Abstract
A boronate affinity monolith with improved affinity and selectivity for glycoproteins was prepared starting from two monomers. The first is 3-aminopropyltriethoxysilane-methacrylic acid (APTES-MAA), and the other is a polyhedral oligomeric silsesquioxane (POSS) monomer. In the next step, 3-(acrylamido)benzeneboronic acid was adopted as boronate affinity ligand, and ethylene glycol dimethacrylate as the crosslinker, and iso-propanol and octanol as binary porogens. The synergistic effect of APTES-MAA and POSS warrants good affinity and selectivity for glycoproteins, which results in a number of attractive features including (a) a wide operation pH range (from 5 to 8); (b) higher enrichment factors ranging from 19.3 to 20.6; (c) greater recoveries of glycoproteins between 95.8 and 107.1%; (d) lower relative standard deviations of ≤4.2%. Compared to the corresponding APTES-MAA/POSS-free monolith, the new boronate material had 1.7-fold increased glycoprotein recovery from complex samples. Glycoproteins in 500-fold diluted serum samples can be enriched by the boronate monolith.

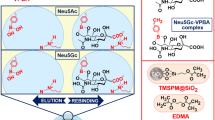
Schematic representation of the preparation of 3-aminopropyltriethoxysilane-methacrylic acid/polyhedral oligomeric silsesquioxanes boronate affinity monolith. This sorbent exhibits high selectivity and wide pH operation range for capturing glycopeptides.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Glycoproteins play a pivotal role in biological and clinical significance, such as disease detection, inter and intracellular signaling, cell regulation and so on [1,2,3]. At the same time, it is difficult to detect glycoproteins even with the most sensitive mass spectrum without separation and enrichment, because of the serious hindrance of high abundance components in complex samples [4, 5]. Therefore, it is extremely important to develop highly specific methods for enriching and isolating the low-abundance glycoproteins. At present, there are many methods for enriching glycoproteins selectively, such as boronate affinity [6] and lectin affinity chromatography [7], and hydrazide chemistry [8]. etc. Among them, boronate affinity material is widely studied because it can reversibly combine with cis-diols of glycoproteins by pH controlling [9,10,11,12]. However, the boronate affinity materials made with conventional approaches require operation condition at pH above their pKa values (ca. 8–9), thus suffer the risk of degradation of labile compounds at such conditions.
A number of approaches have been developed to fabricate boronate acid-functionalized materials with lower pKa values that can give rise to strong binding under mild acidic and neutral conditions [13,14,15,16,17,18,19]. For example, electron-with-drawn groups e.g., sulfonyl [15, 20] and carbonyl [21] were introduced into boronate ligand to reduce the pKa values of the resultant boronate affinity materials. In addition, with an amine [22] or oxygen [17] incorporated into the boronic acid molecules, the resulting boronate material had lower pKa values by forming an intramolecular boron-nitrogen/oxygen coordination. However, a multi-step synthesis method was adopted because no commercial monomer was available, making the preparation of boronate affinity materials time-consuming, inconvenient and difficult. When another molecule containing an amino group adjacent to the boron atom in configuration was introduced, an intermolecular B-N coordination can be formed, leading to improved affinity by one-step synthesis [16]. However, as a new field in boronate affinity materials, there are a fewer monomers available for the intermolecular B-N coordination and further exploration is needed.
Organic-inorganic hybrid monomer is new functional monomer combining the advantages of organic and silica monomer, which can lead to monolithic column with high mechanical stability, less shrinkage and pH stability [23]. For instance, a hybrid functional monomer (3-aminopropyltriethoxysilane-methacrylic acid-methacrylic acid, APTES-MAA) can be utilized to prepare molecularly imprinted polymer. The APTES-MAA monomer can be synthesized easily through a dehydration condensation between APTES and MAA [24]. The structures of APTES-MAA permits silicate ester bond, helping monomer embedded in the silica matrix after condensation, and acylamino group forming B-N coordination with boronate ligands. One can expect that a mechanism of self-assembled molecular team with lower pKa may take place between cis-diol compounds and the boronate materials. However, to the best of our knowledge, there was no report about on the boronate affinity materials made using hybrid monomer APTES-MAA.
Polyhedral oligomeric silsesquioxanes (POSS) are nanoscale building blocks with a unique three-dimensional cage structure [25], which are composed of well-defined nano-cluster with an inorganic rigid silica core and eight organic functional group. These POSS nanoparticles can be easily incorporated into polymer materials by copolymerization or blending with tailorable interfacial properties, thereby avoiding the rigorous and complicated operation of the “sol-gel” method to prepare hybrid materials. Some novel properties of composite materials are altered by the incorporation of POSS, e.g., improved mechanical and solvent resistant properties [26]. Studies have also reported that POSS endows molecularly imprinted polymers with improved imprinting effect by suppressing non-selective binding sites [27], and Si-O-Si groups in silica cages ensured effective resistance to non-glycoproteins [28]. Therefore, based on the superiority and applicability of the above POSS doped materials, it is expected that the separation and enrichment of glycoproteins with the POSS-based boronate affinity materials for extraction can be further improved.
In this study, a new boronate affinity monolith was first prepared by a one-step procedure in situ polymerization. APTES-MAA was used to form B-N bonding, 3-(acrylamido)benzeneboronic acid (AAPBA) as boronic acid ligands, POSS monomer as co-monomer and ethylene dimethacrylate (EDMA) as crosslinker. The synergistic effect of APTES-MAA and POSS to improve the affinity of boronate material was demonstrated. By utilizing the synergistic effect, we hereby developed boronate monolith having (i) good solvent resistance and pH stability, (ii) greater rigidity, and (iii) binding glycoproteins at a wide range of pH (5–8). Finally, the resulting boronate monolith was applied to the selective enrichment of glycoproteins from human serum samples by polymer monolith microextraction (PMME).
Experimental
Materials
Azobisisobutyronitrile (AIBN, AR grade), 3-aminopropyltriethoxysilane (APTES, 98%) and 3-(acrylamido)phenylboronic acid (AAPBA, 98%) were obtained from J&K CHEMICA Co., Ltd. (Beijing, China, www.jkchemical.com/index.aspx). Ethylene glycol dimethacrylate (EDMA, 98%) and 3-(trimethoxysilyl) propylmethacrylate (γ-MPS, 98%) were purchased from Sigma–Aldrich (St. Louis, MO, USA, www.sigmaaldrich.com). Methacrylic acid (MAA) was obtained from Tianjin Kemiou Chemical Reagent (Tianjin, China, www.chemreagent.com). POSS monomer was supplied by Hybrid Plastics Reagent Co., Ltd. (Hattiesburg, MS, USA, hybridplastics.com). Bovine serum albumin (BSA, Mw 66.4 kDa), cytochrome C (Cyt C, Mw 12.4 kDa), transferrin (Trf, Mw 76.7 kDa), horseradish peroxidase (HRP, Mw 40.0 kDa), and ovalbumin (OVA, Mw 45 kDa) were ordered from Macklin Biochemical Technology Co., Ltd. (Shanghai, China, www.macklin.cn). Coomassie brilliant blue G-250 was purchased from Dingguo Changsheng Biotechnology Co., Ltd. (Beijing, China, www.dingguo.com). SDS-PAGE Gel kit and the samples of healthy human serum were obtained from Beijing Solarbio Science & Technology Co., Ltd. (Beijing, China, solarbiojx.foodmate.net). Fused-silica capillaries (250 μm ID, 375 μm OD) were purchased from Xinnuo Optic Fiber Plant (Hebei, China, www.11467.com/handan/co/68426.htm). All other analytical grade reagents were supplied by Tianjin Chemical Reagent Co. Ltd. (Tianjin, China, www.2121853.atobo.com.cn).
Synthesis of APTES-MAA
As indicated in Fig. 1a, in general, MAA (3.46 mL) was mixed with APTES (6 mL). After stirring with the glass rod for 5 min, the mixing solution was purged with nitrogen for 5 min to remove dissolved oxygen and sealed, followed by reaction at 60 °C for 24 h. Then a vinyl-containing alkoxysilane (APTES-MAA) was formed (Fig. S1, in the Electronic Supporting Material (ESM)).
Preparation of poly(AAPBA-co-APTES-MAA-co-POSS-co-EDMA)
Before in situ polymerization, the walls of the capillary were vinylized by γ-MPS to allow covalent attachment of the monolith according to the previous method [11]. As shown in Table S1 (in ESM), AAPBA and AIBN (2 mg) were dissolved with a mixture of iso-propanol (420 mg) and 1-dodeanol (48 mg). Then, POSS monomer, the cross-linker of EDMA and APTES-MAA were added into the mixture. After ultrasonication for 2 min, the mixture was introduced into the modified capillary (20 cm length) with the ends sealed. The polymerization was heated at 60 °C in water bath for 2 h (Fig. 1b). To remove unreacted reagents, the resulting poly(AAPBA-co-APTES-MAA-co-POSS-co-EDMA) column was washed with acetonitrile and methanol/acetic acid (9: 1, v/v), respectively.
Sample preparation
Stock solutions of BSA, Cyt C, Trf, HRP and OVA were dissolved in sodium phosphate buffer (100 mM, pH 8) at a concentration of 2 mg· mL−1. By diluting the stock solutions with phosphate buffer solution mentioned above, the desired concentration of protein solutions was obtained. Healthy human serum sample was diluted 500-fold with phosphate buffer solution (100 mM, pH 8). All samples were stored at 4 °C prior to analysis.
PMME procedures and SDS-PAGE analysis
The PMME procedures consisted of preconditioning, sample loading, washing and desorption. The PMME device was prepared according to the previous work [11]. Briefly, one end of the pinhead of 1.0 mL syringe was coupled seamlessly to the bororate capillary with 20 cm. A digital injection pump (RSP04-B, RISTRON, Zhejiang, China, www.chinamot.com) was used to pump solution during the PMME process at a constant rate.
Before PMME procedures, the monolith was firstly equilibriated with 100 μL methanol and 100 μL phosphate buffer solution (100 mM, pH 8) at 5 μL· min−1, respectively. The monolithic material was then loaded with the sample and rinsed with 100 μL phosphate buffer solution (100 mM, pH 8) and 100 μL phosphate buffer solution (100 mM, pH 3) at a flow rate of 3 μL· min−1, respectively. Elution solution was final delivered with phosphate buffer solution (100 mM, pH 3) at a flow rate of 3 μL· min−1. The eluate collected was subjected to SDS-PAGE and quantitative analysis.
The quantitative analysis was conducted according to Bradford method [29]. The dye Coomassie blue G250 was mixed with the sample at a volume ratio of 1/10 and then incubated for 2 min at room temperature. Absorbance was measured by UV-2201 spectrophotometer at 595 nm (T6 New Century, Beijing, China, www.pgeneral.com).
Results and discussion
Affinity of poly(AAPBA-co-APTES-MAA-co-POSS-co-EDMA) monolith
To obtain a best poly(AAPBA-co-APTES-MAA-co-POSS-co-EDMA) monolith, the following parameters were optimized: (a) the content of AAPBA; (b) the content of APTES-MAA; (c) the content of POSS; (d) the content of EDMA. Corresponding figures and data can be found in Fig. S2 (in ESM). Shortly, the best polymer conditions were: (a) content of AAPBA: 24.8%; (b) optimal the content of APTES-MAA: 6.2%; (c) optimal content of POSS: 3.7%; (d) optimal content of EDMA: 65.3%.
To clarify the cooperation effect of POSS and APTES-MAA as the functional monomer on boronate affinity, the PMME performance of glycoproteins from the boronate affinity monolith synthesized with different monomers was studied (Fig. 2). The AAPBA-free monolith made using POSS and APTES-MAA as the monomer present a limited recovery of 1% (C4), indicating that POSS and APTES-MAA have no affinity to glycoproteins with the very small non-specific adsorption. In the absence of POSS or APTES-MAA, the resulting boronate affinity monolith (C1) shows a low recovery of OVA (38.8%). After the incorporation of APTES-MAA into boronate affinity monolith (C2), the recovery of OVA increases to 53.7%. When POSS is replaced by APTES-MAA, the recovery of OVA on the resulting boronate affinity monolith (C5) reduces to 9%. In contrast, the recovery of the boronate affinity monolith synthesized by the dual-monomers (APTES-MAA/POSS) (C3) is the highest (98.9%) among all of the boronate affinity monoliths.
Selectivity of boronate material
In order to verify the specific selectivity of the resultant bororate monolith, PMME was carried out using a mixed solution of glycoproteins (OVA, Trf and HRP) and non-glycoproteins (Cyt C and BSA) and the eluent was used to SDS-PAGE analysis. As shown in Fig. 3a, BSA and Cyt C were no retention on the resultant boronate affinity monolith basically (lane 2). In contrast, Trf, OVA and HRP were well captured and released on the boronate monolith (lane 2 and 4). In addition, different proteins were used to test the selectivity of the APTES-MAA/POSS-containing and APTES-MAA/POSS-free boronate affinity monolith (Fig. 3b). The recovery of glycoprotein on the boronate affinity monolith synthesized by the bi-monomers (APTES-MAA / POSS) (C3) is about 1.7 times higher than that on the APTES-MAA/POSS-free boronate affinity monolith (C1). The recovery of non-glycoprotein on the resulting monolithic column is also significantly low. In summary, the results above indicated that the boron-affinity monolith synthesized by the bimonomers (APTES-MAA/POSS) has a significantly increased selectivity for glycoproteins.
SDS-PAGE analysis of proteins mixture before and after extraction with APTES-MAA/POSS boronate affinity monolith (a). Lane 1: proteins mixture solution and each protein at a concentration of 100 μg· mL−1; lane 2: the sampling step solution passing through the APTES-MAA/POSS-doped boronate affinity monolith; lane 3: the washing step solution; lane 4: the eluate; lane 5: marker. Comparison of APTES-MAA/POSS-doped and bare boronate affinity monolith on extraction of different proteins (b). Conditions: extraction volume: 1.0 mL, other was same as Fig. 2
To investigate the interference effect of other polyols and saccharides, OVA was chosen as the test protein, while fructose and catechol were interfering substances. When the weight ratio of the interferents to the targets increased from 1:0 to 1:1000, only 7.9% of the recovery of OVA decreased (Fig. S4a, in ESM). Furthermore, the effect of the interfering substances on the extraction of glycoprotein is similar at different loading pHs (Fig. S4b, in ESM). The experimental results show the greater selectivity of the resultant monolith to glycoproteins even in the presence of polyols and saccharides.
Characterization of poly(AAPBA-co-APTES-MAA-co-POSS-co-EDMA) monolith
As shown in scanning electron microscope (Fig. 4), it is found that all the monolith was tightly bound to the inner wall of the capillary. Large marcopores and microglobules are found in all of the monoliths. The APTES-MAA/POSS-containing boronate affinity monolith has smaller particles and uniform through-pores compared to other monolithic columns.
The images of scanning electron microscope of poly (AAPBA-co-EDMA) monolith (C1) (a-1, a-2), poly(AAPBA-co-APTES-MAA-co-EDMA) monolith (C2) (b-1, b-2), poly(AAPBA-co-APTES-MAA-co-POSS-co-EDMA) monolith (C3) (c-1, c-2), poly(APTES-MAA-co-POSS-co-EDMA) monolith (C4) (d-1,d-2) and poly(AAPBA-co-POSS-co-EDMA) monolith (C5) (e-1, e-2)
All the polymers studied show type IV isotherm measured by nitrogen adsorption method (Fig. S5, in ESM), which display closure point at lower p/p0 and no limit of adsorption at higher p/p0. According to the IUPAC classification, the polymers can be attributed to the type of H3 loops, which suggests the feature of the structure of slit-like mesopore [30]. As shown in Table S2 (in ESM), the boronate affinity monolith prepared with APTES-MAA/POSS has lower BET surface area and average pore diameter. Compared with the POSS-free boronate affinity monolith (C2), the BET surface area and total pore volume of the POSS incorporated boronate affinity monolith (C3) increase by 27% and 23%, respectively. Thus, the synergistic effect of APTES-MAA and POSS rather than specific surface area contributes to the increased affinity of the resulting boronate affinity monolith.
Optimization of PMME conditions
Effect of the solution pH
pH value is one of the greatest important factors in reversible combination between boronic acid ligands and glycoprotein. In this experiment, the effect of the pH of loading buffer on the recovery of OVA were investigated. Extraction sample was prepared with different pH 4.0, 5.0, 6.0, 7.0, 8.0, and 9.0 of phosphate buffer solution since the formation of ester between glycoprotein and boronate ion requires sufficient hydroxide ions. It should be noted that at pH 5.0 high recovery of OVA was still achieved. Such a binding pH is rather low thus the applicable range of samples using the boronate material can be expanded to blood, tear, saliva and urine. When the pH was more than 8.0, the recovery showed a decrease trend. This may be caused by the hydrolysis of the resulting siloxanes hybrid monolithic column at higher pH [18] (Fig. S6a, in ESM).
Elution phosphate buffer solution was prepared with different pH 2.0, 3, 3.5, 4.0 and 4.5, respectively. When the pH of phosphate buffer solution were 2.0 and 3.0, a relatively high recovery of OVA can be achieved. The recovery at pH 2 is only 0.6% higher than the recovery at pH 3 (Fig. S6b, in ESM). However, the resultant monolith may be washed out at pH 2. As a result, pH 3.0 of the elution buffer was chosen for subsequent experiments.
Effect of extraction and desorption volume
Important factors for protein extraction also include desorption and extraction volume. As shown in Fig. S6c, d (in ESM), the effect of the loading amount and desorption volume on the recovery for OVA were studied in the range of 20–120 μg and 25–200 μL, respectively. When the loading amount of the sample was higher than 60 μg, the recovery of OVA will reduce because adsorption reaches saturation of the resultant monolith, suggesting the binding capacity of the resultant monolith to OVA of 60 μg. When the desorption volume was higher than 50 μL, no obvious change can be observed in terms of the recovery of OVA. As a result, the desorption volume of extraction procedure was set as 50 μL.
Method analysis
The method of the glycoprotein extraction of the APTES-MAA/POSS boron-affinity monolith was validated by linear range, determination coefficient (R2), enrichment factor (EF) and reproducibility. Glycoproteins exhibited good linearity with satisfactory determination coefficient (R2) (above 0.9988) (Table S3, in ESM). Under the above optimized extraction conditions, the enrichment factors of the monolith was 19.5, 19.3 and 20.6 for OVA, OVT and Trf, respectively. Simultaneously, the on column and column-to-column repeatability of the poly(AAPBA-co-APTES-MAA-co-POSS-co-EDMA) monolith were characterized by relative standard deviations (RSD) for the recovery of different proteins (less than 5%). The recovery of glycoproteins were in the range between 95.8% and 107.1%.
Table 1 compared the performance of a number of boron-affinity materials in extracting glycoproteins [6, 10, 11, 31,32,33,34]. The extraction method in this work had higher enrichment factor than that in ref. [10], and better reproducibility than that in ref. [11]. Even if the method described by Liu et al. [31] had better reproducibility and low loading pH, the solution used in our method did not contain any organic solvents and avoided the risk of degradation of glycoproteins under alkaline conditions. In addition, our method had a wider operating pH than other methods. In summary, our method indicated great potential for enrichment of glycoproteins in real sample.
Fabrication of monolithic chip column and evaluation
In order to explore the application of solid phase adsorbents further, a glass-chip (Jiangsu, China, www.fht360.com/company/62850.htmlht) with straight microchannel (250 μm wide, 50 μm deep and 3 cm long) was fabricated in this study. The microchannel was processed in the same manner as the capillary vinylation described above. Subsequently, the microchannel was filled with pre-polymerization mixture of optimal formulation using injection pump and sealed with tape. The chip was exposed to UV light for 15 min in a reactor equipped with two 365 nm, 8-W UV tubes (EA-180,WESTBURY, USA, www.spectroline.com) (Fig. S7, in ESM). Finally, The resulting monolithic chip was flushed with ACN to remove unreacted reagents.
The performance of glycoprotein extraction on monolithic chip was characterized by in-batch reproducibility (n = 5). OVA was selected as the test protein. The recovery of OVA was in the range of 88.9–105.7% with RSDs of 5.8%. The results show that the extraction performance of the monolithic chip is comparable to that of monolithic capillary column. Therefore, the material can be well applied to chip.
Application in human serum
Encouraged by the results of the specific capture of glycoproteins, the affinity monolith was further assessed by separating and enriching glycoproteins from normal human serum samples selectively. It is well known that the normal human serum contain a great deal of glycoproteins and non-glycoproteins, which provides a complex matrix for verifying the practicability of the resulting boronate affinity-based PMME. The normal human serum sample was diluted to 500-fold with phosphate buffer solution (100 mM, pH 8). After PMME procedure with the boronate affinity monolith, SDS-PAGE analysis was used to analyze PMME performance. The normal human serum without extraction was shown in lane 7 (20-fold dilution) and lane 6 (500-fold dilution) (Fig. 5). Only the proteins at a high concentration was dyed. Thus, four bands can be clearly seen from normal human serum without treatment (Lane 7) and corresponded to protein fractions: transferrin (Trf, 76.7 kDa), human serum albumin (HSA, 66.4 kDa), Vitamin D binding protein (DBP, 51.0 kDa), apolipoprotein A-I (Apo A-I, 27.9 kDa). Enrichment of 500-fold diluted normal human serum (lane 6) by the resultant monolith revealed that non-glycoproteins were directly eluted from the monolith (lanes 4 and 5). After switching to an acidic phosphate buffer solution, Trf and DBP were efficiently separated and concentrated (lane 3), which further demonstrated that only glycoproteins can be isolated and enriched.
SDS-PAGE analysis of extraction for glycoproteins samples in normal human serum by the affinity monolith. Line 1: marker; lane 2: 1 mg· mL−1 Trf standards; lane 3: the eluate; lane 4: the washing step solution; lane 5: the sampling step solution passing the monolith; lane 6: normal human serum without extraction (500-fold dilution); lane 7: normal human serum without treatment (20-fold dilution). Extraction conditions are the same as Fig. 3
Despite the obvious advantages, it should be noted that the extraction process and detection are off-line and the flow rate of the extraction is lower, which results in larger sample consumption and longer extraction times. Further improvement of this method is in progress.
Conclusion
A novel APTES-MAA/ POSS boronate affinity monolith was successfully prepared and used for glycoproteins extraction. In comparison of the recovery of glycoproteins from three boronate affinity monoliths of the poly(AAPBA-co-APTES-MAA-co-EDMA), (AAPBA-co-POSS-co-EDMA) and (AAPBA-co-EDMA), it was found that using synergistic effect of APTES-MAA / POSS monomers to improve the affinity of boronate affinity monolith is possible. The resultant monolith exhibited excellent specificity for glycoproteins at neutral buffer and acid media and eluted by organic-solvent-free solutions. At the same time, the incorporation of POSS into boronate affinity matrix can increase the rigidity of the boronate monolith. Compared with conventional poly(AAPBA-co-EDMA) system, the new strategy presented here expands the applicable range of samples to blood, tear, saliva and urine. Furthermore, the method provided a one-step synthesis for organic-inorganic hybrid boronate materials. Thus, the affinity monolith should have the great potential for glycoproteomics and glycomics studies.
References
Sun X, Jian Y, Wang H, Ge S, Yan M, Yu J (2019) Ultrasensitive microfluidic paper-based electrochemical biosensor based on molecularly imprinted film and boronate affinity sandwich assay for glycoprotein detection. ACS Appl Mater Interfaces 11:16198–16206
Chang L, Wu H, He X, Chen L, Zhang Y (2017) A highly sensitive fluorescent turn-on biosensor for glycoproteins based on boronic acid functional polymer capped Mn-doped ZnS quantum dots. Anal Chim Acta 995:91–98
Tian Y, Zhang H (2013) Characterization of disease-associated N-linked glycoproteins. Proteomics 13:504–511
Alley WR Jr, Mann BF, Novotny MV (2013) High-sensitivity analytical approaches for the structural characterization of glycoproteins. Chem Rev 113:2668–2732
Li D, Chen Y, Liu Z (2015) Boronate affinity materials for separation and molecular recognition: structure, properties and applications. Chem Soc Rev 44:8097–8123
Sun XY, Ma RT, Chen J, Shi YP (2017) Boronate-affinity based magnetic molecularly imprinted nanoparticles for the efficient extraction of the model glycoprotein horseradish peroxidase. Microchim Acta 184(10):3729–3737
Nauom S, da Silva Neto BR, Ribeiro MS, Pedersoli WR, Ulhoa CJ, Silva RN, Monteiro VN (2019) Biochemical and molecular study of trichoderma harzianum enriched secretome protein profiles using lectin affinity chromatography. Appl Biochem Biotechnol 187:1–13
Zhang Y, Kuang M, Zhang L, Yang P, Lu H (2013) An accessible protocol for solid-phase extraction of N-linked glycopeptides through reductive amination by amine-functionalized magnetic nanoparticles. Anal Chem 85:5535–5541
Mayang YC, He XW, Chen LX, Zhang YK (2017) Detection of transferrin by using a surface plasmon resonance sensor functionalized with a boronic acid monolayer. Microchim Acta 184:2749–2757
Zhou C, Chen X, Du Z, Li G, Xiao X, Cai Z (2017) A hybrid monolithic column based on boronate-functionalized graphene oxide nanosheets for online specific enrichment of glycoproteins. J Chromatogr A 1498:90–98
Zhou XJ, Mo CE, Chen M, Huang YP, Liu ZS (2018) Improving affinity of boronate capillary monolithic column for microextraction of glycoproteins with hydrophilic macromonomer. J Chromatogr A 1581-1582:8–15
Sun XY, Ma RT, Chen J, Shi YP (2018) Magnetic boronate modified molecularly imprinted polymers on magnetite microspheres modified with porous TiO2 (Fe3O4@pTiO2@MIP) with enhanced adsorption capacity for glycoproteins and with wide operational pH range. Microchim Acta 185(12):565
Ren L, Liu Z, Liu Y, Dou P, Chen HY (2009) Ring-opening polymerization with synergistic co-monomers: access to a boronate-functionalized polymeric monolith for the specific capture of cis-diol-containing biomolecules under neutral conditions. Angew Chem Int Ed 48:6704–6707
Ren L, Liu Z (2011) A self-assembled molecular team of boronic acids at the gold surface for specific capture of cis-diol biomolecules at neutral pH. Chem Commun 47:2255–2257
Liu Y, Ren L, Liu Z (2011) A unique boronic acid functionalized monolithic capillary for specific capture, separation and immobilization of cis-diol biomolecules. Chem Commun 47:5067–5069
Yang Q, Huang D, Jin S, Zhou H, Zhou P (2013) One-step synthesis of an organic-inorganic hybrid boronate affinity monolithic column with synergistic co-monomers. Analyst 138:4752–4755
Li H, Wang H, Liu Y, Liu Z (2012) A benzoboroxole-functionalized monolithic column for the selective enrichment and separation of cis-diol containing biomolecules. Chem Commun 48:4115–4117
Li D, Li Q, Wang S, Ye J, Nie H, Liu Z (2014) Pyridinylboronic acid-functionalized organic-silica hybrid monolithic capillary for the selective enrichment and separation of cis-diol-containing biomolecules at acidic pH. J Chromatogr A 1339:103–109
Liu Y, Lu Y, Liu Z (2012) Restricted access boronate affinity porous monolith as a protein a mimetic for the specific capture of immunoglobulin G. Chem Sci 3:1467–1471
Dremina ES, Li X, Galeva NA, Sharov VS, Stobaugh JF, Schöneich C (2011) A methodology for simultaneous fluorogenic derivatization and boronate affinity enrichment of 3-nitrotyrosine-containing peptides. Anal Biochem 418:184–196
Matsumoto A, Yoshida R, Kataoka K (2004) Glucose-responsive polymer gel bearing phenylborate derivative as a glucose-sensing moiety operating at the physiological pH. Biomacromolecules 5:1038–1045
Wulff G (1982) Selective binding to polymers via covalent bonds. The construction of chiral cavities as specific receptor sites. Pure Appl Chem 54:2093–2102
Alves F, Nischang I (2015) Radical-mediated step-growth: preparation of hybrid polymer monolithic columns with fine control of nanostructural and chromatographic characteristics. J Chromatogr A 1412:112–125
Tang W, Li G, Row KH, Zhu T (2016) Preparation of hybrid molecularly imprinted polymer with double-templates for rapid simultaneous purification of theophylline and chlorogenic acid in green tea. Talanta 152:1–8
Chen Y, Chen M, Chi J, Yu X, Chen Y, Lin X, Xie Z (2018) Aptamer-based polyhedral oligomeric silsesquioxane (POSS)-containing hybrid affinity monolith prepared via a "one-pot" process for selective extraction of ochratoxin A. J Chromatogr A 1563:37–46
Bagheri H, Soofi G, Javanmardi H, Karimi M (2018) A 3D nanoscale polyhedral oligomeric silsesquioxanes network for microextraction of polycyclic aromatic hydrocarbons. Mikrochim Acta 185:418
Wang X, Yang FF, Zhang LP, Huang YP, Liu ZS (2018) A polyhedral oligomeric silsesquioxane/molecular sieve codoped molecularly imprinted polymer for gastroretentive drug-controlled release in vivo. Biomater Sci 6:3170–3177
Zhang Y, Zhuang Y, Shen H, Chen X, Wang J (2017) A super hydrophilic silsesquioxane-based composite for highly selective adsorption of glycoproteins. Microchim Acta 184(4):1037–1044
Kruger NJ (1994) The Bradford method for protein quantitation. Methods Mol Biol 32:9–15
Sing KSW (1982) Reporting physisorption data for gas/solid systems with special reference to the detemination of surface area and porosity. Pure Appl Chem 54:2201–2218
Liu C, Deng Q, Fang G, Huang X, Wang S (2014) Facile synthesis of graphene doped poly(ionic liquid) boronate affinity material for specific capture of glycoproteins. J Mater Chem B 2:5229–5237
Yang F, Mao J, He XW, Chen LX, Zhang YK (2013) Synthesis of boronate-silica hybrid affinity monolith via a one-pot process for specific capture of glycoproteins at neutral conditions. Anal Bioanal Chem 405:6639–6648
Jin S, Liu L, Zhou P (2018) Amorphous titania modified with boric acid for selective capture of glycoproteins. Microchim Acta 185(6):308
Wu Q, Jiang B, Weng Y, Liu J, Li S, Hu Y, Yang K, Liang Z, Zhang L, Zhang Y (2018) 3-Carboxybenzoboroxole functionalized polyethylenimine modified magnetic graphene oxide nanocomposites for human plasma glycoproteins enrichment under physiological condition. Anal Chem 90:2671–2677
Acknowledgments
This work was supported by National Natural Science Foundation of China (Grant No. 21775109).
Author information
Authors and Affiliations
Corresponding authors
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
ESM 1
(DOCX 1070 kb)
Rights and permissions
About this article
Cite this article
Shen, YF., Yuan, FF., Liu, XY. et al. Synergistic effect of organic-inorganic hybrid monomer and polyhedral oligomeric silsesquioxanes in a boronate affinity monolithic capillary/chip for enrichment of glycoproteins. Microchim Acta 186, 812 (2019). https://doi.org/10.1007/s00604-019-3938-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00604-019-3938-z