiotics tetracyclines. Bioorg Med Chem 16:7245–7253
Handy SM, Yakes BJ, DeGrasse JA, Campbell K, Elliott CT, Kanyuck KM, DeGrasse SL (2013) First report of the use of a saxitoxin-protein conjugate to develop a DNA aptamer to a small molecule toxin. Toxicon 61:30–37
Kulbachinskiy AV (2007) Methods for selection of aptamers to protein targets. Biochem Mosc 72:1505–1518
Funding
This work was financially supported by the National Natural Science Foundation of China (31860647).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
All experimental animal procedures were performed in compliance with the China Code of Practice Animals for the Care and Use of Animals for Scientific Purposes. The animal welfare was approved by Animal Experimental Ethical Review Form of the First Affiliated Hospital of Medical College, Shihezi University.
Conflict of interest
The authors declare that they have no competing of interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
ESM 1
(DOCX 251 kb)
Rights and permissions
About this article
Cite this article
Lu, C., Liu, C. & Shi, G. Colorimetric enzyme-linked aptamer assay utilizing hybridization chain reaction for determination of bovine pregnancy-associated glycoproteins. Microchim Acta 187, 316 (2020). https://doi.org/10.1007/s00604-020-04301-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00604-020-04301-y