Abstract
An optical nanoprobe consisting of gold nanoclusters (AuNCs) and gold nanoparticles (AuNPs) is described for ultrasensitive detection of heparin (Hep). Polyethyleneimine (PEI) induces the aggregation of AuNPs which results in a color change from wine red (peak at 520 nm) to blue (peak at 610 nm). In parallel, the fluorescence of AuNCs (with excitation/emission maxima at 370/610 nm) is weakened. However, in the presence of Hep (which is strongly negatively charged), it will electrostatically bind to positively charged PEI and then will prevent aggregation. Hence, the color changes from blue (aggregated) to red (non-aggregated). In parallel, fluorescence remains unchanged. Hep can be quantified by using the nanoprobe in the range of 4–220 ng·mL−1, with the detection limits as low as 1.6 (colorimetry) and 3.4 ng·mL−1 (fluorometry). The assay was applied to the detection of Hep in (spiked) human serum with satisfactory results.

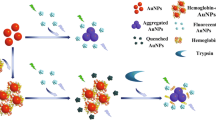
Schematic illustration for colorimetric and fluorometric determination of heparin based on the use of a nanoprobe consisting of gold nanoclusters (AuNCs) and gold nanoparticles (AuNPs) with polyethyleneimine (PEI) as the mediator.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Heparin (Hep) is a highly sulfated glycosaminoglycan with the highest negative charge density among natural biopolymers [1]. It plays an important role in the regulation of diverse physiological processes [2,3,4] and is extensively used as an anticoagulant or antithrombotic agent in clinical operations [3]. However, overuse can cause unpredictable adverse effects [5]. Traditional clinical laboratory assays for Hep include the activated clotting time assay (ACT), activated partial thromboplastin time assay (APTT), chromogenic antifactor Xa, and electrochemical approaches [6,7,8,9,10]. Although these methods have made great progress in terms of high selectivity and comparative reliability, most of them suffer from such drawbacks as high costs, sophisticated instruments, and especially requirements of pretreatment and postanalytical variabilities. Therefore, it is very urgent to develop simple, rapid, sensitive, and cost-effective methods to detect Hep.
Considerable efforts have been dedicated to constructing nanomaterial-based optical probe for Hep, including colorimetric [11,12,13,14,15] and fluorimetric [16,17,18,19,20,21,22,23,24,25,26,27,28], due to the merit of low cost, high sensitivity, and convenient operation. By taking advantage of high extinction coefficients and localized surface plasmon resonance (LSPR) effect [29, 30], gold nanoparticles (AuNPs) have been exploited as colorimetric probes for the detection of Hep. They are mainly based on distinct color changes associated with the turnover process from the dispersed to aggregated state through electrostatic interaction between the negatively charged Hep and cationic surface modifiers anchored onto AuNPs. However, many environmental variables may cause undesirable aggregation of AuNPs, making the detection results not very accurate. On the other hand, a series of fluorimetric probes based on luminescent NPs including quantum dots, carbon dots (C-dots), silicon NPs, and metal nanoclusters [16,17,18,19,20,21,22,23,24,25,26,27,28], have also been well established for Hep assay. For example, our group has reported a fluorescence assay for Hep based on the higher affinity of cetyltrimethyl ammonium bromide (CTAB) with Hep than with gold nanoclusters (AuNCs) [24]. Unfortunately, it acted in the on-off mode, which may not be reliable especially in complex biological fluids. Importantly, most of these optical nanoprobes only focus on a single response for the target, which can easily be disturbed by the surroundings and possible falsified signals, eventually affecting the accuracy of the experimental results. To address this issue, multimodal optical detection systems, for example, with colorimetric and fluorometric dual readout, are highly demanded because mutual correction between multiple signals from more than one transduction channel will make the results more convincing [31,32,33].
In this work, we present a nanoprobe with both colorimetric and fluorometric (“off-on” signal change) readout for selective and sensitive detection of Hep based on AuNCs coupled with AuNPs (Scheme 1). This optical detection platform combines the advantages of the convenience, high sensitivity, and low cost of the fluorometry and colorimetry. More importantly, the simultaneous optical signal changes can enhance the detection accuracy and are very favorable for practical applications, for instance, human serum analysis.
Experimental
Chemicals and materials
Hydrogen tetrachloroaurate(III) trihydrate (HAuCl4·3H2O, 99%), glutathione (GSH), polyethyleneimine (PEI), trisodium citrate, and acetonitrile were provided by Sinopharm Chemical Reagent Co. Ltd. (Shanghai, China, www.reagent.com). Heparin (Hep, 150 USP units·mg−1) was purchased from Adamas Reagent Co. Ltd. (Shanghai, China, adamas.company.lookchem.cn). Anions including Na2SO4, Na2CO3, Na4P2O7, Na2C2O4, NaAc, NaCl, NaHCO3, and Na2HPO4 were obtained from Shanghai Qingxi Technology Co. Ltd. (Shanghai, China, www.qingxihb.com). Biological reagents including glucose (C6H12O6), sodium hyaluronate (HA), adenosine triphosphate (ATP), bovine serum albumin (BSA) and all amino acids were bought from Aladin Reagent Co. Ltd. (Shanghai, China, www.aladdin-e.com). Amino acids used here include tryptophan (Trp), phenylalanine (Phe), lysine (Lys), glycine (Gly), glutamate (Glu), proline (Pro), glutamine (Gln), Aspartic acid (Asp), threonine (Thr), valine (Val), isoleucine (Ile), histidine (His), methionine (Met), cysteine (Cys), serine (Ser), alanine (Ala), tyrosine (Tyr). All chemicals used were at least of analytical grade. Ultrapure water (18.2 MΩ cm−1) was used to prepare all the solution in this study.
Instruments
Fluorescence spectra were performed on a fluorescence spectrometer (F-4600, Hitachi, Japan, hha.hitachi-hightech.com) equipped with a Xenon lamp source and a 1.0 cm quartz cell. UV-vis absorption spectra were conducted on a UV-2550 spectrophotometer (Shimadzu, Japan, www.shimadzu.com). Fourier transform infrared (FT-IR) spectra were recorded with KBr pellets on a Nicolet 5700 FTIR Spectrometer (Nicolet, USA, www.thermonicolet.co). The data of zeta potential were collected on NPA152 Nanoparticle size analyzer (Microtrac Inc., USA, www.microtrac.com). Transmission electron microscopy (TEM) was carried out with a JEM-2100 transmission electron microscope (JEOL Ltd. Japan, www.jeol.co.jp).
Preparation of AuNPs
All pieces of the experimental glassware used were cleaned with aqua regia solution (HCl: HNO3, 3:1) and then rinsed thoroughly with H2O and finally dried at 100 °C before use. Citrate-capped AuNPs were synthesized based on the Turkevich’ method [34]. The procedures for the preparation of 13-nm AuNPs was described as follows: 45 mL solution containing 1.0 mM of HAuCl4 was prepared and heated under reflux to boiling, and then 5 mL of 38.8 mM trisodium citrate was quickly added to the solution under vigorous stirring, and the mixture was allowed to continue for another 30 min. The wine-red solution was cooled to room temperature and stored at 4 °C for further use. The concentration of AuNPs calculated according to Beer’s law was 4.8 nM.
Synthesis of GSH-AuNCs
GSH-AuNCs were synthesized as reported previously [24]. Briefly, HAuCl4 (1 g) was formulated into an aqueous solution (4 mM, 1 L). Then 50 mL of the above solution and the GSH aqueous solution (6 mM, 50 mL) were mixed to form a 100 mL solution. The mixture was immediately transferred to a 250 mL round bottom flask, and heated in an oil bath at 90 °C for 6.5 h. After naturally cooling to room temperature, it was purified by adding acetonitrile, and the precipitate was obtained by centrifugation (12, 000 rpm, 10 min). The above centrifugation operation was repeated several times, except that acetonitrile was replaced with a mixed solution of acetonitrile and ultrapure water (v/v, 3:1). Finally, the purified precipitate were dried under vacuum and stored at 4 °C prior to use.
Procedures for the detection of Hep
First, 1700 μL of phosphate buffer (0.01 M, pH 7.0) containing various concentrations of Hep and PEI (50 ng·mL−1) was mixed with 300 μL AuNPs, and the resulting mixture was equilibrated at room temperature. Then 1 mL of 0.3 mg·mL−1 AuNCs was added into 1 mL of the AuNPs. After incubating for 15 min, UV-vis absorption and fluorescence spectra were conducted accordingly. For the UV-vis absorption measurement, the absorbances at 520 and 610 nm were recorded, and their ratio was calculated for the calibration plot. For the fluorescence measurement, the excitation and emission wavelengths were set at 370 and 610 nm, respectively.
Human serum analysis
Human serum was acquired from Nanchang University Hospital from healthy donors. Prior to analysis, a simple pretreatment was performed. Briefly, acetonitrile (2.0 mL) was added to the serum (0.5 mL). After shaking thoroughly, it was centrifuged (12, 000 rpm, 15 min). The supernatant was then aspirated and diluted to 25 mL. Thus, a diluted serum sample from which the protein was removed was obtained.
To evaluate the reliability in human serum analysis, the standard addition method was used to detect Hep. Simply, Hep at known concentrations (62.5, 125, and 188 ng·mL−1) and PEI (50 ng·mL−1) was first mixed separately, and then the mixture was pipetted into the pretreated serum sample (50 μL). The subsequent procedures were carried out similar to those described above. The recovery results were calculated based on the calibration curves obtained in aqueous solution.
Results and discussion
Choice of materials
In the present detection system, glutathione protected AuNCs (GSH-AuNCs) were selected as the fluorometric reporter. AuNCs are a newly-emerged class of promising fluorophores with intriguing properties such as tunable photoluminescence, high quantum yield, large Stokes shift, and excellent biocompatibility [35]. These features make them very suitable as the fluorometric reporter. Besides, AuNPs functioned as the colorimetric reporter and furthermore as the fluorescence switch for GSH-AuNCs due to the distance-dependent optical properties. With respect to other materials such as C-dots, ZnO, MoS2 or other oxides, when they act as the fluorometric reporters, their emission wavelengths are often shorter than 500 nm [27], which result in little spectral overlap with the absorption bands of AuNPs either in the dispersed or aggregation state. On the other hand, they do not have high extinction coefficients or localized surface plasmon resonance (LSPR) effect like AuNPs [29, 30], so they are not suitable as the colorimetric reporters.
Working principle of the assay
As depicted in Scheme 1, the positively charged polyethyleneimine (PEI) can induce the aggregation of citrate-capped AuNPs through electrostatic interaction followed by a color change from wine red to blue. The absorbance band of aggregated AuNP overlaps the emission spectrum of AuNCs, and the adsorption of PEI on AuNP surface also facilitates the contact with negatively charged AuNCs. Therefore, an efficient inner filter effect (IFE) occurs with AuNCs acting as the donors and AuNP acting as the acceptors, leading to the fluorescence quenching. However, in the presence of PEI and the target Hep simultaneously, Hep deactivates the aggregation agent PEI because they have a higher affinity than that between PEI and AuNPs. AuNPs are still well dispersed in solution with wine red color. When AuNCs are added, a sufficient distance is maintained with AuNPs owing to strong electrostatic repulsion and the emission spectrum does not overlap with the absorption peak of dispersed AuNPs; hence, IFE does not occur. As a result, the fluorescence of AuNCs changes little.
Mechanistic investigation of the assay
As illustrated in Scheme 1, PEI served as a medium to tune the inter-particle distance among AuNPs regarding the color change and to modulate the occurrence of IFE between AuNPs and AuNCs leading to the fluorescence quenching or recovery of AuNCs. Citrate-stabilized AuNPs are negatively charged (ζ = −15.6 mV), while PEI is positively charged (ζ = +13.9 mV). As shown in Fig. 1, PEI can easily induce the aggregation of citrate-capped AuNPs due to electrostatic interaction, which results in a clear color change from red to blue. Concurrently, the characteristic SPR peak at 520 nm of AuNPs decreases and a new peak at 610 nm emerges (black line). Because the maximum emission peak of the GSH-AuNCs locates at about 610 nm (blue line), the absorption spectrum of aggregated AuNPs largely overlaps with the emission spectrum of AuNCs, indicating the potential of an efficient IFE process. In addition, since the GSH-AuNCs also bear negative charges (ζ = −9.18 mV) due to the presence of carboxyl groups, polycationic PEI attached onto AuNPs makes AuNPs and AuNCs close to each other and thus ensures the IFE process to occur. These results virtually contribute to the decrement of the fluorescence of AuNCs (orange line). However, Hep, a highly negatively charged macromolecule (ζ = −27.7 mV), preferentially interacted with PEI due to stronger electrostatic interaction than that between AuNPs and PEI as well as hydrogen bonding between amino groups of PEI and hydroxyl groups of Hep [23]. In this case, AuNPs are still in a dispersed state, retaining wine red, only showing the absorption band at 520 nm (red line). In addition, the distance between AuNPs and AuNCs enlarges due to strong electrostatic repulsion. Therefore, IFE is invalidated and the fluorescence of AuNCs slightly changed (green line). It should be noted that in the presence of AuNPs, PEI, or Hep alone, the fluorescence of AuNCs keeps almost constant (Fig. S1). The phenomenon is further confirmed by TEM (Fig. 2). Upon addition of PEI into the mixture of AuNPs and AuNCs, AuNPs are highly aggregated (Fig. 2A). When PEI and Hep are added into the detection system at the same time, the solution is retained in a dispersed state owing to the interaction between PEI and Hep (Fig. 2B). These results strongly support our principle of detection of Hep that PEI may lead to the aggregation of AuNPs accompanied with the corresponding color change and fluorescence quenching of AuNCs while Hep can reverse the color change of AuNPs and restore the fluorescence of AuNCs by altering the inter-particle distance.
UV-vis absorption spectra of AuNPs in the presence of PEI (red line) and PEI with Hep (black line). The inset shows the corresponding photographs: a AuNPs+PEI, b AuNPs+(PEI + Hep). Fluorescence spectra of AuNCs mixing with AuNPs upon addition of PEI or PEI with Hep. The inset shows the corresponding photographs: c AuNCs, d AuNCs+AuNPs+PEI, e AuNCs+AuNPs +(PEI + Hep)
Optimization of method
The following parameters were optimized: (a) Concentration of AuNPs; (b) concentration of PEI; (c) pH value; (d) incubation time. Respective data and Figures are given in the Electronic Supporting Material. The following experimental conditions were found to give best results: (a) Best concentration of AuNPs: 0.36 nM; (b) Best concentration of PEI: 50 ng·mL−1; (c) Optimal pH: 7.0; (d) Optimal incubation time: 15 min.
Colorimetric and fluorimetric detection of Hep
Under the optimal conditions, AuNPs and AuNCs were incubated with different concentrations of Hep and 50 ng·mL−1 PEI, stirring and standing 15 min. As shown in Fig. 3a, as the concentration of Hep increases, the absorption peak of AuNPs at 610 nm ascribed to the aggregation state drops gradually, while the intensity of characteristic SPR peak centered at 520 nm increases a little. Figure 3b shows a good linear relationship between the ratio of A520nm to A610nm (A520nm/A610nm) and the concentration of Hep from 4 to 220 ng·mL−1 (A520nm/A610nm = 0.017 CHep/(ng·mL−1) + 1.01, R2 = 0.995). The limit of detection (LOD) was calculated to be 1.6 ng·mL−1. At the same time, the fluorescence recovery of AuNCs due to competitive interaction of Hep with PEI can also be demonstrated by the turn-on fluorescence response. As described in Fig. 3c, the fluorescence intensity increases gradually with increasing the concentration of Hep. And the fluorescence recovery correlates well to a concentration range of 4 to 220 ng·mL−1 (F = 0.699 CHep/(ng·mL−1) + 108, R2 = 0.994) with a LOD of 3.4 ng·mL−1 (Fig. 3d). These results suggest that the nanoprobe with two signals can be used for sensitive detection of Hep. Compared with many reported optical nanoprobes for Hep (Table S1), the nanoprobe shows better or comparable detection sensitivity. This assay is also rapid and complex synthetic or modification procedures are not involved [11, 12, 24]. With colorimetric and fluorimetric readout, this assay is highly reliable for the quantification of Hep levels by eliminating possible false response in a single signal system. It should be noted that the linear range of this assay is not wide enough relative to some other assays.
a UV-vis absorption spectra of the AuNP-based detection system containing AuNCs and PEI (50 ng·mL−1) incubated with various concentrations of Hep. b Linear plot of A520nm/A610nm versus Hep concentration in the range of 4–220 ng·mL−1. c Fluorescence spectra of the AuNC-based detection system containing AuNPs and PEI (50 ng·mL−1) incubated with various concentrations of Hep. d Linear plot of the fluorescence intensity at 610 nm of AuNCs versus Hep concentration in the range of 4–220 ng·mL−1
Selectivity of Hep detection
To evaluate the selectivity of the presented nanoprobe for Hep, we investigated the influences of the potentially interfering substances under the same detection conditions by UV-vis absorption and fluorescence spectra. The main relevant species include common anions (CO32−, HCO3−, Cl−, HPO42−, SO42−, P2O74−, Ac−, C2O42−, citrate) and biomolecules (BSA, ATP, HA, glucose, Trp, Phe, Lys, Gly, Glu, Pro, Gln, Arg, Asp, Thr, Val, Ile, His, Met, Cys, Ser, Ala, Tyr). As shown in Fig. 4a and B, the value of A520nm/A610nm is the biggest only when adding Hep, indicating that the addition of interferents does not lead aggregated AuNPs induced by PEI to dispersed state. Similarly, the results obtained from fluorescence response also reveal that only the presence of Hep caused a remarkable fluorescence increase (Fig. 4c and d). In addition, the tolerance experiments show that the coexistence of these species with Hep poses negligible effects on the colorimetric and fluorimetric responses toward Hep (Fig. S3). We reason that these species cannot form a stronger binding than that between Hep and PEI as Hep has a much higher negative charge density [1]. All the results clearly demonstrate that the detection system has excellent selectivity and reliability toward Hep detection.
a, b Absorbance ratio A520nm/A610nm of the solution containing AuNPs, PEI, AuNCs, and various interferents. c, d Fluorescence recovery ratio (F-F0)/F0 of the solution containing AuNPs, PEI, AuNCs, and various interferents, where F0 and F represent the fluorescence intensities at 610 nm of the mixture of AuNPs, PEI, and AuNCs without and with various interferents, respectively. Concentrations: BSA, ATP and HA, 62.5 ng·mL−1; CO32−, HCO3−, Cl−, HPO42−, SO42−, C2O42−, P2O74−, CH3COO−, citrate, and C6H12O6, 50 μM; amino acids, 5 μM; Hep, 125 ng·mL−1
Detection of Hep in human serum samples
In order to verify the capability of this assay in complex biological environments, the human serum samples were analyzed colorimetrically and fluorometrically with the nanoprobe by spiking Hep at known concentrations. As listed in Table 1, the recoveries of the spiked samples are 96.8% ~ 103% and 96.8% ~ 102%, respectively. The t-test was utilized to analyze the statistical differences, and the results suggest that there is no significant difference between colorimetric and fluorometric methods, indicating that this assay is feasible and reliable for Hep detection in practical samples.
Generally, optical probes that work in the UV are often susceptible to interference from biomatters that always display strong background UV absorption and fluorescence. Fortunately, by virtue of the very high sensitivity of the assay, the interference from biomatters in human serum can be effectively eliminated by diluting the sample. In addition, the maximum emission peak of AuNCs locates at about 610 nm, which is almost in the near infrared region, so the biomatters with background UV absorption cannot screen off the fluorescence of AuNCs. Also, the emitted UV fluorescence of the biomatters cannot affect the fluorescence of AuNCs due to a big difference between their emission wavelengths.
Conclusions
In summary, we have successfully constructed an economical nanoprobe for Hep detection with both colorimetric and fluorometric readout based on the regulation of the electrostatic interaction of AuNPs and AuNCs by PEI. Such colorimetric and fluorometric feature endows this assay with ultrahigh sensitivity and excellent reliability. It is capable of the detection of Hep in human serum matrix by largely offsetting the interference from environmental fluctuations. It is also expected that this nanoprobe by means of AuNCs and AuNPs has great potential for monitoring Hep in other complex samples considering its simplicity and flexibility. More efforts will be made to broaden the linear range of this assay.
References
Hu LZ, Liao H, Feng LY, Wang M, Fu WS (2018) Accelerating the peroxidase-like activity of gold nanoclusters at neutral pH for colorimetric detection of heparin and heparinase activity. Anal Chem 90:6247–6252. https://doi.org/10.1021/acs.analchem.8b00885
Whitelock J, Iozzo RV (2005) Heparan sulfate: a complex polymer charged with biological activity. Chem Rev 105:2745–2764. https://doi.org/10.1021/cr010213m
Mackman N (2008) Triggers, targets and treatments for thrombosis. Nature 451:914–918. https://doi.org/10.1038/nature06797
Williams SJ, Davies GJ (2001) Protein-carbohydrate interactions: learning lessons from nature. Trends Biotechnol 19:356–362. https://doi.org/10.1016/S0167-7799(01)01699-7
Girolami B, Girolami A (2006) Heparin-induced thrombocytopenia: a review. Semin Thromb Hemost 32:803–809. https://doi.org/10.1055/s-2006-955463
Cheng TJ, Lin TM, Wu TH, Chang HC (2001) Determination of Hep levels in blood with activated partial thromboplastin time by a piezoelectric quartz crystal sensor. Anal Chim Acta 432:101–111. https://doi.org/10.1016/S0003-2670(00)01346-5
Simko RJ, Sung FFT, Stanek EJ (1995) Activated clotting time versus activated partial thromboplastin time for therapeutic monitoring of Hep. Ann Pharmacother 29:1015–1021. https://doi.org/10.1177/106002809502901012
Mathison S, Bakker E (1999) Renewable pH cross-sensitive potentiometric Hep sensors with incorporated electrically charged H+ ionophores. Anal Chem 71:4614–4621. https://doi.org/10.1021/ac990387s
Qi H, Zhang L, Yang L, Yu P, Mao L (2013) Anion-exchange-based amperometric assay for heparin using polyimidazolium as synthetic receptor. Anal Chem 85:3439–3445. https://doi.org/10.1021/ac400201c
Guo J, Amemiya S (2006) Voltammetric Hep-selective electrode based on thin liquid membrane with conducting polymer-modified solid support. Anal Chem 78:6893–6902. https://doi.org/10.1021/ac061003i
Jena BK, Raj CR (2008) Optical sensing of biomedically important polyionic drugs using nano-sized gold particles. Biosens Bioelectron 23:1285–1290. https://doi.org/10.1016/j.bios.2007.11.014
Wen SH, Zheng FY, Shen MW, Shi XY (2013) Synthesis of polyethyleneimine-stabilized gold nanoparticles for colorimetric sensing of heparin. Colloid Surface A 419:80–86. https://doi.org/10.1016/j.colsurfa.2012.11.052
Qu F, Liu YQ, Lao HL, Wang YP, You JM (2017) Colorimetric detection of heparin with high sensitivity based on the aggregation of gold nanoparticles induced by polymer nanoparticles. New J Chem 41:10592–10597. https://doi.org/10.1039/c7nj02381b
Bamrungsap S, Cherngsuwanwong J, Srisurat P, Chonirat J, Sangsing N, Wiriyachaiporn N (2019) Visual colorimetric sensing system based on the self-assembly of gold nanorods and graphene oxide for heparin detection using a polycationic polymer as a molecular probe. Anal Methods 11:1387–1392. https://doi.org/10.1039/C8AY02129E
Ma XY, Kou XY, Xu YY, Yang DW, Miao P (2019) Colorimetric sensing strategy for heparin assay based on PDDA-induced aggregation of gold nanoparticles. Nanoscale Adv 1:486–489. https://doi.org/10.1039/C8NA00162F
Liu JM, Chen JT, Yan XP (2013) Near infrared fluorescent trypsin stabilized gold nanoclusters as surface plasmon enhanced energy transfer biosensor and in vivo cancer imaging bioprobe. Anal Chem 85:3238–3245. https://doi.org/10.1021/ac303603f
Ma L, Zhang MY, Yang AJ, Wang Q, Qu F, Qu FL, Kong RM (2018) Sensitive fluorescence detection of heparin based on self-assembly of mesoporous silica nanoparticle-gold nanoclusters with emission enhancement characteristics. Analyst 143:5388–5394. https://doi.org/10.1039/C8AN01556B
Lan J, Zou HY, Wang Q, Zeng P, Li YF, Huang CZ (2016) Sensitive and selective turn off-on fluorescence detection of heparin based on the energy transfer platform using the BSA-stabilized au nanoclusters/amino-functionalized graphene oxide hybrids. Talanta 161:482–488. https://doi.org/10.1016/j.talanta.2016.08.081
Long Q, Zhao JN, Yin BD, Li HT, Zhang YY, Yao SZ (2015) A novel label-free upconversion fluorescence resonance energy transfer-nanoprobe for ultrasensitive detection of protamine and heparin. Anal Biochem 477:28–34. https://doi.org/10.1016/j.ab.2015.02.017
Aparna RS, Devi JSA, Anjana RR, Nebu J, George S (2019) Reversible fluorescence modulation of BSA stabilised copper nanoclusters for the selective detection of protamine and heparin. Analyst 144:1799–1808. https://doi.org/10.1039/C8AN01703D
Cheng Q, He Y, Ge YL, Zhou JG, Song GW (2018) Ultrasensitive detection of heparin by exploiting the silver nanoparticle-enhanced fluorescence of graphitic carbon nitride (g-C3N4) quantum dots. Microchim Acta 185:332. https://doi.org/10.1007/s00604-018-2864-9
Wu XM, Zhang F, Li Y (2018) Facile synthesis of near-infrared emitting dBSA-templated cu nanoclusters for sensitive detection of heparin. J Mater Chem B 6:5466–5475. https://doi.org/10.1039/C8TB01733F
Liang SS, Deng X, Fan YY, Li J, Wang M, Zhang ZQ (2018) A ratiometric fluorometric heparin assay based on the use of CdTe and polyethyleneimine-coated carbon quantum dots. Microchim Acta 185:519–517. https://doi.org/10.1007/s00604-018-3061-6
Li S, Huang PC, Wu FY (2017) Highly selective and sensitive detection of heparin based on competition-modulated assembly and disassembly of fluorescent gold nanoclusters. New J Chem 41:717–723. https://doi.org/10.1039/C6NJ03155B
Liu ZP, Ma Q, Wang XY, Lin ZH, Zhang H, Liu LL, Su XG (2014) A novel fluorescent nanoprobe for detection of heparin and heparinase based on CuInS2 quantum dots. Biosens Bioelectron 54:617–622. https://doi.org/10.1016/j.bios.2013.11.050
Cao YL, Shi S, Wang LL, Yao JL, Yao TM (2014) Ultrasensitive fluorescence detection of heparin based on quantum dots and a functional ruthenium polypyridyl complex. Biosens Bioelectron 55:174–179. https://doi.org/10.1016/j.bios.2013.12.009
Wang RX, Wang XF, Sun YM (2017) Aminophenol-based carbon dots with dual wavelength fluorescence emission for determination of heparin. Microchim Acta 184:187–193. https://doi.org/10.1007/s00604-016-2009-y
Peng X, Long Q, Li HT, Zhang YY, Yao SZ (2015) "turn on-off" fluorescent sensor for protamine and heparin based on label-free silicon quantum dots coupled with gold nanoparticles. Sensor Actuat B-Chem 213:131–138. https://doi.org/10.1016/j.snb.2015.02.070
Liu WJ, Li ZC, Jia HM, Zhang LX, He WW, Meng QB (2019) Shell surface sulfidation mediated the plasmonic response of au@Ag NPs for colorimetric sensing of sulfide ions and sulfur. Appl Surf Sci 481:678–683. https://doi.org/10.1016/j.apsusc.2019.03.175
Rajar K, Sirajuddina BA, Bhanger M, Shaha MT, Shaikha T, Siddiqui S (2018) Succinic acid functionalized silver nanoparticles (Suc-Ag NPs) for colorimetric sensing of melamine. Appl Surf Sci 435:1080–1086. https://doi.org/10.1016/j.apsusc.2017.11.208
Deng JH, Lu QJ, Hou YX, Ug L, Li HT, Zhang YY, Yao SZ (2015) Nanosensor composed of nitrogen-doped carbon dots and gold nanoparticles for highly selective detection of cysteine with multiple signals. Anal Chem 87:2195–2203. https://doi.org/10.1021/ac503595y
Shi YP, Pan Y, Zhang H, Zhang ZM, Li MJ, Yi CQ, Yang MS (2014) A dual-mode nanosensor based on carbon quantum dots and gold nanoparticles for discriminative detection of glutathione in human plasma. Biosens Bioelectron 56:39–45. https://doi.org/10.1016/j.bios.2013.12.038
Zhang XL, Deng JJ, Xue YM, Shi GY, Zhou TS (2016) Stimulus response of au-NPs@GMP-Tb core-shell nanoparticles: toward colorimetric and fluorescent dual-mode sensing of alkaline phosphatase activity in algal blooms of a freshwater lake. Environ Sci Technol 50:847–855. https://doi.org/10.1021/acs.est.5b04600
Kimling J, Maier M, Okenve B, Kotaidis V, Ballot H, Plech A (2006) Turkevich method for gold nanoparticle synthesis revisited. J Phys Chem B 110:15700–15707. https://doi.org/10.1021/jp061667w
Sun J, Jin YD (2014) Fluorescent au nanoclusters: recent progress and sensing applications. J Mater Chem C 2:8000–8011. https://doi.org/10.1039/C4TC01489H
Acknowledgments
This work was supported by the National Natural Science Foundation of China (Nos. 21765014 and 21864018); and the Opening Project of Guangzhou Key Laboratory of Analytical Chemistry for Biomedicine (No. 2018001).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
ESM 1
(DOC 2023 kb)
Rights and permissions
About this article
Cite this article
Zhang, Z., Li, S., Huang, P. et al. Colorimetric and fluorometric aggregation-based heparin assay by using gold nanoclusters and gold nanoparticles. Microchim Acta 186, 790 (2019). https://doi.org/10.1007/s00604-019-3928-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00604-019-3928-1