Abstract
The authors describe an ultrasensitive method for simultaneous detection of neomycin (NEO) and quinolones antibiotics (QNS). It is based on the use of (a) two immuno-nanoprobes (a probe for NEO and a probe for QNS), (b) surface-enhanced Raman scattering (SERS) detection, and (c), a portable lateral flow assay (LFA). The two probes consist of gold nanoparticles (AuNPs) conjugated to the Raman active molecule 4-aminothiophenol (PATP), and to monoclonal antibody against NEO (NEO mAb) or against NOR (NOR mAb). Quantitative detection of NEO and QNS was realized via SERS of the PATP-coated AuNPs captured in the test line of a LFA. Under optimized condition, the visual limits of LFA are 10 ng·mL−1 for NEO and 200 ng·mL−1 for NOR, and with LODs down to 0.37 pg·mL−1 and 0.55 pg·mL−1 by using SERS. The NEO test line is not interfered by the NEO analogues gentamycin, streptomycin and tobramycin, but the NOR test line suffers from different degrees of cross-reactivity (CR) to 12 common other QNS, the CRs ranging from 1.5% to 136%. The recoveries of NEO and NOR from spiked milk samples ranged between 86% and 121%, with relative standard deviations (RSD) from 3% to 6%. The method is highly sensitive, accurate and effective. It may be applied to simultaneous detection of NEO and 8 QNS, including NOR, enoxacin, ciprofloxacin, ofloxacin, fleroxacin, marbofloxacin, enrofloxacin, and pefloxacin.

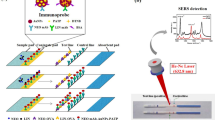
Schematic of a lateral flow assay (LFA) based on an indirect competitive model. By using two test lines, the LFA can detect the neomycin and quinolones antibiotics simultaneously. Based on the surface-enhanced Raman scattering (SERS), the LFA shows high sensitivity to antibiotics with low limit of detection.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Neomycin (NEO) and quinolones (QNS) are classified as broad spectrum antibiotics and widely used in veterinary medicine to treat bacterial infections in animals, due to their effective inhibition of Gram-positive and Gram-negative bacteria [1]. NEO can perturb protein synthesis in bacteria by binding the 30 S subunit of ribosomal RNA, which causes misreading of the genetic code and inhibits translation [2], and QNS exert their bactericidal effects by inhibiting DNA gyrase within susceptible bacteria [3]. However, NEO is potentially neurotoxic and nephrotoxic to humans and animals [4]; QNS can cause nausea, vomiting and slow cartilage’s growth [5, 6]. In more serious cases, the two drugs can be used for human and animals [7]. The residues of the two drugs in animal tissues caused by continuous usage in the growth process may have potential risks to human health, including stimulation of bacterial resistance (e.g. Campylobacter species), alterations in intestinal microflora and hypersensitivity reactions [8]. Therefore, monitoring of antibiotics residue in food is important with regard to public health issues. Currently, the European Union (EU) lays down a set of maximum residue limits (MRLs) for NEO and QNS in different kinds of food [9]. For minimizing the risk of NEO and QNS residue to humans, it is necessary to develop a sensitive method for simultaneous detection of these antibiotics. Moreover, the multiplex detection is a research trend of safety detection and has notable advantages including high efficiency, low cost and low sample consumption.
Up to now, various detection methods have been used for detection of NEO or QNS residue, including high performance liquid chromatography (HPLC) [10, 11], liquid chromatography-mass spectrometry (LC-MS) or LC-MS/MS [12, 13], electrochemical [14, 15], fluorescence [16, 17], enzyme-linked immunosorbent assay (ELISA) [18, 19], lateral flow assay (LFA) [20, 21], among which LFA is the most popular and commercially available technique. The LFA is a paper based sensor, and has the advantages of portable, observable, and operable [22]. The LFA has been employed to monitor high molecular mass analytes such as bacteria, viruses, hormones, and parasite antigens, and many smaller molecular drugs such as pesticide and veterinary medicines [23]. Despite the widespread use of the LFA, it still suffers from certain inadequacies, such as semi-quantitative detection and low-sensitivity. Therefore, it is highly desired to establish a novel LFA with high sensitivity of detection.
Surface-enhanced Raman scattering (SERS) technique, in combination with nanotechnology, has emerged as a promising solution to the aforementioned issues due to its nondestructive and ultrasensitive features [24]. Based on localized plasmon resonance, SERS can enhance the Raman signals of target species adsorbed on Au or Ag metallic nanostructures by as much as 6 to 14 orders of magnitude [25]. The AuNPs has good stability, colour rendering, security and biocompatibility, so we chose the AuNPs as nanomaterials in this study. Moreover, SERS based immunoassay in sandwich format for macromolecules detection [26], and in the competitive format for small molecular examination, have been reported [27]. However, to the best of our knowledge, multi-detection lines of SERS based LFA (SERS-LFA) has not been reported yet.
In this study, we use two monoclonal antibodies (mAbs), NEO mAb and NOR mAb. Especially, the NOR mAb with broad specificity, which can react with 13 QNS (NOR, enoxacin, ciprofloxacin, ofloxacin, fleroxacin, marbofloxacin, enrofloxacin, pefloxacin, sarafloxacin, danofloxacin, lomfloxacin, flumequine, sparfloxacin) at different degrees. And then prepared two immuno-nanoprobes, NEO-probe and NOR-probe, which consist of dual labeled AuNPs carrying with different mAbs and the same Raman reporter PATP. In this paper, by using the two probes, the SERS-LFA realizes the simultaneous detection of NEO and 8 QNS. Meanwhile, the SERS-LFA has high detection sensitivity and satisfactory recovery for milk samples, and also in complete conformity to the standard laid down by the international standardization organization.
Materials and methods
Materials and apparatus
Neomycin, gentamycin, streptomycin, tobramycin, norfloxacin, lomfloxacin, enrofloxacin, dafloxacin, sparfloxacin, marbofloxacin, ciprofloxacin, ofloxacin, sarafloxacin, pefloxacin, enoxacin, flumequine, fleroxacin, bovine serum albumin (BSA) and ovalbumin (OVA) were purchased from Sigma-Aldrich (Milwaukee, USA, www.sigma-aldrich.com). 4-aminothiophenol (PATP) was purchased from Aladdin China Ltd. (Shanghai, China, www.aladdin.com). Chloroauric acid (HAuCl4·4H2O) and trisodium citrate were purchased from Sinopharm Chemical Reagent Co., Ltd. (Shanghai, China, www.sinoreagent.com). The peroxidase-conjugated goat anti-mouse IgG (GaMIgG-HRP) was purchased from Solarbio science and technology Co., Ltd. (Beijing, China, www.solarbio.com). Nitrocellulose membrane, glass fibre, and absorbent pad were purchased from Millipore Sigma (Milwaukee, USA, www.merckmillipore.com). The monoclonal antibodies (NEO mAb and NOR mAb) were produced in the key laboratory of animal immunology of the Chinese ministry of agriculture.
UV-1800 ultraviolet visible spectrophotometer (Shimadzu, Japan, www.shimadzu.com) was used for absorption spectral measurement. Transmission electron microscopy (TEM) images were taken using a Technai G2 S-Twin (FEI, Eindhoven, Netherlands, www.fei.com). An XYZ Bio-strip Dispenser, CM 4000 Cutter, and TSR 3000 membrane strip reader were purchased from Bio-Dot (Irvine, CA, USA, www.biodot.com). Raman spectra were measured using a LabRAM HR 800 Raman spectrometer (HORIBA, Japan, www.horiba.com) equipped with a holographic notch filter and a CCD detector. A long working distance 50× objective was used to collect the Raman scattering signal.
Preparation of AuNPs with SERS
40 nm AuNPs was synthesized as reported by Frens [28]. In this procedure, 49.5 mL of deionized water was heated to boiling, and 0.5 mL of trisodium citrate in 1% solution (weight to volume) was rapidly injected with vigorous magnetic stirring, then the solution was kept boiling for 3 min. 0.5 mL of HAuCl4·4H2O in 0.1% solution (weight to volume) was added, and the mixture reacted for another 15 min until the color turned to wine-red, then cooled and stored at room temperature with 0.05% sodium azide adding.
The Raman reporter-labeled AuNPs were prepared by following a procedure reported by Ni et al. [29] with slight modification. Briefly, a certain amount of 1 mM PATP was added to 1.0 mL of AuNPs, and the mixture was shaken for 12 h, then the PATP-labeled AuNPs (AuNPs-PATP) were separated from the solution by centrifugation at 10000 rpm for 10 min and resuspended with 1.0 mL of DDW (contain 4.0 μL 0.02 M K2CO3).
Preparation of Immuno-nanoprobe
The preparation of NEO-probe was as the follows: 4 μL of the purified NEO mAb (1 mg·mL−1) was added to 1 mL of AuNPs-PATP with gentle agitation. After incubation at room temperature for 30 min, 100 μL of 10% BSA solution in 20 mM sodium borate (pH 9.0) was added to the above NEO-probe solution to make sure that no bare binding sites on AuNPs were left for eliminating nonspecific binding. The mixture was incubated for 3 h at room temperature and then washed using repeated centrifugation (12,000 rpm) at 4 °C for 30 min with 20 mM sodium borate (pH 9.0) containing with 1% BSA and 0.1% sodium azide. The precipitate was then resuspended in 500 μL of TB solution (0.02 M Na2B4O7 containing 1% BSA and 0.03% sodium azide) and stored at 4 °C for use.
The preparation of NOR-probe was similar to that of NEO-probe with some modifications. That is, 5 μL of the purified NOR mAb (8 mg·mL−1) was added to 1 mL of AuNPs-PATP. And in the end, the precipitate was resuspended in 300 μL of TB solution and stored at 4 °C for use.
Assembly of SERS-LFA
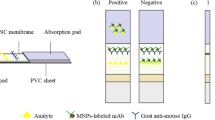
The SERS-LFA is composed of a sample application pad, conjugation pad, nitrocellulose membrane, absorption pad, and a backing card, as illustrated in Fig. 1a. Both the sample pad (15 mm × 300 mm) and conjugation pad (7 mm × 300 mm) were made from glass fibre. The conjugation pad was prepared by dispensing a desired volume of immuno-nanoprobe onto the glass fibre pad using an XYZ Biostrip Dispenser, followed by drying at room temperature before stored at 4 °C. Typically, the SERS-LFA has two test lines and one control line at the center of the nitrocellulose (NC) membrane. NEO-OVA and NOR-OVA were immobilized to form the NEO test line (T1) and NOR test line (T2), respectively. The goat anti-mouse IgG was immobilized to form control line. These reagents were applied in the form of dots at 50 dots/ mL/cm on the membrane. After drying for 1 h at 40 °C, the membrane was blocked with 2% (w/v) BSA and then dried, sealed, and stored under dry condition. The LFA assembly procedure was similar to that described by Zhang et al [30]. The sample pad, conjugate pad, nitrocellulose membrane, and absorption pad were assembled on a plastic backing support board sequentially with a 1–2 mm overlap and covered by color film at both ends. The master card was cut to 3 mm width strips using a CM 4000 Cutter (Bio-Dot). Then strips were sealed in a plastic bag with desiccant and stored at 4 °C.
Sensitivity and specificity assay
A 100 μL composite sample of NEO and NOR at different concentrations (0, 10−5, 10−4, 10−3, 10−2, 0.1, 1, 10, 100 ng·mL−1) was applied to the SERS-LFA. 15 min later, the two test lines were measured using a laser confocal Raman spectrometer. Excitation source was set at 5 mW with a 632.8 nm He-Ne laser radiation and aligned to the center of each test line, then measured using a long working distance 50× objective. The Raman signal was collected in the 1000–1800 cm−1 region with spectral resolution of 1 cm−1, while the integration time was set to 10 s. The Raman intensity at 1078 cm−1 was measured and the average intensity acquired from 10 different spots along the middle part of the test line was used for quantification.
To evaluate the specificity of the assay, 3 other aminoglycosides (gentamycin, streptomycin, tobramycin) structurally similar to NEO, and 12 other FQs (enoxacin, ciprofloxacin, ofloxacin, fleroxacin, marbofloxacin, enrofloxacin, pefloxacin, sarafloxacin, danofloxacin, lomfloxacin, flumequine, sparfloxacin) structurally similar to NOR were used to test the cross-reactivity (CR). All analogues were prepared to the final concentrations of 0, 10−5, 10−4, 10−3, 10−2, 0.1, 1.0, 10, 100 ng·mL−1, and for the same assay procedure applied to the SERS-LFA. The CR value was calculated according to the following equation:
Where IC50 calculated from standard curves is the concentration at which 50% of the antibodies are bound to the analyte [31].
Affix recovery test
The pure milk samples were purchased from a local supermarket and confirmed to be negative by GC-MS. Then, 1.0 mL of NEO and NOR mixture solution at the concentration of 10 ng·mL−1 was added to 1 mL of pure milk. Next, the milk added analytes were centrifuged at 10000 rpm for 10 min at room temperature to discard some fat and precipitates; the supernatant was then diluted to different concentrations by normal saline. The analyses were repeated three times for each concentration.
Results and discussion
Mechanism of SERS-LFA
The principle of the SERS-LFA can be illustrated in Fig. 1. A competitive interaction between frees analyte and coating antigen with labelled immunogold. If sufficient NEO and QNS are present, it will completely block the reaction between coating antigens and immuno-nanoprobes, thus no red line appears; when without NEO and QNS, the immuno-nanoprobes would be trapped by NEO-OVA and NOR-OVA to form two visible test lines; if only NEO is present, the T2 line will appear, and if only QNS existed, the T1 line will appear. Moreover, when the test procedure was properly carried out, whether or not the sample contains analytes, the control line was always visible; otherwise the test strip was invalid. The Raman reporter PATP has the characteristic peak of the Raman signals at 1078 cm−1 and 1588 cm−1, and the peak at 1078 cm−1 is more apparent. Therefore, we choose the intensity of the peak at 1078 cm−1 as a basis for quantitative measurement [32].
Choice of materials
It is known that the LFA based AuNPs has been widely applied for detection in many different fields. When the AuNPs conjugated with antibody, it can be performed with high reproducibility under predetermined conditions [33]. When the SERS is introduced, then a new and more accessible method is created on the existing LFA based AuNPs. Moreover, the SERS intensity of AuNPs also can meets current test requirement. In conclusion, we can use AuNPs as nanomaterials to achieve the SERS-LFA.
In a certain range, Raman intensity increases with the size of AuNPs [34]. However, larger concentrations of AuNPs can cause aggregation, and this can affect stability and performance of the immuno-nanoprobe. In this study, we chose 40 nm AuNPs because they are suitable for the preparation of test strip while maintaining strong Raman signal.
Characterization of AuNPs, AuNPs-PATP and Immuno-nanoprobe
To measure the shape and size of the AuNPs, AuNPs-PATP and immuno-nanoprobe (take NEO-probe for example), the TEM images is usually used for observation. The Fig. 2a and b demonstrate that when the appropriate PATP and AuNPs were combined, the morphology of AuNPs did not change. The average diameters of the AuNPs and AuNPs-PATP were approximately 40 nm, demonstrating that PATP modification did not affect the size of AuNPs. The Fig. 2c presents the size of NEO-probe is about 45 nm. The size of AuNPs-PATP gets bigger when added the NEO mAb, proves that the mAb has been combined with AuNPs-PATP.
The UV-vis spectra (Fig. 2d) were scanned with UV-vis spectroscopy from 400 to 750 nm. The maximum absorption wavelength of AuNPs and AuNPs-PATP are all at 528 nm, and the NEO-probe is at 531 nm, so the maximum absorption wavelength of NEO-probe has a red shift compared with that of the AuNPs and AuNPs-PATP. The result shows the mAb has been absorbed on the AuNPs-PATP successfully.
Feasibility of SERS-LFA
To demonstrate the feasibility of SERS-LFA, the Raman spectra of the SERS-LFA at different conditions were measured. There are four groups of experiments to be conducted: (a) NEO-probe and NOR-probe as mixture probe, NEO-OVA and NOR-OVA coated on the T1 line (a1) and T2 line (a2), respectively; (b) NEO-probe and NOR-probe as mixture probe, Na2CO3-NaHCO3 buffer spread on the T1 line (b1) and T2 line (b2); (c) BSA-AuNPs-PATP (BSA replacing mAb) as a probe, NEO-OVA and NOR-OVA coated on the T1 line (c1) and T2 line (c2), respectively; (d) NEO-probe and NOR-probe as mixture probe, BSA and OVA coated on the T1 line (d1) and T2 line (d2), respectively. The SERS spectra of the above conditions are shown in Fig. 3. The two characteristic peaks of the PATP are at 1078 cm−1 and 1588 cm−1 in Fig. 3 curve (a1) (a2), which did not appear in other curves of Fig. 3, indicating that the SERS signals on the strips are only from mAb-AuNPs-PATP and that the SERS-LFA is feasible.
Optimization of method
The following parameters were optimized: (a) amount of mAbs; (b) concentration of coating antigens; (c) concentration of immuno-nanoprobes. The results are shown in Fig. S2. The following experimental conditions are found to give best results: (a) the optimal volumes of NEO mAb and NOR mAb used for preparing immuno-nanoprobes are determined to be 4 μL and 5 μL; (b) the appropriate concentrations of NEO-OVA and NOR-OVA applied to the test line are determined to be 0.08 mg·mL−1 and 0.35 mg·mL−1; (c) the optimal concentrations of NEO-probe and NOR-probe used to the SERS-LFA are 5 μM and 8 μM, respectively.
Sensitivity of SERS-LFA
Under the optimized detection condition, the SERS-LFA was used for quantitative evaluations of NEO and NOR in sample. As shown in the photographs in Fig. 4a, the visual detection limit of NEO and NOR is estimated to be about 10 ng·mL−1 and 200 ng·mL−1, respectively. So it is difficult to achieve quantitative evaluation accurately by bare eye. Using the SERS technique, it is possible to achieve a quantitative result by monitoring the Raman peak intensities of the corresponding test lines. Fig. 4b and c illustrates the corresponding SERS spectra of NEO and NOR at different concentrations. With the concentration of NEO and NOR increasing, the experiment reveals, the Raman intensity of the test lines decrease gradually.
The corresponding standard curves are displayed in Fig. 4d and e. The corresponding SERS intensity is inversely correlated to the concentrations of NEO and QNS over a wide linear range of 0.0001–1.0 ng·mL−1. The concentrations of analyte that provide a tracer binding inhibition in SERS intensity at 50% and 10% are defined as the IC50 value and the limit of detection (LOD), respectively. From the curve, the IC50 values of NEO and NOR are 0.03 ng·mL−1 and 0.07 ng·mL−1, and the LODs are found to be 0.37 pg·mL−1 and 0.55 pg·mL−1, respectively. The values are lower than the EU defined toxicity level for NEO and QNS in milk, and which were at least 10,000 times more sensitive than the colorimetric method by bare eye.
Specificity of SERS-LFA
The tested analogues can be approximately divided into three groups according to the cross-reactivity results. As can be observed in Table S1, the first group shows the NEO test line has negligible cross-reactivity (<0.05%) with other compounds (gentamycin, streptomycin, tobramycin). However, the NOR test line has different degrees of cross-reactivity to the 12 other QNS. The second group contains the 7 QNS (enoxacin, ciprofloxacin, ofloxacin, fleroxacin, marbofloxacin, enrofloxacin, pefloxacin). They all present a stronger cross-reactivity, the CR ranged from 43% to 136%. The third group contains 5 QNS (sarafloxacin, danofloxacin, lomfloxacin, flumequine, sparfloxacin), which shows the cross-reactivities below 10%. The result demonstrates that the SERS-LFA can complete simultaneous detection of NEO and 8 QNS (NOR, enoxacin, ciprofloxacin, ofloxacin, fleroxacin, marbofloxacin, enrofloxacin, pefloxacin). Apart from NOR, the standard curve of other 7 QNS are given in the Fig. S3.
Application in milk sample
To determine the accuracy of the SERS-LFA, the milk sample added with NEO and QNS (QNS taken NOR for instance) at three levels were analyzed by the SERS-LFA. The intensity of the two test lines was quantified using the Raman spectroscopy. Data representing the means of three determinations are summarized in Table 1. It is found that the recovery of the two analytes from spiked samples ranged from 86% to 121%, with RSD value in range of 3%–6%, indicating that the strip is able to detect effectively the target analytes in real samples.
Methodology comparison
A comparison of some reported nanomaterials based on different methods for determination of antibiotics is presented in Table 2. The parameters for comparison included materials, method and LOD. It can be seen from Table 2 that the LOD value of our work is the lowest. The result indicates that the SERS-LFA can be used for high sensitive and selective detection of antibiotics.
Conclusion
We have presented a SERS-LFA for simultaneous detection of NEO and QNS. The use of immuno-nanoprobes and coating antigens allows an efficient and specific capturing and concentrating of the free analytes. The LODs of NEO and NOR determined from our SERS-LFA were estimated to be 0.37 pg·mL−1 and 0.55 pg·mL−1, respectively, approximately 10,000 times greater sensitivity than the visual detection limit. Due to the broad-specificity of the NOR mAb, the NOR test line has a strong cross-reactivity to the other 7 QNS (enoxacin, ciprofloxacin, ofloxacin, fleroxacin, marbofloxacin, enrofloxacin, pefloxacin) except NOR, the CR ranged from 1.5% to 136%. In consequence, the SERS-LFA can be applied to the simultaneous detection of NEO and 8 QNS including NOR. The recoveries of NEO and NOR in milk are 86%–121%, with RSD values in range of 3%–6% (n = 3) and so is able to simultaneously detect trace amounts of NEO and NOR in milk samples. We demonstrate that the SERS-LFA has high sensitivity, specificity and reliability, which can also serve as a promising model for detecting other residues.
References
Schwarz S, Kehrenberg C, Walsh TR (2016) Use of antimicrobial agents in veterinary medicine and food animal production. Int J Antimicrob Agents 12:431–437
Fourmy D, Recht MI, Puglisi JD (1998) Binding of neomycin-class aminoglycoside antibiotics to the A-site of 16 S Rrna. J Mol Biol 277:347–362
Hoshino K, Kitamura A, Morrissey I, Sato K, Kato J, Ikeda H (1994) Comparison of inhibition of Escherichia Coli topoisomerase IV by quinolones with DNA gyrase inhibition. Antimicrob Agents Chemother 38:2623–2627
Greenberg LH, Momary H (1965) Audiotoxicity and nephrotoxicity due to orally administered neomycin. J Am Med Assoc 194:827–828
Christ W (1990) Central nervous system toxicity of quinolones: human and animal findings. J Antimicrob Chemother 26:219–225
Hildebrand H, Kempka G, Schlüter G, Schmidt M (1993) Chondrotoxicity of quinolones in vivo and in vitro. Arch Toxicol 67:411–415
Cabello FC (2006) Heavy use of prophylactic antibiotics in aquaculture: a growing problem for human and animal health and for the environment. Environ Microbiol 8:1137–1144
Fàbrega A, Sánchezcéspedes J, Soto S, Vila J (2008) Quinolone resistance in the food chain. Int J Antimicrob Agents 31:307–315
EC. Commission regulation (EU) no 508/1999 of 4 march 1999 amending annexes I to IV to council regulation (EEC) no 2377/90 laying down a community procedure for the establishment of maximum residue limits of veterinary medicinal products in foodstuffs of animal origin. Off J Eur Communities, 1999, 60, 22–23
Salama I, Goma MS (2013) Comparative determination of miconazole, nystatin, hydrocortisone and neomycin by HPTLC/HPLC-DAD. Eur J Chem 63:29–34
Gigosos PG, Revesado PR, Cadahía O, Fente CA, Vazquez BI, Franco CM, Cepeda A (2000) Determination of quinolones in animal tissues and eggs by high-performance liquid chromatography with photodiode-array detection. J Chromatogr A 871:31–36
Oertel R, Renner U, Kirch W (2004) Determination of neomycin by LC-tandem mass spectrometry using hydrophilic interaction chromatography. J Pharm Biomed Anal 35:633–638
Karageorgou E, Myridakis A, Stephanou EG, Samanidou V (2013) Multiresidue LC-MS/MS analysis of cephalosporins and quinolones in milk following ultrasound-assisted matrix solid-phase dispersive extraction combined with the quick, easy, cheap, effective, rugged, and safe methodology. J Sep Sci 36:2020–2027
Adams E, Puelings D, Rafiee M, Roets E, Hoogmartens J (1998) Determination of netilmicin sulfate by liquid chromatography with pulsed electrochemical detection. J Chromatogr A 812:151–157
Pellegrini GE, Carpico G, Coni E (2004) Electrochemical sensor for the detection and presumptive identification of quinolone and tetracycline residues in milk. Anal Chim Acta 520:13–18
Zhou GD, Wang F, Wang HL, Kambam S, Chen XQ (2013) Colorimetric and fluorometric detection of neomycin based on conjugated polydiacetylene supramolecules. Macromol. Rapid Commun 34:944–948
Shtykov SN, Smirnova TD, Bylinkin YG, Kalashnikova NV, Zhemerichkin DA (2007) Determination of ciprofloxacin and enrofloxacin by the sensitized fluorescence of europium in the presence of the second ligand and micelles of anionic surfactants. J Anal Chem 62:136–140
Loomans E, Van Wiltenburg J, Koets M, Van Amerongen A (2003) Neamin as an immunogen for the development of a generic ELISA detecting gentamicin, kanamycin, and neomycin in milk. J Agric Food Chem 51:587–593
Huet AC, Charlier C, Tittlemier SA, Singh G, Benrejeb S, Delahaut P (2006) Simultaneous determination of (fluoro)quinolone antibiotics in kidney, marine products, eggs, and muscle by enzyme-linked immunosorbent assay (ELISA). J Agric Food Chem 54:2822–2827
Peng J, Wang YW, Liu LQ, Kuang H, Li AK, CL X (2016) Multiplex lateral flow immunoassay for five antibiotics detection based on gold nanoparticle aggregations. RSC Adv 6:7798–7805
Sheng W, Li Y, Yuan M, Wang S (2011) Enzyme-linked immunosorbent assay and colloidal gold-based immunochromatographic assay for several (fluoro)quinolones in milk. Microchim Acta 173:307–316
Xu Y, Liu M, Kong N, Liu J (2016) Lab-on-paper micro- and nano- analytical devices: fabrication, modification, detection and emerging applications. Microchim Acta 183:1521–1542
Paek SH, Lee SH, Cho JH, Kim YS (2000) Development of rapid one-step immunochromatographic assay. Methods 22:53–60
Lee SJ, Guan ZQ, Xu HX, Moskovits M (2007) Surface-enhanced Raman spectroscopy and nanogeometry: the plasmonic origin of SERS. J Phys Chem C 111:17985–17988
Kneipp K, Kneipp H, Itzkan I, Dasari RR, Feld MS (1996) Population pumping of excited vibrational states by spontaneous surface-enhanced Raman scattering. Phys Rev Lett 76:2444–2447
Lee M, Lee S, Lee J, Lim HW, Seong GH, Lee EK, Chang S, CH O, Choo J (2011) Highly reproducible immunoassay of cancer markers on a gold-patterned microarray chip using surface-enhanced Raman scattering imaging. Biosens Bioelectron 26:2135–2141
Wang YZ, Chen S, Wei C, Xu MM, Yao JL, Li Y, Deng AP, Gu RA (2014) A femtogram level competitive immunoassay of mercury (II) based on surface-enhanced Raman spectroscopy. Chem Commun 50:9112–9114
Frens G (1973) Controlled nucleation for the regulation of the particle size in monodisperse gold suspensions. Nature 241:20–22
Ni J, Lipert RJ, Dawson GB, Porter MD (2014) Immunoassay readout method using extrinsic Raman labels adsorbed on immunogold colloids. Anal Chem 71:4903–4908
Song CM, Zhi AM, Liu QT, Yang JF, Jia GC, Shervin J, Tang L, Hu XF, Deng RG, Xu CL, Zhang GP (2013) Rapid and sensitive detection of β-agonists using a portable fluorescence biosensor based on fluorescent nanosilica and a lateral flow test strip. Biosens Bioelectron 50:62–65
She P, Chu YX, Liu CW, Guo X, Zhao K, Li JG, HJ D, Zhang X, Wang H, Deng AP (2016) A competitive immunoassay for ultrasensitive detection of Hg2+ in water, human serum and urine samples using immunochromatographic test based on surface-enhanced Raman scattering. Anal Chim Acta 906:139–147
Tian XR, Chen L, HX X, Sun MT (2012) Ascertaining genuine SERS spectra of p-aminothiophenol. RSC Adv 2:8289–8292
Paek SH, Lee SH, Cho JH, Kim YS (2000) Development of rapid one-step Immunochromatographic assay. Methods 22:53–60
Krug JT, Wang GD, Emory SR, Nie S (1999) Efficient Raman enhancement and intermittent light emission observed in single gold nanocrystals. J Am Chem Soc 121:9208–9214
Zhu Y, Son JI, Shim YB (2010) Amplification strategy based on gold nanoparticle-decorated carbon nanotubes for neomycin immunosensors. Biosens Bioelectron 26:1002–1008
Sierra-Rodero M, Fernández-Romero JM, Gómez-Hens A (2014) Determination of fluoroquinolone antibiotics by microchip capillary electrophoresis along with time-resolved sensitized luminescence of their terbium (III) complexes. Microchim Acta 181:1897–1904
Xiao C, Liu J, Yang A, Zhao H, He Y (2015) Colorimetric determination of neomycin using melamine modified gold nanoparticles. Microchim Acta 182:1501–1507
Acknowledgments
This work is supported by the National Science & Technology Pillar Program of “12th Five-Year Plan” (2014BAD13B05) and China Agriculture Research System (CARS-36).
Author information
Authors and Affiliations
Corresponding authors
Electronic supplementary material
ESM 1
(DOC 502 kb)
Rights and permissions
About this article
Cite this article
Shi, Q., Huang, J., Sun, Y. et al. A SERS-based multiple immuno-nanoprobe for ultrasensitive detection of neomycin and quinolone antibiotics via a lateral flow assay. Microchim Acta 185, 84 (2018). https://doi.org/10.1007/s00604-017-2556-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00604-017-2556-x