Abstract
The authors report on a loop-mediated isothermal amplification (LAMP) scheme that uses antarctic thermally sensitive uracil-DNA-glycosylase (AUDG) for simultaneous detection of nucleic acids and elimination of carryover contamination. It was applied in a lateral flow assay (LFA) format. The assay has attractive features in that it does not require the use of labeled primers or probes, and can eliminate false-positive results generated by unwanted hybridization between two labeled primers or between a labeled primer and probe. LAMP amplification and AUDG digestion are conducted in a single pot, and the application of a closed-tube reaction prevents false-positives due to carryover contamination. The method was applied to the detection of the human pathogen Streptococcus pneumoniaein in pure cultures and spiked blood samples. This LFA can detect S. pneumoniae in pure cultures with a 25 fg.μL−1 detection limit and in spiked blood samples with a 470 cfu.mL−1 detection limit. Conceivably, this assay can be applied to the detection of various other targets if the specific LAMP primers are available.

ᅟ
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Nucleic acid amplification is a fundamental tool for life sciences fields from clinical diagnostics to basic laboratory research. Among the many techniques developed for analysis and detection of nucleic acids, polymerase chain reaction (PCR)-based methods remain the most popular amplification methodologies for amplifying and analyzing miniscule quantities of nucleic acids [1]. Although PCR-based assays are widely employed in clinical diagnosis, basic research, agriculture, epidemiology and many other fields, these approaches are restricted by the fact that many molecular tools are the methodologically challenging and require the expensive laboratory apparatuses [2, 3].
Sequence-specific isothermal amplification emerges as a promising alternative that eliminates the need for the sophisticated equipment, even basic equipment. These techniques include loop-mediated isothermal amplification (LAMP), multiple cross displacement amplification, cross-priming amplification, helicase-dependent amplification and recombinase polymerase amplification [1, 4,5,6]. In particular, LAMP is the most popular isothermal amplification technique, and has been applied in a number of field and point-of-care diagnostics [7]. Traditionally, LAMP results are confirmed by unaided eye for the formation of a white precipitate yielded from magnesium, by agarose gel electrophoresis, by the use of spectrophotometric instrument to measure turbidity, by visualization of the products under UV irradiation and natural light after adding double-stranded DNA intercalating molecules (such as SYBR green) or colorimetric indicator (such as hydroxynaphthol blue, HNB) [8, 9]. Agarose gel electrophoresis exhibited approximately ten-fold more sensitive than other monitoring techniques, while gel electrophoresis required opening the LAMP reaction vessel. Thus, indicating LAMP results using gel electrophoresis increased the risk of carryover contamination. Moreover, the observation of turbidity or color with the bare eye is potentially subjective, and a sample may be ambiguous to the unaided eye when the concentration of target template is extremely low. In addition, the double strand-binding dyes (such as SYBR Green I) have the tendency to inhibit LAMP amplification reaction at high concentration. Most importantly, a complex mixture of amplification products is produced from LAMP reaction due to the use of four to six primers, thus these monitoring techniques cannot differentiate the specific amplification from non-specific amplification. Hence, newer monitoring techniques are required for further simplifying and speeding up the total time of LAMP methods.
Particularly, lateral flow biosensor (LFB) is a superior nucleic acid analysis tool due to its high specificity and sensitivity, user-friendliness and easy interpretation of results. LFB has been growingly used as an alternative for analyzing LAMP products, which avoids subjectivity associated with the visual interpretation of LAMP results [10,11,12,13,14]. In order to achieve LFB detection, two primers (such as LF/LB or LF/FIP) involved in LAMP reaction are labeled, one with hapten (such as FITC, Dig or Hex) and one with biotin [10, 15]. During the amplification stage, the LAMP amplicons are modified simultaneously. The end of the LAMP amplicon modified with biotin can bind streptaviding-conjugated nanoparticles (such as gold nanoparticles) for visualization. The other end of LAMP amplicons modified with hapten can be captured by an anti-hapten antibody immobilized on the test region of the LFB, and the LAMP results are indicated as a colored band by the bare eye in 2 to 10 min. These strategies eliminate the use of label-probe for lateral flow biosensor, thus no incubation step is required prior to LFB detection. However, the major drawback of traditional strategies is that false positive results can be generated from interaction between two labeled primers, because the hybridization from two labeled primers (one with biotin, and one with hapten) can form a double-labeled detectable product without amplification.
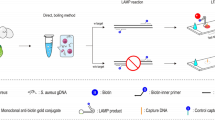
To address the problem, we devised a label-free strategy in the current report (Fig. 1), which didn’t require labeled primers/probes for facilitating LFB detection, thus the detection system was able to effectively remove the false positive results arising from interaction between labeled primers/probes. Moreover, the additional process, such as indicating LAMP result by LFB and gel electrophoresis, require opening of amplification tubes, which easily generates aerosol droplets of distinct sizes that include high concentrations of LAMP amplicons. Due to its high sensitivity, LAMP assay is extremely vulnerable to carryover contamination (aerosol droplets), thus these further steps carry high risk of contamination. Hence, the effective techniques for eliminating false positive results arising from carryover contamination are extremely important for reliable detection of nucleic acids. Toward a strategy to control carryover contamination, we devised a one-pot, closed-vessel enzymatic method that can remove carryover contamination while preserving normal LAMP reaction and providing reliable diagnostic of target sequences in contaminated samples. In this report, a new LAMP technique (named label-free AUDG-LAMP-LFB), which merged label-free LAMP assay with antarctic thermal sensitive uracil-DNA-glycosylase (AUDG) cleavage of carryover contaminants and lateral flow biosensor analysis of amplicons, was developed for rapid, sensitive and reliable identification of target sequences.
a Outline of label-free LAMP with biotin-11-dUTP and FITC-aha-dUTP. b The detailed structure of LFB (top row), and the schematic illustration of the principle of LFB for visualization of LAMP products (bottom row). c Interpretation of the results: (I), positive (two crimson red lines, including test band and control band, appeared on the NC zone of the biosensor); (II), negative (only the control line zone displayed a crimson red band)
Streptococcus pneumoniae (S. pneumoniae), an important human pathogen, is the main cause of community-acquired pneumonia, septicemia, sinusitis, acute otitis media and meningitis. Herein, the specific, reliable and sensitive techniques, which can be promptly completed in the clinical laboratory, are required for early diagnosis and appropriate antibiotic therapy. In seeking such detection assays, we investigate the feasibility of the new LAMP technique (AUDG-LAMP-LFB) devised in the current report, based on the amplification of the specific pneumolysin gene (Ply) [16]. Here, we offer the details of AUDG-LAMP-LFB assay, and demonstrate its applicability by identifying the target pathogen using pure cultures and spiked blood samples.
Materials and methods
Reagents and apparatus
Backing card, sample pad, conjugate pad, nitrocellulose membrane (NC) and absorbent pad were purchased from the Jie-Yi Biotechnology. Co., Ltd. (Shanghai, China; http://www.joey-bio.com/index.html). Dye streptavidin coated polymer nanoparticles (Crimson red, 129 nm) were purchased from Bangs Laboratories, INC. (Indiana, USA, http://www.bangslabs.com). Biotinylated bovine serum albumin (biotin-BSA) and rabbit anti-fluorescein antibody (anti-FITC) and were purchased from Abcam. Co., Ltd. (Shanghai, China; http://www.abcam.cn). dATP, dCTP, dGTP and antarctic thermal sensitive uracil-DNA-glycosylase (AUDG) were purchased from New England Biolabs, INC. (Beijing, China; http://www.neb-china.com). dUTP were obtained from Kuimo. Co., Ltd. (Beijing, China). Fluorescein (FITC)-aha-dUTP and biotin-11-dUTP were obtained from Thermo Scientific. Co., Ltd. (Shanghai, China; https://www.thermofisher.com/cn/zh/home.html). Visual detection reagent (Malachite green, MG) was purchased from Bei-Jing HaiTaiZhengYuan. Co., Ltd. (Beijing, China). DNA extraction kits (QIAamp DNA minikits; Qiagen, Hilden, Germany) were purchased from Qiagen (Beijing, China).
Bacterial strains and DNA preparation
Of the total of 75 bacterial strains used in the current study, including 44 strains of S. pneumoniae and 31 strains of non-S. pneumoniae bacteria, are shown in Table S1. According to the manufacturer’s protocol, DNA templates were extracted using DNA extraction kits (QIAamp DNA minikits, Hilden, Germany) and were tested using ultraviolet spectrophotometer (Nano drop ND-1000, Calibre, Beijing, China) at A260/280. The templates were stored under at −20 °C before the templates were used. The strain of S. pneumoniae ATCC700674 was used for optimal temperature, confirmation performance and sensitivity analysis, and the genomic templates of S. pneumoniae ATCC700674 were serially diluted (2.5 ng, 250 pg, 25 pg, 2.5 pg, 250 fg, 25 fg and 2.5 fg per microliter). A 1 μL aliquot of each dilution was used for confirmation of assay’s sensitivity.
Construction of lateral flow biosensor
Lateral flow biosensor (LFB) was constructed as previously described with some modifications [17]. Briefly, four components, including a buffer loading pad, a conjugate pad, an NC membrane and an absorbent pad, were laminated onto a backing card orderly by overlapping 2 mm among them (Fig. 1b). Dye streptavidin coated polymer nanoparticles (SA-DPNs) were deposited on the conjugate pad, and anti-FITC antibody (0.2 mg.mL−1) and biotin-BSA (2.5 mg.mL−1) were dispensed onto the test line (TL) and control line (CL), respectively.
Design of LAMP assay primers
The LAMP primer pairs (F3, B3, FIP, BIP, LF and LB) were designed using primer software PRIMER EXPLORER 4.0 (Eiken Chemical, Japan) and were listed in Table S2. All LAMP primers were examined for hybrids and hairpin structures using the Integrated DNA Technologies design tool (http:www.idtdna.com/site). Blast analysis was applied for confirming that all LAMP primers were specific for target pathogen. All of the oligomers were synthesized and purified by TsingKe Biotech Co., Ltd. (Beijing, China) at HPLC purification grade.
LAMP and AUDG-LAMP reactions
LAMP was performed in a one-step reaction in a 25-μL mixture containing 2.5 μL 10 X of the supplied buffer (New England Biolabs), 1.6 μM each of FIP and BIP, 0.8 μM each of LF and LB, 0.4 μM each of F3 and B3, 1.4 mM dATP, 1.4 mM dCTP, 1.4 mM dGTP, 0.7 mM dUTP, 0.5 μL of 0.1 mM biotin-11-dUTP, 0.5 μL of 0.1 mM FITC-aha-dUTP, 1 μL (8 U) of Bst 2.0 polymerase (New England Biolabs), 0.8 M betaine (Sigma-Aldrich) and 1 μL DNA template.
AUDG-LAMP was also performed in a one-step reaction in a 25-μL mixture containing 2.5 μL 10 X of the supplied buffer (New England Biolabs), 1.6 μM each of FIP and BIP, 0.8 μM each of LF and LB, 0.4 μM each of F3 and B3, 1.4 mM dATP, 1.4 mM dCTP, 1.4 mM dGTP, 0.7 mM dUTP, 0.5 μL of 0.1 mM biotin-11-dUTP, 0.5 μL of 0.1 mM FITC-aha-dUTP, 1 μL (8 U) of Bst 2.0 polymerase (New England Biolabs), 0.3 μL (0.3 U) of AUDG, 0.8 M betaine (Sigma-Aldrich) and 1 μL DNA template.
Three monitoring techniques, including colorimetric indicator (MG), lateral flow biosensor (LFB) and real-time turbidity (LA-320C), were used for verifying the amplification of LAMP reactions. Furthermore, we tested the optimal amplification of the primer set designed in the report, and the LAMP assays were carried out at a single temperature from 59 °C to 66 °C for 1 h. The amplification mixtures with 1 μL DNA template of K. pneumonia (Isolated strain) and S. aureus (Isolated strain) were used as negative controls (NC), and amplification mixtures with 1 μL of double distilled water (DW) are used as a blank control (BC).
Sensitivity of LAMP assays
To examine the assay’s sensitivity, limit of detection (LoD) of LAMP was confirmed using serial dilutions (2.5 ng, 250 pg, 25 pg, 2.5 pg, 250 fg, 25 fg and 2.5 fg per microliter) of purified templates of S. pneumoniae ATCC700674. A volume of 1 μL of each dilution is used as DNA template for LAMP assay, and the LoD of LAMP was determined as the last dilution of each positive test. All examinations were performed in triplicate.
Simulating carryover contamination
Reaction products of AUDG-LAMP generated from 250 pg.μL in the absence of AUDG was quantitated using ultraviolet spectrophotometer (NanoDrop ND-1000, Calibre, Beijing, China) and was serially diluted from 1 × 10−12 to 1 × 10−19 g.μL−1. The diluted products served as the source of simulating carryover contaminants, and were use as templates in the AUDG-LAMP assays. A volume of 1 μL of each dilution was added into the AUDG-LAMP mixtures.
Prevention of carryover contamination by AUDG-LAMP
In the current study, we then demonstrated the feasibility of the established AUDG-LAMP assay to eliminate the false-positive results due to carryover contaminants in detecting target nucleic acids. The AUDG-LAMP assay and conventional LAMP method (AUDG-LAMP without AUDG enzyme digestion) were compared by adding 1 μL of diluted templates and 1 μL of simulated carryover contamination of 1 × 10−18 g.μL−1 in the same tube. The total mass (1 × 10−18 g ~ a 0.2 μm-diameter aerosol droplet) of the simulated carryover contamination was selected as the source of simulating carryover contaminants for AUDG-LAMP and LAMP reactions, because it dose not be effectively removed by either fibrous pipette tip filters or high efficiency particulate air filters in the biosafety cabinets [18, 19]. Analytical sensitivity of LAMP with and without AUDG cleavage before amplification are compared to confirm whether the AUDG-LAMP assay can efficiently eliminate false positive amplifications.
Specificity of AUDG-LAMP assay
Analytical specificity was determined by performing AUDG-LAMP assay on at least 2.5 ng of DNA templates extracted from a panel of S. pneumoniae and non-S. pneumoniae organisms (Table S1).
Practical application of AUDG-LAMP to S. pneumoniae in blood samples
The human blood samples were acquired from a healthy donor with the written informed consent. Our study was reviewed and approved by the ethics committee of the National Institute for Communicable Disease Control and Prevention, China CDC, according to the medical research regulations of the Ministry of Health China (Approval No. ICDC2014003).
To demonstrate the feasibility of AUDG-LAMP assay, S. pneumoniae strain (ATCC700674) is used to determine the assay’s sensitivity in blood samples. S. pneumoniae strain (ATCC700674) suspension with an OD (optical density) at 0.6 was prepared in 1 mL of sterile saline. According the previous study, a plate-counting method was applied for counting the numbers of colony forming units (CFUs) in the bacteria suspension [20]. In brief, serial 10-fold dilution (10−1-10−7) of the S. pneumoniae suspensions was prepared, and 100 μL of appropriate dilution (10−6) was plated on blood agar (BHI) in triplicate. The numbers of CFU were calculated after 24 h at 37 °C. Then, 100 μL of each suspension were transferred into 900 μL of blood samples, and the numbers of S. pneumoniae ATCC700674 were adjusted to approximate 470,000 CFU.mL−1, 47,000 CFU.mL−1, 4700 CFU.mL−1, 470 CFU.mL−1, 47 CFU.mL−1, 4.7 CFU.mL−1 and 0.47 CFU.mL−1. The spiked contaminated blood samples (100 μL) were subjected to extract genomic templates, and were eluted in 10 μL of Qiagen elution buffer. 2 μL of extracted templates was added into AUDG-LAMP reaction mixtures, and non-contamination blood samples were used as negative control (NC). The experiment was independently conducted in triplicate.
Results
The establishment of label-free LAMP-LFB assay
A schematic illustration of the principle of label-free LAMP-LFB technique is displayed in Fig. 1. In the LAMP system, biotin-11-dUTP and FITC-aha-dUTP are added into LAMP reaction mixtures (Fig. 1a). LAMP primers anneal to target templates during the amplification stage, and are extended by Bst 2.0 polymerase. As a result, biotin-11-dUTP and FITC-aha-dUTP are simultaneously incorporated into the LAMP products. Thus, a double-labeled detectable amplicon, which is modified with FITC and biotin, are successfully constructed during the reaction stage.
A schematic illustration of the principle of lateral flow lateral for visualization of LAMP products is exhibited in Fig. 1b. The biosensor detected LAMP amplicons through selective recognition of FITC labels, which are incorporated into LAMP products by using FITC-aha-dUTP. The biotin labels, which are incorporated into LAMP amplicons by using biotin-11-dUTP, bind dye streptavidin coated polymer nanoparticles (SA-DPNs) for visualization. Firstly, the LAMP reaction products (0.5 μL) are dropped to sample pad of LFB, then diluent buffer also is added to sample application zone of the biosensor. The diluent buffer can rehydrate the immobilized detector reagents (SA-DPNs) because the buffer can move along the dipstick by capillary. FITC- and biotin-labeled amplicons are specifically captured by the immobilized anti-FITC at the test line (TL), and the visual reagents (SA-DNPs) can rapidly accumulate in the region of the biosensor through biotin/streptavidin interaction, resulting in a crimson red band on the test zone. Moreover, the excess SA-DPNs are captured by immobilized biotin-BSA at the control line (CL), which verifies the proper function of the LFB. As a result, the colorimetric signal was easily visible to the bare eye within 2 min (Fig. 1c).
Confirmation and detection of LAMP products
To examine the feasibility of LAMP primer set designed in this study, the LAMP reactions were conducted in the presence or absence of templates within 1 h at a fixed temperature (63 °C). The positive LAMP vessel was seen by bare eye as light green, whereas the negative and blank controls were observed as colorless (Figure S1A). By dipstick, it was seen that two crimson red bands (TL and CL) were observed in the positive amplification, whereas only a crimson red band (CL) was observed in negative and blank controls (Figure S1B). These results indicated that the primer set designed in the current report was a good candidate for establishment of AUDG-LAMP-LFB approach for target nucleic acid detection.
The temperature optimization of the LAMP primer set
To confirm the optimal assay temperature during the reaction stage, the LAMP assays were conducted using the S. pneumoniae (ATCC70076) templates at the level of 250 pg per tube to determine the optimal reaction temperature. Eight distinct temperatures (from 59 °C to 66 °C at 1 °C interval) were examined and real-time measurement of turbidity was applied to monitor the LAMP reactions. As shown in Figure S2, the typical kinetics graphs corresponding to eight reaction temperatures were produced and the better results were obtained from the assay temperature of 61 °C to 65 °C. The amplification temperature of 63 °C was selected for performing the rest of experiments conducted in this report.
Analytical sensitivity of LAMP-LFB assay in pure culture
To confirm the LoD of the LAMP assay, we conducted LAMP amplifications using serial dilutions (from 2.5 ng to 2.5 fg per microliter) of pure S. pneumoniae (ATCC700674) templates. By LFB, the sensitivity of LAMP assay for detecting Ply gene was 25 fg of genomic templates per reaction (Fig. 2a). Using the LAMP reaction by self-trail, the LoD of LAMP was further tested by real-time turbidity analysis and direct visual inspection of amplification products with MG reagents (Fig. 2b and c). By real-time turbidity and MG reagents, the analytical sensitivity of LAMP method was also 25 fg per tube, which was in complete accordance with LFB detection.
AUDG-LAMP assay
To eliminate the false-positive results arising from carryover contamination, we devise the AUDG-LAMP technique, which is a one-pot, closed-vessel method for simultaneous detection of target nucleic acids and prevention of carryover contamination (Fig. 3, Table S3). In the AUDG-LAMP system, dUTP is incorporated into all LAMP amplicons after amplifying the target nucleic acids (First stage). To digest the dUTP incorporated products, AUDG treatment is performed before AUDG-LAMP amplification (Second stage). Importantly, the natural DNAs cannot be digested during the stage because they do not contain dUTP. Furthermore, two additional components, including FITC-aha-dUTP and biotin-11-dUTP, are included in the reaction mixtures to construct the detectable double-labeled amplicons.
A total of two stages are required by AUDG-LAMP assay for preventing carryover contamination. In the presence of Bst 2.0 polymerase and dUTP, all AUDG-LAMP products are modified with dUTP during the first stage. All subsequent AUDG-LAMP amplifications are treated with AUDG enzyme to digest carryover contaminants by removing uracil from amplicons from previous reactions during the second stage, but having no effect on natural templates (target sequences). Then, the AUDG is rapidly heat-inactivated and the cleaved contaminants are degraded during the AUDG-LAMP reaction stage (63 °C), ensuring that only the target sequences are amplified. To construct the biotin- and FITC-attached duplex amplicons and enable them analysis on the biosensor, two additional components (biotin-11-dUTP and FITC-aha-dUTP) are included in the AUDG-LAMP reaction mixtures
To confirm that simulated carryover contamination (dUTP-incorporated products) from previous reactions has the ability to contaminate new LAMP reactions. We compared the sensitivity of the AUDG-LAMP and LAMP methods using serial diluted LAMP amplicons with concentrations ranging from 1 × 10 − 12 to 1 × 10 − 19 g.μL−1. As shown in Fig. 4, the AUDG-LAMP technique only detected 1 × 10 − 12 g of simulated carryover contamination per vessel (Fig. 4a). In absence of AUDG treatment, the LAMP method detected as little as 1 × 10 − 18 g of simulated carryover contamination per vessel, which was equivalent to a 0.2 μM-diameter aerosol droplet (Fig. 4b). These results demonstrated that marginal amounts of amplicons (1 × 10 − 18 g~0.2 μM-diameter aerosol droplet), which cannot be efficiently blocked by fibrous pipette tip filters, is capable of causing false-positive results. Herein, AUDG is able to eliminate the amplification up to 105-fold higher concentrations of carryover contaminant products (1 × 10 − 18 g), which significantly abates the likelihood of false-positive amplifications of LAMP assay.
Sensitivity test of AUDG-LAMP (a) and label free LAMP (b) using 10-fold serial dilutions of simulated carryover contamination (dUTP-incorporated amplicons DNA, concentration diluted from 1 × 10−12, 1 × 10−13, 1 × 10−14, 1 × 10−15, 1 × 10−16, 1 × 10−17, 1 × 10−18 and 1 × 10−19 g.μL−1) as determined using LFB (top row), real time turbidity (middle row) and MG (bottom row)
To further confirm that AUDG-LAMP assay can reduce the likelihood of false-positive amplifications due to the carryover contaminations, the sensitivity test of AUDG-LAMP and LAMP assays are conducted using serial dilution of the S. pneumoniae (ATCC 700674). Moreover, the contaminants (dUTP incorporated LAMP products) at the level of 1 × 10 − 18 g are simultaneously added into amplification reactions. In the LAMP assay without AUDG cleavage, all examined samples show positive results, even containing the samples with undetectable level of S. pneumoniae (ATCC700674) templates (less than 25 fg per reaction), which are considered as false-positive results (Fig. 5a). In the AUDG-LAMP assay with AUDG digestion, the sensitivity of AUDG-LAMP assay is complete accordance with the aforementioned sensitivity examination (Figs. 2 and 5b). These results suggested that the sensitivity of LAMP method with AUDG treatment was not correctly verified. It also suggests that the false-positives amplifications resulting from carryover contamination can be prevented by AUDG-LAMP method.
Sensitivity examination of LAMP (a) and AUDG-LAMP (b) assay using serial dilutions (2.5 ng.μL−1, 250 pg.μL−1, 25 pg.μL−1, 2.5 pg.μL−1, 250 fg.μL−1, 25 fg.μL−1 and 2.5 fg.μL−1,) of ATCC700674 and 1 × 10−18 g.μL−1 of simulated carryover contamination (dUTP-incorporated amplicons DNA) as determined using LFB (top row), real-time turbidity (middle row) and MG (bottom row)
Specificity of the AUDG-LAMP technique
To confirm the assay’s specificity, 44 S. pneumoniae strains and 31 non-S. pneumoniae strains are tested. Positive results were obtained after 60 min of incubation in S. pneumoniae strains determined, whereas other non-S. pneumoniae strains produced negative results (Table S1). Theses results indicated that the AUDG-LAMP technique devised here was very specific to S. pneumoniae.
Practical application of the AUDG-LAMP to S. pneumoniae in blood samples
To confirm the applicability of AUDG-LAMP approach as a nucleic acid analysis tool, the spiked blood samples with S. pneumoniae were determined using AUDG-LAMP assay. As shown in Fig. 6, AUDG-LAMP approach generated positive results when the contaminated numbers of S. pneumoniae were more than 470 CFU per milliliter (~ more than 9.4 CFU per tube). No positive results were produced from non-contaminated blood samples (Negative control).
Monitoring techniques, including later flow biosensor (a), real-time turbidity (b) and colorimetric indicator (MG, c), were applied for analyzing the amplification products. The serial dilutions of target templates were subjected to AUDG-LAMP reactions. Strips (a) /Signals (b) /Tubes (c) 1-8 represented the DNA levels of 9400 CFU, 940 CFU, 94 CFU, 9.4 CFU, 0.94 CFU, 0.094, 0.0094 CFU per reaction, negative control (non-contaminated blood samples). The DNA levels of 9400 CFU, 940 CFU, 94 CFU and 9.4 CFU per reaction produced the positive reactions
Discussion
Isothermal amplification techniques have been devised for providing simplified formats of nucleic acid analysis and paving the way for molecular diagnosis in various fields [21]. Among these methodologies, LAMP, as a promising low-cost amplification technique, has been used to detect a variety of pathogens, including bacteria, viruses, parasites and fungi [9]. However, analysis of LAMP products has been a pivotal concern as simplifying detection tools is a major concern. The monitoring techniques, such as colorimetric agents, fluorescent agents, agarose gel electrophoresis, turbidimeters, have been employed to detect LAMP amplicons [8]. Unfortunately, these analysis methods are not specific to target amplicons, thus cannot differentiate specific amplification and non-specific amplification. Moreover, these monitoring approaches require special reagents (such as fluorescent or colorimetric agents), complex instruments (such as turbidimeters) or an additional analysis procedure (gel electrophoresis), thus resultant instrumental restraint can restrict the uptake of LAMP technology in field, point-of-care and ‘on-site’ settings.
The disadvantages posed by conventional detection methods have spurred the researchers to develop other superior monitoring techniques. In particular, LFBs have been increasingly used as alternative tools for analyzing LAMP products. This report describes a LAMP assay based double-labeled amplicons, which can be detected by biosensor. In the LAMP-LFB system, no labeled primer or probe was required, and the detectable LAMP amplicons is formed from FITC-aha-dUTP and biotin-11-dUTP during the amplification stage (Fig. 1a). The biotin labels, which are incorporated into LAMP amplicons using biotin-11-dUTP, can bind dye streptavidin coated polymer nanoparticles for visualization. The hapten (FITC) labels, which are incorporated into LAMP products using FITC-aha-dUTP, are captured by an anti-body immobilized on the test zone of the dipstick (Fig. 1b), and the analysis results are displayed as a crimson red band visible by the bare eye within 2 min (Fig. 1c). Comparing with conventional analysis techniques, the advantages of the lateral flow strip include disposable format, rapidity, ease-of-use, little training to operate and relatively low cost. Most importantly, the LAMP-LFB assay can eliminate the false-positive results yielding from the hybridization between two labeled primers or between a labeled primer and probe, because the LAMP-LFB assay do not require the labeled primers and probes.
Although LAMP assay has been widely applied in molecular diagnostics, the isothermal amplification technique occasionally suffers from false-positive results arising from carryover contamination. Particularly, the further procedures (such as confirming LAMP results by LFB or agarose gel electrophoresis) require the opening of the reaction vessel, thus the aerosol droplets of distinct sizes that contain high concentrations of products are produced. Our present study demonstrated that a trace amount of contaminants (1 × 10 − 18 g/reaction) can generate unwanted amplification due to the high sensitivity of the LAMP assay (Fig. 4), thus avoiding carryover contamination is an extremely pivotal factor for reliable and accurate detection. In this study, we successfully eliminated the carryover contamination using AUDG enzyme and dUTP, and the dUTP was first incorporated instead of dTTP into all amplicons (Fig. 3, First stage). Next, we performed AUDG cleavage and LAMP amplification in a one-pot process. Prior to LAMP reaction, we treat the amplification mixture with AUDG (a heat-labile enzyme) at room temperature for five minutes. The enzyme can specifically cleave uracil bases from any contaminating products. Importantly, the natural templates, which are uracil-free DNA, remain completely unaffected. During the reaction stage, these abasic sites hamper replication by Bst 2.0 polymerase, and cause rapid degradation of contaminating amplicons via hydrolysis at the phosphate backbone, thus effectively eliminating contaminants from re-amplification. In the AUDG-LAMP-LFB system, the AUDG is a heat-labile enzyme, and is automatically and rapidly deactivated when the amplification is carried out at an elevated temperature (i.e., 63 °C), thus the use of the enzyme enables the AUDG-LAMP assay to be conducted in a single closed tube (Fig. 3, Second stage). As such, genuine LAMP products subsequently yielded from the target sequences during the reaction are not cleaved, permitting amplification to proceed normally.
As a poof-of-concept, S. pneumoniae is used as the model for demonstrating the applicability of AUDG-LAMP-LFB technique (Table S3) [22]. Our present report shows that AUDG-LAMP-LFB’s LoD for S. pnumoniae detection is 25 fg of genomic template per vessel in pure culture (Figs. 2 and 5). The results using strips are in complete accordance with colorimetric reagent (MG) and real-time turbidity (Figs. 2, 5 and 6). Although AUDG-LAMP-LFB products can be detected equally with other methods employed in this study, the LFB is likely the preferred detection methods as reporting the results is less subjective and do not require special instruments. For the analytical specificity examination, the wanted results were yielded for S. pneumoniae strains, and no positive results were obtained in the assay of non-S. pneumoniae strains (Table S2). Furthermore, the practical application of AUDG-LAMP-LFB assay was successfully examined by detecting S. pneumoniae in blood samples with high sensitivity and specificity (Fig. 6).
In sum, the present report developed a new assay on the basis of standard LAMP technique, named label-free AUDG-LAMP-LFB. The assay has the ability to effectively remove the false-positive results generating from unwanted hybridization (between two labeled primers or between the labeled primer and probe) because the analysis strategy did not require labeled primers and probes. Moreover, the assay also can eliminate false-positives arising from carryover contaminants by using AUDG enzyme, and is conducted in a one-pot, closed-tube reaction. For demonstration purpose, S. pneumoniae was detected by AUDG-LAMP-LFB assay to verify the availability of target detection. The performance of AUDG-LAMP-LFB technique in detecting S. pneumoniae from blood samples and pure culture was successfully evaluated. In particular, the AUDG-LAMP-LFB technique, as the proof-of-concept assay, can be reconfigured to detect various targets by redesigning the specific LAMP primers.
References
Wang Y, Wang Y, Ma A-J, Li D-X, Luo L-J, Liu D-X, Jin D, Liu K, Ye C-Y (2015) Rapid and sensitive isothermal detection of nucleic-acid sequence by multiple cross displacement amplification. Sci Rep 5:11902
Yan L, Zhou J, Zheng Y, Gamson AS, Roembke BT, Nakayama S, Sintim HO (2014) Isothermal amplified detection of DNA and RNA. Mol BioSyst 10(5):970–1003. https://doi.org/10.1039/c3mb70304e
Wang X, Liu W, Yin B, Sang Y, Liu Z, Dai Y, Duan X, Zhang G, Ding S, Tao Z (2017) An isothermal strand displacement amplification strategy for nucleic acids using junction forming probes and colorimetric detection. Microchim Acta 184(6):1603–1610. https://doi.org/10.1007/s00604-017-2158-7
Zhao Y, Chen F, Li Q, Wang L, Fan C (2015) Isothermal Amplification of Nucleic Acids. Chem Rev 115(22):12491–12545. https://doi.org/10.1021/acs.chemrev.5b00428
Zhou L, Wang J, Chen Z, Li J, Wang T, Zhang Z, Xie G (2017) A universal electrochemical biosensor for the highly sensitive determination of microRNAs based on isothermal target recycling amplification and a DNA signal transducer triggered reaction. Microchim Acta 184(5):1305–1313. https://doi.org/10.1007/s00604-017-2129-z
Xu M, He Y, Gao Z, Chen G, Tang D (2015) Isothermal cycling and cascade signal amplification strategy for ultrasensitive colorimetric detection of nucleic acids. Microchim Acta 182(1):449–454. https://doi.org/10.1007/s00604-014-1385-4
Tanner NA, Evans TC (2014) Loop-mediated isothermal amplification for detection of nucleic acids. Curr Protoc Mol Biol 105:15.14. 1–15.14. 14
Zhang X, Lowe SB, Gooding JJ (2014) Brief review of monitoring methods for loop-mediated isothermal amplification (LAMP). Biosens Bioelectron 61:491–499. https://doi.org/10.1016/j.bios.2014.05.039
Rafati A, Gill P (2015) Microfluidic method for rapid turbidimetric detection of the DNA of Mycobacterium tuberculosis using loop-mediated isothermal amplification in capillary tubes. Microchim Acta 182(3):523–530. https://doi.org/10.1007/s00604-014-1354-y
Wang Y, Li H, Wang Y, Zhang L, Xu J, Ye C (2017) Loop-mediated isothermal amplification label-based gold nanoparticles lateral flow biosensor for detection of enterococcus faecalis and staphylococcus aureus. Front Microbiol 8:192
Yongkiettrakul S, Jaroenram W, Arunrut N, Chareanchim W, Pannengpetch S, Suebsing R, Kiatpathomchai W, Pornthanakasem W, Yuthavong Y, Kongkasuriyachai D (2014) Application of loop-mediated isothermal amplification assay combined with lateral flow dipstick for detection of Plasmodium falciparum and Plasmodium vivax. Parasitol Int 63(6):777–784
Plaon S, Longyant S, Sithigorngul P, Chaivisuthangkura P (2015) Rapid and sensitive detection of Vibrio alginolyticus by loop-mediated isothermal amplification combined with a lateral flow dipstick targeted to the rpoX gene. J Aquat Anim Health 27(3):156–163
Mori Y, Kanda H, Notomi T (2013) Loop-mediated isothermal amplification (LAMP): recent progress in research and development. J Infect Chemother 19(3):404–411
Santiago-Felipe S, Tortajada-Genaro LA, Puchades R, Maquieira Á (2016) Parallel solid-phase isothermal amplification and detection of multiple DNA targets in microliter-sized wells of a digital versatile disc. Microchim Acta 183(3):1195–1202. https://doi.org/10.1007/s00604-016-1745-3
Nurul Najian AB, Engku Nur Syafirah EAR, Ismail N, Mohamed M, Yean CY (2016) Development of multiplex loop mediated isothermal amplification (m-LAMP) label-based gold nanoparticles lateral flow dipstick biosensor for detection of pathogenic Leptospira. Anal Chim Acta 903:142–148. https://doi.org/10.1016/j.aca.2015.11.015
Salo P, Ortqvist A, Leinonen M (1995) Diagnosis of bacteremic pneumococcal pneumonia by amplification of pneumolysin gene fragment in serum. J Infect Dis 171(2):479–482
Wang Y, Wang Y, Xu J, Ye C (2016) Development of multiple cross displacement amplification label-based gold nanoparticles lateral flow biosensor for detection of shigella spp. Front Microbiol 7:1834. https://doi.org/10.3389/fmicb.2016.01834
Le Rouzic E (2006) Contamination-pipetting: relative efficiency of filter tips compared to Microman® positive displacement pipette. Nat Methods 3(6). https://doi.org/10.1038/nmeth887
Barhate RS, Ramakrishna S (2007) Nanofibrous filtering media: filtration problems and solutions from tiny materials. J Membr Sci 296(1):1–8
Wang Y, Wang Y, Zhang L, Li M, Luo L, Liu D, Li H, Cao X, Hu S, Jin D, Xu J, Ye C (2016) Endonuclease restriction-mediated real-time polymerase chain reaction: a novel technique for rapid, sensitive and quantitative detection of nucleic-acid sequence. Front Microbiol 7:1104. https://doi.org/10.3389/fmicb.2016.01104
Zhao H, Dong J, Zhou F, Li B (2015) G-quadruplex − based homogenous fluorescence platform for ultrasensitive DNA detection through isothermal cycling and cascade signal amplification. Microchim Acta 182(15):2495–2502. https://doi.org/10.1007/s00604-015-1608-3
Hagiwara E, Baba T, Shinohara T, Kitamura H, Sekine A, Komatsu S, Ogura T (2017) Ten-year trends and clinical relevance of the antimicrobial resistance genotype in respiratory isolates of streptococcus pneumoniae. Chemotherapy 62(4):256–261. https://doi.org/10.1159/000470828
Acknowledgements
We acknowledge the financial supports of the grants (Mega Project of Research on the Prevention and Control of HIV/AIDS, Viral Hepatitis Infectious Diseases 2013ZX10004-101 to Changyun Ye) from the Ministry of Science and Technology, People’s Republic of China, and grant (2015SKLID507 to Changyun Ye) from State Key Laboratory of Infectious Disease Prevention and Control, China CDC.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The author(s) declare that they have no competing interests.
Electronic supplementary material
ESM 1
(DOC 5184 kb)
Rights and permissions
About this article
Cite this article
Wang, Y., Wang, Y., Li, D. et al. Detection of nucleic acids and elimination of carryover contamination by using loop-mediated isothermal amplification and antarctic thermal sensitive uracil-DNA-glycosylase in a lateral flow biosensor: application to the detection of Streptococcus pneumoniae. Microchim Acta 185, 212 (2018). https://doi.org/10.1007/s00604-018-2723-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00604-018-2723-8