Abstract
Magnetic nanoparticles (MNPs) modified with β-cyclodextrin and mono-6-deoxy-6-(1-methylimidazolium)-β-cyclodextrin tosylate (an ionic liquid), which called MNP-β-CD and MNP-β-CD-IL, were coated into the capillary inner wall. Compared to an uncoated capillary, the new systems show good reproducibility and durability. The systems based on the use of MNP-β-CD or MNP-β-CD-IL as stationary phases were established for enantioseparation of Dns-modified amino acids. Improved resolutions were obtained for both CEC systems. Primary parameters such as running buffer pH value and applied voltage were systematically optimized in order to obtain optimal enantioseparations. Under the optimized conditions, the capillaries exhibited excellent chiral recognition ability for six Dns-amino acids (the DL-forms of alanine, leucine, lsoleucine, valine, methionine, glutamic acid) and provided a promising way for the preparation of chiral column.

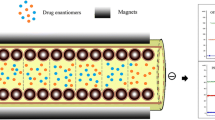
Schematic presentation of the open-tubular capillary electrochromatography systems with MNP-β-CD and MNP-β-CD-IL as stationary phases for enantioseparation of dansylated amino acids.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Capillary electrophoresis (CE) is a powerful technology for enantioseparation owing to its simplicity, high efficiency, low cost, as well as its flexibility of separation mode [1, 2]. Capillary electrochromatography (CEC), exhibiting both high selectivity of liquid chromatography and high separation efficiency of capillary electrophoresis, is a miniaturized separation technique and has been widely used in many fields [3,7,5]. According to different preparation methods of stationary phase in capillaries, the separation modes in CEC can be divided into three types: the open-tubular CEC (OT-CEC), the packed CEC (P-CEC) and the monolithic CEC. Comparing with the other separation modes, OT-CEC has several advantages such as none eddy diffusion, rapid analysis, variety of available modification, the relatively simple preparation [6]. However, the low column capacity and phase ratio of the small surface area of the coatings limits the development of OT-CEC.
Nanomaterials have a high surface to volume ratio and special physico-chemical properties. This is important for the application in separation science. When used in CE, nanomaterials are suspended in background as pseudostationary phase [7,13,14,10]. Moreover, they can be physically absorbed or covalently bonded to stationary phase as component of the CEC column [11,17,18,14]. Ferrite oxide magnetic (Fe3O4) has been widely used for many applications, because it is easy to obtain, synthetize and surface modify, and maintain superparamagnetic [15]. In general, MNPs were introduced into the analytical process to improve the resolution of the chromatographic and electrophoretic separation. It may due to the above unique advantages as nanomaterial and the additional magnetic property. In 2007, Yukihiro Okamato etal prepared avidin immobilized magnetic particles packed capillary by applied an external magnetic field and successfully obtained the enantioseparation of ketoprofen in a chiral CEC system [16]. As a simple and convenient method, the physical magnetic attraction approach to preparing functionalization magnetic packed columns gradually got to gain more attention in separation science [17,23,24,20].
We synthesized magnetite nanoparticle coated with β-cyclodextrin (MNP-β-CD) and magnetite nanoparticle coated with mono-6-deoxy-6-(1-methylimidazolium)-β-cyclodextrin tosylate (MNP-β-CD-IL) nanoparticles via the co-precipitation method, and then prepared MNP-β-CD and MNP-β-CD-IL open-tubular columns in bare capillary with external magnetic field. Ionic liquids (ILs) are a broad class of organic salts consisting of organic cation and organic anion or inorganic anion, which melt at or below 100 °C. Compared with the traditional preparation methods, the method of preparing the coated column with magnetic is quite simple. The preparation of the coated capillary was discussed and the column characteristic was systematically investigated. Moreover, we studied the inner surface of capillary with SEM. The MNPs can be obviously observed in columns. Six dansylated DL-amino Acids was successfully separated in CEC with MNP-β-CD and MNP-β-CD-IL open-tubular columns.
Materials and methods
Materials and chemicals
Iron(II) sulfate (FeSO4∙7H2O, 98%), ammonium hydroxide solution (25%) were purchased from Nanjing Chemical Regent Co., Ltd. (Nanjing, China, https://www.pvc123.com/b-zhouzijian/). while iron(III) chloride hexahydrate (FeCl3∙6H2O, 98%) was purchased from Sinopharm Chemical Regent Co., Ltd. (Nanjing, China, http://en.reagent.com.cn/). β-cyclodextrin (≥ 98%) was purchased from Zibo Qianhui Fine Chemical Co. Ltd. (Shandong, China, http://cn.commerce.com.tw/), recrystallized and dried twice at 100 °C for 24 h before use. 1-Methylimidazole was purchased from J&K Scientific Co. Ltd. (Beijing, China, http://jkchemical.labscn.com.cn/).Cylindrical permanent magnets of 10 mm d. × 3 mm h. (magnetic flux density of each one is 0.22 T) were purchased from Ningbo YongCi magnetic materials Co. Ltd. Amino acids (DL-alanine, DL-leucine, DL-lsoleucine, DL-valine, DL-methionine, DL-glutamic acid) were obtained from Shanghai Yuanye Biological Technology Co., Ltd. (Shanghai, China, http://www.shyuanye.com/). Dansyl chloride (99%) and Lithium carbonate (99.99%) were purchased from Shanghai Aladdin Bio-Chem Technology Co., Ltd. (Shanghai, China, http://www.aladdin-e.com/). Acetone, thiourea, acetonitrile and Dimethyl formamide (DMF), phosphoric acid and sodium hydroxide were purchased from Nanjing Chemical Reagent (Nanjing, China, https://ncrcl.en.china.cn/). Nylon filters (0.45 μm) were purchased from Jiangsu Hanbon Science and Technology (Nanjing, China, http://hanbon.company.weiku.com/). Tris (hydroxymethyl) aminomethane (Tris) were purchased from Shanghai Huixing Biochemistry Reagent (Shanghai, China, http://shhuixing.company.lookchem.cn/). Double distilled water was used in all the experiments. Calculation for chiral separation were Rs = 2(t2 - t1)/(w1 + w2) and α = t2/t1, where t1 and t2 are the migration times of the two enantiomers, and w1 and w2 are the widths of their peaks at the baseline.
Apparatus
The morphology of the prepared column and magnetic nanaoparticles were investigated by SEM (Hitachi, S-4800, Japan). TEM (FEI, Tecnai G2 F30 S-TWIN, America) and FT-IR (ALPHA, BRUKER, Germany) were used to characterize the magnetic nanoparticles. All electrophoretic experiments were performed on an Agilent 3D CE system (Agilent Technologies, Waldbronn, Germany), which consisted of a sampling device, a power supply, a photodiode array UV detector (wavelength range from 190 to 600 nm) and a data processor. The whole system was driven by Agilent ChemStation software (Revision B.02.01) for system control, data collection and analysis. Fused-silica capillaries of 75 μm i.d. × 365 μm o.d. were purchased from Hebei Yongnian County Reafine Chromatography Ltd. (Hebei, China).
A new capillary was conditioned by successive flushes with 1 M NaOH, 0.1 M NaOH and distilled water under a pressure of 940 mbar for 30 min, respectively. All separations were carried out at 25 °C. Separation voltage was 5–20 kV. The samples of Dns-amino acids were injected by pressure method for 3 s (injection pressure 50 mbar). Thiourea was used as neutral marker to determine the electroosmotic flow (EOF). Buffer was 40 mM Tris/ H3PO4 solution (if not stated otherwise). The running buffer was freshly prepared with a specified pH, and then adjusted to a desired pH value by adding a small volume of H3PO4 (20%, v/v). All solutions were filtered through a 0.45 μm pore membrane filter and degassed by sonication before use. The concentration of all enantiomers is 0.5 mg·mL−1. The wavelength of UV detection was set at 254 nm for all the Dns-AAs.
Preparation of β-CDs modified Fe3O4 nanoparticle-coated open-tubular capillary and characterization
A 40 cm (31.5 cm effective length) × 75 μm i.d. uncoated fused-silica capillary was used in our expriments. Firstly, the capillary was orderly washed with 1.0 M NaOH (30 min), 0.1 M NaOH (30 min) and water (30 min), and then purged with nitrogen for 2 h. To supply a local stable magnetic field to the capillary, 10 pairs of cylindrical permanent magnets (diameter 10 mm × height 3 mm) were oppositely nipped to the capillary (as shown in Figure s1). The length of capillary covered by magnets was 10.0 cm, which was adjusted to CE instrument. The first pair of magnets was placed at a distance of 15 cm from the inlet of the capillary, and the last pairs were placed at a distance of 6.5 cm from the detection window. The capillary was flushed with 1 mg·mL−1 of β-CDs functionalized Fe3O4 magnetic nanoparticle suspension (dispersed in alcohol, ultrasonic dispersion for 30 min) for 10 min under a pressure of 940 mbar, and H2O for 2 min to wash away the un-adsorbed nanoparticles. The above steps were repeated four times. The nanoparticles stationary phase can be easily removed and recycled when the permanent magnets are removed. The effective length of nanoparticle coating was 10.0 cm.
Results and discussion
Characterization of β-CDs modified Fe3O4 nanoparticles coated open-tubular capillary
The FT-IR spectra of the pure Fe3O4 nanoparticles and cyclodextrins modified Fe3O4 nanoparticles (MNP-β-CD and MNP-β-CD-IL) were carried out to identify the coating of β-CD and β-CD-IL on the surface of Fe3O4 nanoparticles. As shown in Figure s2 and s3, MNP, MNP-β-CD and MNP-β-CD-IL exhibited obvious Fe-O bond at 570 cm−1 -590 cm−1 and a broad absorption peak of around 3200 cm−1-3350cm−1which was assigned to the -OH stretching vibration from hydroxyl groups [21]. Comparing the MNPs, the peaks at 1067 cm−1 and 1087 cm−1 corresponded to the skeletal vibration involving α-1, 4 linkage of β-cyclodextrins in functionalized MNPs. Moreover, MNP-β-CD-IL showed the apparent bands at 1650 cm−1 (C-N-H), about 1400 cm−1 (C=C in imidazolium, CH2 bending vibration) [22]. Overall, these absorption bands, observed in FT-IR spectrum of MNP-β-CD and MNP-β-CD-IL, confirmed the coating of β-CD and β-CD-IL on the surface of MNP nanoparticles. The morphology changes of the pure and functionalized magnetic nanoparticles were analyzed by TEM. As shown in Figure s3, the average diameter of all the three kinds of magnetic nanoparticles was about between 8~12 nm. While Figure s4 presents the particle size distribution graph of MNP-β-CD and MNP-β-CD-IL. The average particle size of two functionalized nanoparticles was about 70 nm, which was bigger than the results in TEM due to the conglomeration of nanoparticles. Further, the morphology of both MNP-β-CD coating capillary and MNP-β-CD-IL coating capillary was evaluated by SEM. Figure 1 shows the formation of the functionalized MNPs coating on the inner wall of the capillary. There were no significant differences between the two coating columns. The coating thickness of MNP-β-CD modified capillary was about 5 μm - 15 μm, while the coating thickness of MNP-β-CD-IL modified capillary was about 5 μm - 20 μm.
Evaluation of the column characteristic
Measurement of EOF
In capillary electrophoresis, the value and direction of the electroosmotic flow (EOF) are determined by the state of the functional groups on the inner wall of the capillary. By studying the change of EOF under different buffer pH, we can understand the change of the inner wall of capillary and further characterize the capillary. In this study, EOF was measured at pH 5.0–7.0 with thiourea as EOF marker in the uncoated capillary and functionalized MNPs coated columns. The results are shown in Fig. 2. With the increasing of the buffer pH, the value of EOF in all the three columns increased. And the variation extent of EOF in functionalized MNPs coating columns are both smaller than that in the uncoated capillary, which means that improved reproducibility can be obtained in the functionalized MNPs coating columns. Compared to MNP-β-CD modified capillary, the the variation extent of EOF in MNP-β-CD-IL modified capillary is smaller. It was attributed to the existence of imidazolium cations in MNP-β-CD-IL which would effectively inhibit the electroosmotic flow.
Reproducibility
It is not easy to get the high reproducibility of EOF in an uncoated capillary. The comparison of run-to-run, day-to-day and column-to-column reproducibility (n = 5) among the bare column and the β-cyclodextrins functionalized Fe3O4 nanoparticles based columns was evaluated by calculating the RSD of the retention time under the experimental condition (buffer pH 6.0, applied voltage 15 kV). The results were shown in Table 1. The run-to-run RSDs of the retention time were 2.56% in MNP-β-CD caipllary and 2.28% in MNP-β-CD-IL capillary, which were less than 3.21% in bare capillary. Finally, it can be concluded that those new magnetic nanoparticles-based CEC systems can provide satisfactory reproducibility. The difference may be explained by that the silicon hydroxyl on the inner surface of the coated column was covered by the nanoparticles, which makes the capillary wall less sensible to the change of buffer composition in analysis process.
Enantioseparation of Dan-amino acids
The MNP-β-CD and MNP-β-CD-IL coated enantioseparation CEC systems were established with six Dns-amino acids. The chiral separation results in different capillary columns were shown in Table 2 and Fig. 3. As observed, the good baseline separations of six Dns-amino acids were obtained in both new coated CEC systems. According to the previous reported [23,29,25], the MNP-β-CD-IL coating can influence the formation of the inclusion complex between enantiomers and chiral selectors. Comparing with the MNP-β-CD coated system, the better resolution and higher selectivity of all the tested dns-amino acid were achieved in MNP-β-CD-IL coated CEC system, which might attribute to the different of the chiral recognition ability between β-CD and β-CD-IL. For all selected amino acid, the enantiomer will enter the cavity of the cyclodextrin to form a complex. Different complexes formed by single enantiomers will lead to a differential migration under the electric field, resulting in separation.
Enantioseparation electropherograms of Dns-Amino acids on MNP-β-CD coated capillary and MNP-β-CD-IL coated capillary by CEC. Conditions: 30 mM sodium citrate/citric acid buffer; buffer pH 6.0; applied voltage, 15 kV; capillary temperature, 25 °C; concentration of Dns-amino acid is 0.5 mg·mL−1; other conditions see Materials and methods
Optimization of method
The following parameters were optimized: (a) Effect of buffer pH; (b) Effect of applied voltage. Respective data and Figures are given in the Electronic Supporting Material (Table s1 and Figure s5). The following experimental conditions were found to give best results: (a) Best buffer pH value: pH 6.0; (b) Optimal voltage: 15 kV.
Comparison with other methods
In the previous work, various magnetic-based nanoparticles have been successfully applied in CEC for enantioseparation [16, 18, 20, 26,27,28,29,30]. To further demonstrate the superiority of our systems, we compared our method with previously reported research. As shown in Table 3, high enantiorecognition capability and excellent reproducibility for analytes were obtained in our systems compared with other methods. The baseline chiral separations of six dns-amino acids were obtained in both MNP-β-CD and MNP-β-CD-IL coating open-tubular capillary electrochromatography systems.
Conclusions
Two novel open tubular capillary columns based on β-CDs modified magnetic nanoparticles were prepared for chiral separation. They were constructed by using the magnetic effect of permanent magnets to immobilize the magnetic nanoparticles onto the inner wall of the capillary. Compared with the traditional preparation methods, the method of preparing the coating column with magnetic is quite flexible. We studied the inner surface of capillary with SEM. The MNPs can be obviously observed in columns. Six Dns-DL-Amino Acids was successfully separated in CEC systems with MNP-β-CD and MNP-β-CD-IL open-tubular columns. Several parameters such as chiral running buffer pH and applied voltage were systematically optimized. The results indicate that the application of magnetic nanoparticles as coating materials is a promising way in chiral separation science. The novel system provides many more possibilities and potential to separate other compounds, such as amino acid and their derivatives, small molecular drugs and some kind of biogenic amines.
Abbreviations
- CEC:
-
capillary electrochromatography
- MNP-β-CD:
-
magnetite nanoparticle coated with β-cyclodextrin
- MNP-β-CD-IL:
-
magnetite nanoparticle coated with mono-6-deoxy-6-(1-methylimidazolium)-β -cyclodextrin tosylate nanoparticles
- β-CDOTs:
-
mono-6-O-Tosyl-β-cyclodextrin
- β-CD-IL:
-
6-MI-β-CD+OTs−, mono-6-deoxy-6-(1-methylimidazolium)-β -cyclodextrin tosylate.
References
Wang J (2005) Electrochemical detection for capillary electrophoresis microchips: a review. Electroanalysis 17:1133–1140
Ramos-Payán M, Ocaña-Gonzalez JA, Fernández-Torres RM, Llobera A, Bello-López MÁ (2018) Recent trends in capillary electrophoresis for complex samples analysis: a review. Electrophoresis 39:111–125
Zhang K, Gao R (2001) Capillary Electrochromatography. Methods Mol Biol 52:197
Yang L, Guihen E, Holmes JD, Loughran M, O'Sulliva GP, Glennon JD (2005) Gold nanoparticle-modified etched capillaries for open-tubular capillary electrochromatography. Anal Chem 77:1840–1846
Tanaka N, Nagayama H, Kobayashi H et al (2015) Monolithic silica columns for HPLC, micro-HPLC, and CEC. J Sep Sci 23:111–116
Guihen E, Glennon JD (2004) Recent highlights in stationary phase design for open-tubular capillary electrochromatography. J Chromatogr A 1044:67–81
Nilsson C, Birnbaum S, Nilsson S (2007) Use of nanoparticles in capillary and microchip electrochromatography. J Chromatogr A 1168:212–224
Řezanka P, Ehala S, Koktan J et al (2012) Application of bare gold nanoparticles in open-tubular CEC separations of polyaromatic hydrocarbons and peptides. J Nanopart Res 35:73–78
Hua X, Du Y, Chen J et al (2013) Evaluation of the enantioselectivity of carbon nanoparticles-modified chiral separation systems using dextrin as chiral selector by capillary electrokinetic chromatography. Electrophoresis 34:1901–1907
Gong ZS, Duan LP, Tang AN (2015) Amino-functionalized silica nanoparticles for improved enantiomeric separation in capillary electrophoresis using carboxymethyl-β-cyclodextrin (CM-β-CD) as a chiral selector. Microchim Acta 182:1297–1304
Liu Z, Du Y, Feng Z (2018) Enantioseparation of drugs by capillary electrochromatography using a stationary phase covalently modified with graphene oxide. Microchim Acta 184:583–593
Zhang Y, Wang W, Ma X, Jia L (2016) Polydopamine assisted fabrication of titanium oxide nanoparticles modified column for proteins separation by capillary electrochromatography. Anal Biochem 512:103–109
Liu S, Peng J, Liu Z, Liu Z, Zhang H, Wu R’ (2016) One-pot approach to prepare Organo-silica hybrid capillary monolithic column with intact mesoporous silica nanoparticle as building block. Sci Rep 6:34718–34728
Xu S, Mo R, Jin C, Cui X, Bai R, Ji Y (2017) Mesoporous silica nanoparticles incorporated hybrid monolithic stationary phase immobilized with pepsin for enantioseparation by capillary electrochromatography. J Pharm Biomed Anal 140:190–198
Ríos Á, Zougagh M (2016) Recent advances in magnetic nanomaterials for improving analytical processes. Trends Anal Chem 84:72–83
Okamoto Y, Ikawa Y, Kitagawa F, Otsuka K (2007) Preparation of fritless capillary using avidin immobilized magnetic particles for electrochromatographic chiral separation. J Chromatogr A 1143:264–269
Wang Y, Zhang Z, Zhang L, Li F, Chen L, Wan QH (2007) Magnetically immobilized beds for capillary electrochromatography. Anal Chem 79:5082–5086
Qu P, Lei J, Zhang L, Ouyang R, Ju H (2010) Molecularly imprinted magnetic nanoparticles as tunable stationary phase located in microfluidic channel for enantioseparation. J Chromatogr A 1217:6115–6121
Zhu Y, Zhou C, Qin S, Ren Z, Zhang L, Fu H, Zhang W (2012) A novel open-tubular capillary electrochromatography with magnetic nanoparticle coating as stationary phase. Electrophoresis 33:340–347
Wu LL, Liang RP, Chen J et al (2017) Separation of chiral compounds using magnetic molecularly imprinted polymer nanoparticles as stationary phase by microchip capillary electrochromatography. Electrophoresis 39:356–362
Hu J, Shao D, Chen C, Sheng G, Li J, Wang X, Nagatsu M (2010) Plasma-induced grafting of cyclodextrin onto multiwall carbon nanotube/iron oxides for adsorbent application. J Phys Chem B 114:6779–6785
Shan C, Ma Z, Tong M, Ni J (2015) Removal of hg(II) by poly(1-vinylimidazole)-grafted Fe3O4@SiO2 magnetic nanoparticles. Water Res 69:252–260
Tang W, Ong TT, Muderawan IW, Ng SC (2007) Effect of alkylimidazolium substituents on enantioseparation ability of single-isomer alkylimidazolium-beta-cyclodextrin derivatives in capillary electrophoresis. Anal Chim Acta 585:227–233
Yu J, Zuo L, Liu H, Zhang L, Guo X (2013) Synthesis and application of a chiral ionic liquid functionalized β-cyclodextrin as a chiral selector in capillary electrophoresis. Biomed Chromatogr 27:1027–1033
Li J, Yu T, Xu G, Du Y, Liu Z, Feng Z, Yang X, Xi Y, Liu J (2018) Synthesis and application of ionic liquid functionalized Î2-cyclodextrin, mono-6-deoxy-6-(4-amino-1,2,4-triazolium)-Î2-cyclodextrin chloride, as chiral selector in capillary electrophoresis. J Chromatogr A 1559:178–185
Zhu Y, Zhang L, Qian J, Zhang W (2013) The characteristics of open-tubular capillary electrochromatography columns with series/mixed stationary phases constructed with magnetic nanoparticle coating. Talanta 104:173–179
Liang RP, Wang XN, Wang L, Qiu JD (2014) Enantiomeric separation by microchip electrophoresis using bovine serum albumin conjugated magnetic core-shell Fe3 O4 @au nanocomposites as stationary phase. Electrophoresis 35:2824–2832
Wang XN, Liang RP, Meng XY, Qiu JD (2014) One-step synthesis of mussel-inspired molecularly imprinted magnetic polymer as stationary phase for chip-based open tubular capillary electrochromatography enantioseparation. J Chromatogr A 1362:301–308
Carrasco-Correa EJ, Ramis-Ramos G, Herrero-Martínez JM (2015) Hybrid methacrylate monolithic columns containing magnetic nanoparticles for capillary electrochromatography. J Chromatogr A 1385:77–84
Qu P, Lei J, Zhang L, Ouyang R, Ju H (2010) Molecularly imprinted magnetic nanoparticles as tunable stationary phase located in microfluidic channel for enantioseparation. J Chromatogr A 1217:6115–6121
Acknowledgements
This work was supported by the Natural Science Foundation of Jiangsu Province (Program No.BK20141353) and the Project of the Priority Academic Program Development of Jiangsu Higher Education Institutions (PAPD).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The author(s) declare that they have no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
ESM 1
(DOCX 1.88 MB)
Rights and permissions
About this article
Cite this article
Yang, X., Sun, X., Feng, Z. et al. Open-tubular capillary electrochromatography with β-cyclodextrin-functionalized magnetic nanoparticles as stationary phase for enantioseparation of dansylated amino acids. Microchim Acta 186, 244 (2019). https://doi.org/10.1007/s00604-019-3318-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00604-019-3318-8