Abstract
Fluorescent polymer dots (PDs) with maximum excitation/emission wavelengths of 410/515 nm were prepared in water solution from 1,4-benzoquinone and ethylenediamine. The green fluorescence of these PDs is screened off by the red-colored oxidation product (PPDox, maximum absorption at 510 nm) formed by horseradish peroxidase (HRP)-catalyzed oxidation of p-phenylenediamine (PPD). It causes the reduction of the fluorescence intensity of the PDs due to spectral overlap and an inner filter effect (IFE). If glucose is enzymatically oxidized under the formation of H2O2, the formed H2O2 can be quantified by the above IFE. The assay for HRP activity and glucose have detection limits of 0.2 U·L−1 and 0.1 μM, respectively. The nanoprobe was further extended to an immunosorbent assay (ELISA) for the determination of insecticidal Cry1Ab/Ac protein with a detection limit of 0.25 ng·mL−1. The ELISA was applied to rice leaf analysis.

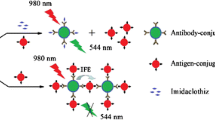
Schematic representation of fluorometrict enzyme-linked immunosorbent assay for Cry1Ab/Ac protein detection based on horseradish peroxidase (HRP)-triggered fluorescence quenching of polymer dots (PDs). Quenching is caused by an inner filter effect (IFE) caused by PPDox, the oxidation product of p-phenylenediamine (PPD).
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Horseradish peroxidase (HRP), an important enzyme obtained from horseradish [1], is frequently utilized in sensing various important substances in clinical chemistry [2], environmental chemistry [2] and food industry [3]. Glucose is also closely related to HRP-catalyzed cycle in physiology [4,5,6]. Although plenty of methods for the detection of glucose have been developed, including colorimetry [7], fluorescent spectrometry [8] and electrochemistry methods [9], etc. [10], only a few detection strategies for HRP was reported [11,12,13].
Among the analytical techniques, the fluorescent spectrometry appears to be an effective and low-cost detection tool due to its excellent fluorescence brightness, extraordinary photostability, excellent biocompatibility and good sensitivity [14, 15]. Fluorescent sensors have been commonly used for chemical and biological analysis with the detecting mechanisms including electronic energy transfer (EET), fluorescence resonance energy transfer (FRET), photoinduced electron transfer (PET), etc [14, 16, 17]. The fluorescent sensor based on these mechanisms usually involved complicated fabrication such as the bond linkage between the receptor and fluorophore. However, the sensor fabrication was commonly costly and time-consuming, which seriously restricted their practical applications. Inner filter effect (IFE) was generally produced by the absorption of the excitation and/or emission light of the absorber. As a non-irradiation energy conversion pattern, it had been used for fluorescent detection [18,19,20]. The detection methods based on IFE would be more flexible and efficient since no restrictive chemical connection between the fluorophore and the absorber was needed.
As a new emerging kind of fluorescent nanomaterials with low cytotoxicity, excellent optical properties, good water solubility and biocompatibility, polymer dots (PDs) have been widely used in the detection of various analytes [15, 21, 22]. In this paper, PDs, which were prepared by one-pot aqueous synthetic route from 1,4-benzoquinone and ethylenediamine, displayed a maximum excitation wavelength at 410 nm and a maximum emission wavelength at 515 nm. The oxidation of PPD by HRP/H2O2 leads to a red-colored product (Bandrowski’s base, PPDox, Scheme 1A) with a maximum absorption at 510 nm. The band overlaps both the fluorescence excitation and emission spectra of PDs. Thus, fluorescence is turned off due to IFE, which can directly demonstrate the activity of HRP. On the other hand, glucose can be converted into H2O2 by the catalytic action of glucose oxidase (GOx) (Scheme 1A), and this paves the way for the quantification of glucose.
To improve the resistance to insects, a few genetically modified crops such as Bacillus thuringiensis (Bt) gene-embedded crops have been authorized by many countries. However, the cultivation of genetically modified crops should be managed by the authorized institutes. Unreasonable cultivation may contaminate non-transgenic varieties and cause unexpected biosafety and ecological environment problem [23, 24]. Sensitive detection methods for Bt proteins should be explored since their content is quite low in crops. The insecticidal proteinic toxin Cry1Ab/Ac is the most common Bt protein in genetically modified crops [25, 26]. In this work, the PD-based nanoprobe was successfully extended into the HRP-based fluorescent enzyme-linked immunosorbent assay (ELISA) in the determination of Cry1Ab/Ac protein (Scheme 1B). The excellent performance in real rice leaves analysis indicated that the PD-based fluorescence nanoprobe exhibited a promising prospect in related biological detection applications.
Experimental section
Materials and apparatus
All the materials and apparatus were presented in supplementary material.
Preparation of PDs
The fluorescent PDs were synthesized according to our previous report [27]. The detailed information for preparation of PDs was shown in supplementary material.
Procedure for the determination of HRP activity
Different concentrations (0, 0.0002, 0.01, 0.02, 0.04, 0.06, 0.08, 0.1, 0.12, 0.14, 0.16, 0.2, 2, 10, 20, 50, 100, 125 and 200 U·mL−1) of HRP solution, H2O2 solution (10 mM) and PPD solution (10 mM) were prepared with phosphate buffered saline (PBS, pH = 7.0, 10 mM). HRP solution (150 μL) with different concentrations was added into a centrifugal tube. To which, 50 μL of H2O2 solution, 50 μL of PPD solution and 250 μL of PBS solution were added in sequence. The resulting mixture was incubated for 15 min at ambient temperature. 300 μL of reaction solution was added to 200 μL PD solution. Finally, the fluorescence intensity of the reaction system was recorded under the excitation wavelength of 410 nm.
The selectivity of the detecting system for HRP activity was investigated with several common biomolecules, including bovine serum albumin (BSA), α-L-fucosidase (AFu), alkaline phosphatase (ALP), α-glucosidase, β-glucosidase, α-amylase, lipase and metal salts (FeCl3, CaCl2, NaCl, MgCl2) as controls following the above procedure.
Procedure for the determination of glucose
Glucose solution with different concentrations (0, 0.1, 1, 10, 50, 100, 250, 500, 1000, 2500, 5000, 7500 and 10,000 μM), GOx solution (20 U·mL−1), HRP solution (1 μg·mL−1) and PPD solution (15 mM) were prepared with PBS solution (10 mM pH = 7.0). Glucose solution (75 μL) with different concentrations was added into 75 μL of GOx solution and reacted for 30 min at 37 °C. Then, 450 μL of HRP solution, 150 μL of PPD solution, 750 μL of PBS solution were added to the reaction mixture in sequence. The resulting mixture was incubated for 15 min at room temperature. 300 μL of reaction solution was mixed with 200 μL of PD solution and the fluorescence intensity was recorded with the excitation wavelength of 410 nm.
Fluorescence immunoassay for Cry1Ab/Ac protein
The determination of Cry1Ab/Ac protein was performed by using a commercial Cry1Ab/Ac ELISA kit with PPD instead of 3,3′,5,5′-tetramethylbenzidine (TMB) as reaction substrate. The analysis was performed as following: firstly, 100 μL of Cry1Ab/Ac protein standards with different concentrations (0, 0.5, 0.75, 1.0, 2.5, 5.0, 7.5, 10, 12.5, 20, 25 and 30 ng·mL−1) were added to wells coated with anti-mouse Cry1Ab/Ac, and wells were incubated for 45 min at 25 °C. Then, wells were rinsed with Tween in phosphate buffered saline (PBS-T) solution for five times. 100 μL of HRP-conjugated antibody was dropped and wells were incubated for 30 min at 25 °C to form the sandwich immunocomplex before being washed with PBS-T solution for five times. Subsequently, PPD (100 μL, 15 mM), H2O2 (100 μL, 15 mM) and PBS solution (pH 7.0, 100 μL, 10 mM) were added in sequence and wells were incubated for 20 min at 37 °C. Then, the reaction solution (250 μL) was added to PD solution (75 μL) and the fluorescence intensity was measured with the excitation wavelength of 410 nm.
The determination of Cry1Ab/Ac protein in real rice leaves was also investigated by this method. The Cry1Ab/Ac protein from rice leaves was extracted as following: 1.0 g of fresh rice leaves was weighed, smashed, added into 10 mL of sample extracting solution, shook for 5 min, and centrifuged at 4000 r·min−1 (max relative centrifugal force 15,100 g) for 3 min. The supernatant was collected and submitted for fluorescence immunoassay according to the same procedure above by just adding the supernatant instead of the Cry1Ab/Ac protein standard solutions.
Results and discussion
Principle of HRP activity assay
Figure 1a is a schematic illustration for the mechanism of PD-based nanoprobe for HRP activity detection. HRP/H2O2-catalyzed oxidation of PPD produced colored PPDox with a wide absorption peak around 510 nm, which overlapped the fluorescence emission and excitation spectra of PDs simultaneously (Fig. 1b) and resulted in a fluorescence quenching of PDs due to IFE.
a Principle of HRP activity determination based on IFE, b UV-Vis absorption spectra of PPD solution (a) and PPDox solution (b), fluorescence excitation (c) and emission spectra (d) of PD solution, c UV-Vis absorbance spectra of PPD solution (a), PPD solution + H2O2 solution (b), PPD solution + HRP solution (c), H2O2 solution + HRP solution (d), and PPD solution + HRP solution + H2O2 solution (e) in 10 mM pH 7.0 PBS. d Fluorescence spectra of PD solution (a), PD solution mixed with PPD solution (b), PPD solution + H2O2 solution (c), PPD solution + HRP solution (d), H2O2 solution + HRP solution (e), and PPD solution + HRP + H2O2 solution (f) in 10 mM pH 7.0 PBS
To prove the reliability of this design, the UV-vis absorption spectrum of PPD solution (dissolved in 10 mM PBS solution, pH = 7.0) was measured. As shown in Fig. 1c line a, colorless PPD solution showed a strong UV-vis absorption wavelength at 305 nm. Under the same conditions, 10 mM H2O2 solution and 4 U·mL−1 HRP solution was added into the PPD solution, respectively. The UV-vis absorption spectra of PPD solution did not significantly change (Fig. 1c lines b, c). These solutions had no interference on the fluorescence of PDs (Fig. 1d lines a, b, c, d, e). However, a new strong UV-vis absorption peak appeared at 510 nm when H2O2 solution and HRP solution was simultaneously added to PPD solution (Fig. 1c line e). As expected, a significant fluorescence quenching of PDs was observed after the addition of the three-component mixture into PD solution (Fig. 1d line f). These results validated the reliability of our nanoprobe. It is noteworthy that PPD solution must be freshly prepared since it would be oxidized if exposing to the air for a long time.
The zeta potential of PD solution and PPDox solution were respectively measured as −18.3 mV and − 22.95 mV (Fig. S4A), which indicated that there was no electrostatic interaction between the negatively charged PDs and PPDox. Therefore, the interaction was not possible due to FRET or PET mechanism, which was occurred in the case of opposite charges [28, 29]. The fluorescence decay curve of PDs was not changed in the presence of PPDox in this study (Fig. S4B), which indicated that there was no energy transfer process [30, 31]. Both results indicated that the fluorescence quenching between PDs and PPDox was arisen from IFE.
The determination of HRP activity
To optimize the enzymatic hydrolysis time, the time-dependent fluorescence intensity ratios I/I0 of the reaction system were investigated. I/I0 value reached the plateau at 15 min with different concentrations of HRP (Fig. 2a). Subsequently, the performance of PD-based nanoprobe for HRP activity detection was investigated under the optimized reaction time of 15 min. The fluorescence spectra (Fig. 2b) illustrated that the fluorescence intensity of sensing system was effectively reduced with the HRP concentration increased from 0 to 200 U·mL−1. Figure 2d shows the regression line between the relative fluorescence intensity (I0-I) of PDs (Fig. 2c) and the HRP activity from 0.0002 to 0.2 U·mL−1. A regression equation was obtained as y = 13,141.53x + 7683 and the linear correlation coefficient was 0.9911. The HRP activity detection limit was calculated to be 0.2 U·L−1 based on the 3σ method.
a The relationship between relative fluorescence intensities and incubation time in different concentration of HRP, b Fluorescence spectra (λex/λem = 410/515 nm) of the detecting system for different HRP concentrations with 10 mM PPD and H2O2 incubated for 20 min at 37 °C, c Plots of I0-I vs concentrations of HRP from 0.0002 to 200 U·mL−1, d Linear range of I0-I against concentrations of HRP from 0.0002 to 0.2 U·mL−1. I and I0 was the fluorescence intensity of the detecting system in the presence and absence of HRP, respectively
Selectivity of HRP activity determination
The selectivity of this detecting system was performed by using several common biomolecules, including BSA, AFu, ALP, α-glucosidase, β-glucosidase, α-amylase, lipase and metal ions (Fe3+, Ca2+, Na+, Mg2+) as controls. Figure 3 demonstrates that these potential interferences did not show noticeable influence on the detecting system either in the absence (red histogram) or presence of HRP (black histogram), which indicated that our detecting system was highly selective.
Relative fluorescence intensities of detecting system in the presence of various proteins or metal ions (250 U·L−1 for enzymes and 1 mmol/L for metal ions) with (red columns) and without (black columns) HRP (20 U·mL−1) after incubation for 15 min, where I and I0 was the fluorescence intensity of the detecting system in the presence and absence of interferents, respectively
Fluorescent detecting of glucose
The detecting system of HRP activity was on account of HRP/H2O2-induced oxidation PPD into PPDox, while H2O2 can also be generated via the enzymatic oxidation of glucose by GOx. Therefore, the detecting system was further extended to glucose content measurement. As the concentrations of glucose increased, the fluorescence of PDs was quenched gradually (Fig. 4a). As shown in Fig. 4b, a good regression line between the relative fluorescence intensity (I0-I) of PDs and the concentration of glucose from 0.1 to 1000 μM (R2 = 0.9916) was exhibited. The detection limit for glucose was calculated as 0.1 μM (3σ method). Compared to other methods for the determination of glucose, this assay showed a lower detection limit (Table S1).
a Fluorescence spectra (λex/λem = 410/515 nm) of the detecting system with the increasing concentrations of glucose (from top to bottom: 0, 0.1, 1, 10, 50, 100, 250, 500, 1000, 2500, 5000, 7500 and 10,000 μM). b Linear range of I0-I against concentrations of glucose from 0.1 to 1000 μM, where I0 and I was the fluorescence intensity of the detecting system in the absence and presence of glucose, respectively
Fluorescent immunoassay for Cry1Ab/Ac protein
Considering HRP was one of the most widely used enzymes in ELISA, we attempted to extend this assay to HRP-enabled fluorescence ELISA for Cry1Ab/Ac protein detection. As shown in Scheme 1, the antigen was Cry1Ab/Ac protein, the primary antibody was anti-mouse Cry1Ab/Ac, and the secondary antibody was anti-rabbit Cry1Ab/Ac labeled with HRP. After the sandwich immunocomplex was formed, HRP bound on the wells triggered the conversion of PPD into PPDox in the presence of H2O2, resulting in the fluorescence quenching of PDs. As the concentration of Cry1Ab/Ac protein increased, the fluorescence intensity of PDs gradually declined (Fig. 5a). A good regression line between the relative fluorescence intensity (I0-I) of PDs and the concentration of Cry1Ab/Ac protein was obtained within the range of 0.5~12 ng·mL−1 (R2 = 0.992) (Fig. 5b). The limit of detection was 0.25 ng·mL−1 (3σ method). Compared with the results measured by commercial Cry1Ab/Ac ELISA kit (Fig. S5), this fluorescence ELISA assay offers a slightly wider range of Cry1Ab/Ac protein concentration.
a Fluorescence spectra (λex/λem = 410/515 nm) of the detecting system with the increasing concentrations of Cry1Ab/Ac protein (from top to bottom: 0, 0.5, 0.75, 1.0, 2.5, 5.0, 7.5, 10, 12.5, 20, 25 and 30 ng·mL−1). b Linear range of I0-I against concentrations of Cry1Ab/Ac protein from 0.5 to 12.5 ng·mL−1. I and I0 was the fluorescence intensity of the detecting system in the presence and absence of Cry1Ab/Ac protein, respectively
Rice is an important crop and widely planted in the world. In order to further evaluate the reliability and practicability, the detection method was used to detect the Cry1Ab/Ac protein in the rice leaf extraction. The results are given in Table 1, which indicated that this PDs assay is potential to be an alternative to the conventional TMB-based colorimetric method in real sample analysis.
Conclusion
An efficient polymer dot-based fluorescent nanoprobe was built for the determination of HRP activity and glucose. The detection was achieved by a turn-off pattern based on IFE effect between PDs and PPDox oxidized from PPD. The HRP assay was successfully applied in the HRP-based fluorescent ELISA for Cry1Ab/Ac protein determination and rice leaves analysis. The practicability of this assay demonstrated its potential in the detection of other important protein by the combination of ELISA. This PD-based fluorescence nanoprobe threw a light on the practical application of nanomaterials in bioanalysis.
References
Veitch NC (2004) Horseradish peroxidase: A modern view of a classic enzyme. Phytochemistry 65(3):249–259
Satvekar RK, Rohiwal SS, Raut AV, Karande VA, Tiwale BM, Pawar SH (2014) A silica-dextran nanocomposite as a novel matrix for immobilization of horseradish peroxidase, and its application to sensing hydrogen peroxide. Microchim Acta 181(1–2):71–77
Li DY, Ying YB, Wu J, Niessner R, Knopp D (2013) Comparison of monomeric and polymeric horseradish peroxidase as labels in competitive ELISA for small molecule detection. Microchim Acta 180(7–8):711–717
Cheng X, Huang Y, Yuan C, Dai K, Jiang H, Ma JM (2019) Colorimetric detection of α-glucosidase activity based on the etching of gold nanorods and its application to screen anti-diabetic drugs. Sensor Actuat B Chem 282:838–843
Tronstad C, Elvebakk O, Staal OM, Kalvøy H, Høgetveit JO, Jenssen TG, Birkeland KI, Martinsen ØG (2019) Non-invasive prediction of blood glucose trends during hypoglycemia. Anal Chim Acta 1052:37–48
Khan MI, Zhang Q, Wang YX, Saud S, Liu WW, Liu SR, Kong H, Wang CH, Uzzaman A, Xiao H, Fan LY, Cao CX (2019) Portable electrophoresis titration chip model for sensing of uric acid in urine and blood by moving reaction boundary. Sensor Actuat B Chem 286:9–15
Darabdhara G, Boruah PK, Das MR (2019) Colorimetric determination of glucose in solution and via the use of a paper strip by exploiting the peroxidase and oxidase mimicking activity of bimetallic cu-Pd nanoparticles deposited on reduced graphene oxide, graphitic carbon nitride, or MoS2 nanosheets. Microchim Acta 186(1):13
Liu JW, Luo Y, Wang YM, Duan LY, Jiang JH, Yu RQ (2016) Graphitic carbon nitride nanosheets-based ratiometric fluorescent probe for highly sensitive detection of H2O2 and glucose. ACS Appl Mater Inter 8(49):33439–33445
Karuppiah C, Palanisamy S, Chen SM, Veeramani V, Periakaruppan P (2014) Direct electrochemistry of glucose oxidase and sensing glucose using a screen-printed carbon electrode modified with graphite nanosheets and zinc oxide nanoparticles. Microchim Acta 181(15–16):1843–1850
Hovancová J, Šišoláková I, Oriňaková R, Oriňak A (2017) Nanomaterial-based electrochemical sensors for detection of glucose and insulin. J Solid State Electrochem 21(8):2147–2166
Parween S, Nahar P (2015) Femtogram detection of horseradish peroxidase by a common desktop scanner. J Biosci Bioeng 119(1):113–116
Huang S, Wang LM, Huang CS, Su W, Xiao Q (2016) Amino-functionalized graphene quantum dots based ratiometric fluorescent nanosensor for ultrasensitive and highly selective recognition of horseradish peroxidase. Sensor Actuat B-Chem 234:255–263
Wang Q, Xue R, Guo H, Wei Y, Yang W (2018) A facile horseradish peroxidase electrochemical biosensor with surface molecular imprinting based on polyaniline nanotubes. J Electroanal Chem 817:184–194
Wu JS, Liu WM, Ge JC, Zhang HY, Wang PF (2011) New sensing mechanisms for design of fluorescent chemosensors emerging in recent years. Chem Soc Rev 40(7):3483–3495
Ma JM, Cheng X, Peng FF, Zhang N, Li RF, Sun LH, Li ZL, Jiang H (2019) A polymer dots fluorescent sensor for detection of alkaline phosphatase activity and inhibitor evaluation. J Mater Sci 54:10055–10064
Sutariya PG, Soni H, Gandhi SA, Pandya A (2019) Novel luminescent paper based calix [4] arene chelation enhanced fluorescence-photoinduced electron transfer probe for Mn2+, Cr3+ and F. J Lumin 208:6–17
Liu SY, Wang H, He T, Qi L, Zhang ZQ (2016) Sensitive fluorimetric assays for α-glucosidase activity and inhibitor screening based on β-cyclodextrin-coated quantum dots. Luminescence 31(1):96–101
Chen S, Yu YL, Wang JH (2018) Inner filter effect-based fluorescent sensing systems: A review. Anal Chim Acta 999:13–26
Zhang J, Zhou R, Tang D, Hou X, Wu P (2018) Optically-active nanocrystals for inner filter effect-based fluorescence sensing: Achieving better spectral overlap. TrAC Trend Anal Chem 110:183–190
Wang T, Zeng LH, Li DL (2017) A review on the methods for correcting the fluorescence inner-filter effect of fluorescence spectrum. Appl Spectrosc Rev 52(10):883–908
Zhu SJ, Song YB, Shao JR, Zhao XH, Yang B (2015) Non-conjugated polymer dots with crosslink-enhanced emission in the absence of fluorophore units. Angew Chem Int Ed 54(49):14626–14637
Nasirian V, Chabok A, Barati A, Rafienia M, Arabi MS, Shamsipur M (2017) Ultrasensitive aflatoxin B1 assay based on FRET from aptamer labelled fluorescent polymer dots to silver nanoparticles labeled with complementary DNA. Microchim Acta 184(12):4655–4662
Liu MM, Huang R, Weisman A, Yu XY, Lee SH, Chen YL, Huang C, Hu SH, Chen XH, Tan WF, Liu F, Chen H, Shea KJ (2018) Synthetic polymer affinity ligand for bacillus thuringiensis (Bt) Cry1Ab/ac protein: The use of biomimicry based on the Bt protein–insect receptor binding mechanism. J Am Chem Soc 140(22):6853–6864
Wang X, Chen X, Xu J, Dai C, Shen W (2015) Degradation and detection of transgenic Bacillus thuringiensis DNA and proteins in flour of three genetically modified rice events submitted to a set of thermal processes. Food Chem Toxicol 84:89–98
Jiang Y, Ling L, Zhang L, Wang K, Li X, Cai M, Zhan M, Li C, Wang J, Cao C (2018) Comparison of transgenic Bt rice and their non-Bt counterpart in yield and physiological response to drought stress. Field Crops Res 217:45–52
Zhu M, Li M, Li G, Zhou Z, Liu H, Lei H, Shen Y, Wan Y (2015) Nanobody-based electrochemical immunoassay for bacillus thuringiensis Cry1Ab toxin by detecting the enzymatic formation of polyaniline. Microchim Acta 182:2451–2459
Cheng X, Huang Y, Li DY, Yuan C, Li ZL, Sun LH, Jiang H, Ma JM (2019) A sensitive polymer dots fluorescent sensor for determination of α-L-fucosidase activity in human serum. Sensor Actuators B Chem 288:38–43
Kong WH, Wu D, Xia L, Chen XF, Li GL, Qiu NN, Chen G, Sun ZW, You JM, Wu YN (2017) Carbon dots for fluorescent detection of α-glucosidase activity using enzyme activated inner filter effect and its application to anti-diabetic drug discovery. Anal Chim Acta 973:91–99
Li GL, Kong WH, Zhao M, Lu SM, Gong PW, Chen G, Xia L, Wang H, You JM, Wu YN (2016) A fluorescence resonance energy transfer (FRET) based "turn-on" nanofluorescence sensor using a nitrogen-doped carbon dot-hexagonal cobalt oxyhydroxide nanosheet architecture and application to alpha-glucosidase inhibitor screening. Biosens Bioelectron 79:728–735
Zhang MW, Cao XY, Li HK, Guan FR, Guo JJ, Shen F, Luo YL, Sun CY, Zhang LG (2012) Sensitive fluorescent detection of melamine in raw milk based on the inner filter effect of au nanoparticles on the fluorescence of CdTe quantum dots. Food Chem 135:1894–1900
Liu HJ, Li M, Xia YN, Ren XQ (2017) A turn-on fluorescent sensor for selective and sensitive detection of alkaline phosphatase activity with gold nanoclusters based on inner filter effect. ACS Appl Mate Inter 9:120–126
Acknowledgments
This study was funded by the Fundamental Research Funds for the Central Universities, China (2662018JC011 and 2662019PY023).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
ESM 1
(DOCX 1852 kb)
Rights and permissions
About this article
Cite this article
Cheng, X., Sun, L., Li, R. et al. Organic polymer dot-based fluorometric determination of the activity of horseradish peroxidase and of the concentrations of glucose and the insecticidal protein toxin Cry1Ab/Ac. Microchim Acta 186, 731 (2019). https://doi.org/10.1007/s00604-019-3831-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00604-019-3831-9