Abstract
A novel covalent organic framework based magnetic adsorbent was developed for magnetic solid phase extraction (MSPE) of polycyclic aromatic hydrocarbons (PAHs). Covalent organic framework-LZU1 (= Lan Zhou University-1) was covalently immobilized onto polyethyleneimine-functionalized magnetic nanoparticles (COF-LZU1@PEI@Fe3O4), and the resulting material was characterized by transmission electron microscopy and Fourier transform infrared spectroscopy. The effects of the pH value of sample solution, percentage of acetonitrile, extraction time and sampling volume on MSPE of six PAHs were investigated. The COF-LZU1@PEI@Fe3O4 displays high extraction efficiency for the PAHs such as pyrene, benzo[a]pyrene, fluoranthene, benz[a]anthracene, benzo[a]fluorathene and dibenz[a,h]anthracene. Following desorption with acetonitrile, the PAHs were quantified by HPLC. The MSPE-HPLC method shows low limit of detection (0.2–20 pg mL−1), wide linear range and good reproducibility (relative standard deviations <4.4% for intra-day and inter-day precision). The method was successfully applied to determine PAHs in environmental samples. Good recoveries were obtained, ranging from 90.9 to 107.8% for water samples and 85.1 to 105.0% for soil samples.

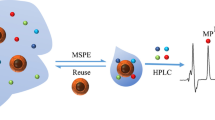
Schematic of the preparation of magnetic nanoparticles modified with a covalent organic framework (COF-LZU1) for extraction of polycyclic aromatic hydrocarbons prior to quantitation by HPLC. PEI: Polyethyleneimine.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Covalent organic frameworks (COFs) are a class of crystalline porous materials that consist of light elements (C, H, B, N, O etc.) and are connected through strong covalent bonds [1,2,3,4,5,6,7]. These materials possess much fascinating properties including high specific surface area, excellent thermal stability, high porosity, and low density. With these unique properties, COFs have aroused intensive interest of scientists for the great potential application in diverse areas [2, 4], such as gas storage [8,9,10], gas adsorption [11, 12], photoelectricity [13, 14], catalysis [15, 16] and chromatographic separation [17,18,19]. However, there are still few reports focusing on their performance in the field of sample pretreatment. Metal–organic frameworks (MOFs) are another novel class of porous materials similar to COFs, which have shown to be promising materials as sorbents for sample preparation in many pioneer works [20,21,22,23,24]. As the analogues of MOFs, COFs should possess great application potential in sample preparation. Hydrazone covalent organic frameworks have been synthesized and applied to the solid-phase microextraction (SPME) of pyrethroids [25] and pesticides [26] in vegetables. Therefore, it is possible to develop the application of COFs as promising adsorbent in sample pretreatment.
Since the first discovery of COF materials in 2005 [1], a variety of COFs have been reported. Imine-linked COF-LZU1 (Lan Zhou University-1) was first designed and synthesized by Wang’ group in 2011 [15], which was constructed with 1,3,5-triformylbenzene and 1,4-diaminobenzene through Schiff base reaction. COF-LZU1 has a two-dimensional (2D) layered-sheet structure and possesses large number of benzene rings and imine groups. Unlike boron-containing COFs that linked by boroxine or boronate-ester groups, COF-LZU1 is highly stable in water and most organic solvents [15]. Niu et al. explored its performance as the stationary phase of capillary electrochromatography for separating organic molecules [19]. The results indicated that COF-LZU1 can offer strong π stacking interaction and hydrophobic effect with the analytes. This suggests that it may be an excellent enrichment media for compounds with abundant π system.
Sample preparation is a critical step prior to analysis, especially when complex samples are present. Over the past few decades, many new sample pretreatment techniques with low solvent consumption and low sample handling have been developed and applied to extract target compounds from different matrices [27, 28]. Among these techniques, magnetic solid-phase extraction (MSPE) has attracted increasing attention due to its large adsorption capacity and high extraction efficiency [29]. Additionally, magnetic nanoparticles (MNPs) can be rapidly separated from sample matrix via an external magnet, which would simplify operation procedure and shorten analysis time. Considering of the features of COF-LZU1 and MSPE, we attempted to prepare a COF-LZU1 functionalized MNPs and apply to magnetic solid-phase extraction. In order to achieve the immobilization of COF-LZU1, polyethyleneimine-functionalized Fe3O4 nanoparticles (PEI@ Fe3O4) [30] were chosen as the magnetic support. With the numerous active amino groups in the structure of PEI, COF-LZU1 can be covalently bonded to the surface of PEI@Fe3O4 through Schiff base reaction.
Polycyclic aromatic hydrocarbons (PAHs) are well-known environment pollutants, which are considered to be carcinogenic and mutagenic to human beings. It is of great importance to monitor and control the amount of PAHs in environment. Since the concentration of PAHs in environmental samples is usually in trace levels, it is necessary to enrich PAHs by efficient sample pretreatment method before instrumental analysis [31,32,33,34]. Considering the properties of COF-LZU1 and PAHs, COF-LZU1 based MSPE method was expected to exhibit high extraction efficiency and capacity for analysis of PAHs in environment.
Herein, in this work, we reported the fabrication of a novel COF-LZU1 functionalized MNPs (COF-LZU1@PEI@Fe3O4) and its application for the MSPE of PAHs. Fe3O4 nanoparticles were first coated with PEI, then COF-LZU1 was grown on the surface of MNPs attributing to the covalent bonding between the PEI layer and COF-LZU1. The morphology and surface properties of nanoparticles were characterized by transmission electron microscopy (TEM) and Fourier-transformed infrared spectroscopy (FT-IR). COF-LZU1@PEI@Fe3O4 were used as magnetic adsorbent for extraction of PAHs from environmental samples. The extraction performance was systematically investigated. To the best of our knowledge, it is the first time that COF-LZU1 is immobilized on solid support for sample preparation.
Experimental
Chemicals and materials
1,3,5-Triformylbenzene, 1,4-diaminobenzene, pyrene (PYR), benzo[a]pyrene (B[a]PY), phenanthrene, anthracene, naphthalene, 1-naphthol were purchased from Sigma–Aldrich (MO, USA, www.sigma-aldrich.com). Fluoranthene (FLU), benz[a]anthracene (B[a]AN), benzo[a]fluorathene (B[a]FL), dibenz[a,h]anthracene (D[a,h]AN) were obtained from TCI (Shanghai, China, www.tcichemicals.com). 4-Phenylphenol, 4-vinylbiphenyl and 1,4-dioxane, polyethyleneimine (PEI, Mw 70,000 g mol−1, 50% (w/v) aqueous solution) were bought from Aladdin Reagent (Shanghai, China, www.aladdin-e.com). Methanol and acetonitrile were HPLC grade (Tedia, OH, USA, http://www.tedia.com.cn). Other reagents were analytical grade. Purified water was obtained from a Milli-Q system (MA, USA, http://www.emdmillipore.com/US/en).
Instrumentation
The chromatographic analysis was performed by a Shimadzu 20A HPLC system (Tokyo, Japan, http://www.shimadzu.com.cn), which equipped with two 20A pumps, a six-port valve, a 20A UV detector, and a 10A fluorescent detector. The chromatographic separation was carried out by a C18 column (150 mm × 4.6 mm i.d., 5 μm particle size, GL Science, Tokyo, Japan, www.shimadzu-gl.com.cn). The mobile phase consisted of methanol and water (89/11, v/v) and the flow rate was 1.0 ml min−1. The detection wavelength of fluorescent detector was set at 290 nm (exiting wavelength) and 430 nm (emission wavelength), while the detection wavelength of UV detector was set at 254 nm. The column temperature for HPLC separation was set at 30 °C.
Fourier-transformed infrared spectroscopy (FT-IR) characterization was performed on Thermo Nexus 470 FT-IR system (MA, UAS, http://www.thermonexus.com). The transmission electron microscopy (TEM) image was obtained by a JEM-2100 (HR) transmission electron microscope (TEM) (JEOL, Tokyo, Japan, https://www.jeol.co.jp/en/).
Preparation of COF-LZU1@PEI@Fe3O4
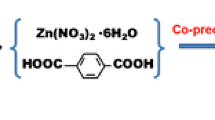
PEI@Fe3O4 was prepared following Amal’s method [30]. Briefly, FeCl2∙4H2O (0.7 g) was firstly dissolved in 80 mL of distilled water. Then 10 mL of KNO3 (2.0 M), 10 mL of NaOH (1.0 M) and PEI (1.7 g) were added to this solution sequentially under nitrogen atmosphere. After being stirred for 2 h at 90 °C, the synthesized nanoparticles were collected by a magnet, followed by washing with water for several times.
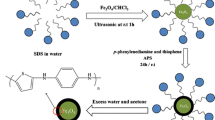
1,3,5-Triformylbenzene (3 mg), 1,4-diaminobenzene (3 mg) were dissolved in 3 mL of 1,4-dioxane and followed by the addition of 60 μL of 3 M aqueous acetic acid. PEI@ Fe3O4 (100 mg) were suspended in above solution with ultrasonication. For modification of COF-LZU1, the temperature was rose to 150 °C and reacted for 24 h. Finally, the COF-LZU1@PEI@Fe3O4 were washed with ethanol thoroughly and dried in the oven (60 °C).
COF-LZU1@PEI@Fe3O4 based MSPE procedures
PAHs standard were dissolved in 20 mM phosphate buffer (pH 9, containing 1% acetonitrile, v/v) at certain concentration. COF-LZU1@PEI@Fe3O4 (5 mg) were carefully weighed and mixed with 20 mL of sample solution. The mixture was stirring for 30 min with the assistant of a magnetic stirring apparatus (two bottles containing the mixture and stirring bar were placed together and stirring). Before collection of nanoparticles, the stirring bar was removed by a magnet under stirring. After that, the nanoparticles were separated from the solution by an external magnet. The step of desorption was carried out by sonication for 3 min with 200 μL acetonitrile. Then the eluent (20 μL) was injected into the HPLC system for analysis.
Sample preparation
To investigate the application in real samples of this method, water and soil samples were collected. Tap water was obtained from laboratory and lake water was from East Lake, Wuhan. Water samples were filtered through 0.45 μm nylon membrane and then added 1% acetonitrile (v/v). Two soil samples were collected, one was from lakeshore of East Lake (soil A) and the other one was from the land beside a local road in Wuhan (soil B). The soil samples were firstly dried and grounded into powder. 5 g soil powder were mixed with 20 mL acetonitrile and ultrasonicated for 1 h. The supernatant was then collected after centrifuged at 10000 rpm (6932 g) for 10 min. The solution was condensed to dryness through a rotary vacuum evaporation, followed by being redissolved in 20 mL acetonitrile for soil A sample and 1 mL for soil B sample. 50 μL solution of soil A sample and 150 μL acetonitrile were diluted to 20 mL with 20 mM phosphate buffer (pH 9) and loaded for MSPE. 20 μL soil B sample solution and 180 μL acetonitrile were diluted to 20 mL with 20 mM phosphate buffer (pH 9) and loaded for MSPE.
Results and discussion
Choice of materials
COF-LZU1 possesses many remarkable characteristics: excellent thermal stability, low density, high surface area, and permanent porosity. The high surface area and porosity can improve the loading capacity of adsorbent. It is highly stable in water and most organic solvents [15]. COF-LZU1 is prepared from 1,3,5-triformylbenzene and 1,4-diaminobenzene through Schiff base reaction. There are rich benzene rings and imine groups in its structure. Thus it can offer strong π stacking interaction and hydrophobic effect with compounds with abundant π system. All these characteristics make it an attractive material for solid phase extraction. Moreover, as a new class of covalent porous crystalline polymers, it is significant to broaden the application of COFs.
Preparation of COF-LZU1@PEI@Fe3O4
A COF-LZU1-modified magnetic nanoparticles was prepared in this work, as shown in Fig. 1. Fe2+ was firstly formed Fe(OH)2 in the presence of NaOH, then heated at 90 °C after the addition of PEI solution. During the formation of Fe3O4 nanoparticles, PEI was self-assembled on the surface via electrostatic interaction. Amino groups of PEI layer were active to participate the formation of COF-LZU1 through the Schiff-base reaction, resulting in the immobilization and growth of COF-LZU1 on magnetic nanoparticles. The aromatic rings and imine groups of COF-LZU1 can offer strong π stacking interaction and hydrophobic effect with the analytes. Therefore, COF-LZU1@PEI@Fe3O4 can exhibit high extraction efficiency for PAHs.
The structure and the thickness of COF-LZU1 would affect the interaction between the target compounds and the COF-LZU1@PEI@Fe3O4. Some parameters that can affect modification of COF-LZU1 were investigated. These include the reaction temperature, the concentration of ligands and the volume of aqueous acetic acid as can be seen in the supporting information. The optimized conditions were selected for the coating of COF-LZU1 as follows: reaction temperature: 150 °C, concentration of ligands: 1 mg mL−1 and volume of 3 M aqueous acetic acid: 60 μL.
Characterization of COF-LZU1@PEI@Fe3O4
FT-IR spectroscopy was employed to characterize the functional moieties of the COF-LZU1@PEI@Fe3O4 (Fig. 2). The absorption band at 581 cm−1 is assigned to stretching vibration of Fe-O for Fe3O4 nanoparticles. The absorption bands at 3404 cm−1,1688 cm−1 are caused by stretching of N-H and C = O, respectively. The peak at 1614 cm−1 corresponds to the C = N stretching for imine. The peaks at 614 cm−1, 679 cm−1,831 cm−1 are characteristic absorption of C-H for substituted aromatic. The FT-IR analysis result indicates the formation of COF-LZU1, suggesting the COF-LZU1 has been successfully modified on the surface of Fe3O4.
The morphology of PEI@Fe3O4 and COF-LZU1@PEI@Fe3O4 nanoparticles were investigated by TEM. As shown in Fig. 3, the obtain nanoparticles are cubic in shape and well-dispersed, with a mean diameter about 40 nm (average face-centered diagonal). It can be seen that the surface of PEI@Fe3O4 was smooth (Fig. 3b). After modification of COF-LZU1, a thin and rough polymer layer is observed (Fig. 3d), indicating that the COF-LZU1 is immobilized onto the surface of PEI@Fe3O4.
Optimization of sampling conditions
As mentioned above, the aromatic rings and imine groups of COF-LZU1 can offer strong π-interaction and hydrophobic effect with the analytes. Therefore, six PAHs compounds were selected to test the enrichment performance of COF-LZU1@PEI@Fe3O4 due to their hydrophobic properties and demands of low-content determination. To achieve higher extraction efficiency, the following parameters were optimized: (a) pH of sample solution; (b) acetonitrile content; (c) extraction time; (d) sampling volume. Respective data and Figure are given in the Electronic Supporting Material. The following experimental conditions were found to give best results: (a) A sample pH value of 9.0; (b) acetonitrile content of 1%; (c) extraction time of 30 min; (d) sample volume of 20 mL.
Enrichment performance of COF-LZU1@PEI@Fe3O4
Extraction performance of COF-LZU1@PEI@Fe3O4 was evaluated under the optimized conditions. Sample solution containing 0.5 ng mL−1 was extracted by COF-LZU1@PEI@Fe3O4, then analyzed with HPLC (Fig. 4b). For comparison, the six PAHs standard solution (0.5 ng mL−1) was directly injected into HPLC system for analysis (Fig. 4a). Significant extraction efficiency is observed for COF-LZU1@PEI@Fe3O4. Enrichment factors (FE) of COF-LZU1@PEI@Fe3O4 towards PAHs were determined and calculated by: FE = C g /C 0; where C0 is defined as the concentration of PAHs in the initial sample solution and Cg is the PAHs concentration in desorption solution after MSPE. FE for six PAHs are calculated to be 39 for FLU,40 for PYR,72 for B[a]AN,86 for B[a]FL,86 for B[a]PY, 90 for D[a,h]AN (theoretical enrichment factor is 100-fold), as shown in Table S1.
To further understand the adsorption mechanism of COF-LZU1@PEI@Fe3O4 for analytes, other PAHs and aromatic compounds were also loaded onto MSPE, the extraction factors are listed in Table S1. FE is positively related mostly to the number of condensed rings and log K OW of compounds, for example, FE(B[a]PY) ˃FE(PYR) ˃FE(phenanthrene), which suggests that the adsorption of COF-LZU1@PEI@Fe3O4 is mainly attributed to π stacking and hydrophobic interaction. However, 4-phenylphenol and 1-naphthol with lower log K OW value (3.20 and 3.09 respectively) shows more adsorption on COF-LZU1@PEI@Fe3O4 than naphthalene, which possesses a log K OW value of 3.33. It seems that the hydrogen bonds interaction is involved in the adsorption of –OH groups containing compounds by forming O-H∙∙∙N = C. B[a]FL and D[a,h]AN possess same log K OW value, while D[a,h]AN shows more adsorption on COF-LZU1@PEI@Fe3O4 than B[a]FL, which suggests that molecular weight is also involved in the adsorption besides π stacking and hydrophobic interaction.
Reusability of COF-LZU1@PEI@Fe3O4
Reusability is an important factor for evaluating the efficiency of sorbents. In the process of MSPE, the coating of COF-LZU1@PEI@Fe3O4 might be destroyed during stirring and ultrasonication, further affected the reuse of COF-LZU1@PEI@Fe3O4. In order to investigate the reusability, COF-LZU1@PEI@Fe3O4 were used six times for the extraction of PAHs (the nanoparticles were washed with acetonitrile for three times and dried in the oven before the next use). The conservation rate was calculated by the ratio of peak area after extraction to the peak area after first extraction. The conservation rate maintains at 91.0–96.2% for sixth use (Fig. S5), indicating the mechanical stability and great reusability of COF-LZU1@PEI@Fe3O4.
Analytical performance
The COF-LZU1@PEI@Fe3O4-based MSPE was coupled with HPLC and applied to determination of PAHs in aqueous samples. Parameters for analytical performance including linearity, limit of detection (LOD), limit of qualification (LOQ) and the repeatability were investigated, the results are listed in Table S2. Over the range of 2–1000 pg mL−1 for FLU, B[a]AN and B[a]FL, 50–5000 pg mL−1 for PYR, 0.5–500 pg mL−1 for B[a]PY, 10–2000 pg mL−1 for D[a,h]AN, all six PAHs exhibit good linearity, with correlation coefficients (R) higher than 0.9989. LODs (S/N = 3) is 0.5 pg mL−1 for FLU, B[a]AN and B[a]FL, 20 pg mL−1 for PYR, 0.2 pg mL−1 for B[a]PY, 5 pg mL−1 for D[a,h]AN. The LOQs (S/N = 10) of six PAHs are in a range of 0.5–50 pg mL−1, which is sufficient for trace analysis of PAHs in real samples. The repeatability was evaluated by calculating the relative standard deviations (RSDs) of a series of replicate experiments. RSDs are 1.8–2.9% for intra-day precision, 2.1 to 4.4% for inter-day precision and 2.6–4.1% between batches, which indicates good repeatability for this COF-LZU1@PEI@Fe3O4-based MSPE-HPLC method.
This method is compared with other MSPE methods for the enrichment and determination of PAHs [32, 34,35,36,37,38]. As shown in Table 1, the sensitivity of this method is comparable or superior to other MSPE methods with less amount of adsorbent and lower sample volume consumption.
Application in environmental samples
The MSPE-HPLC method was applied to enrich and determine of PAHs in real samples. PAHs are potent carcinogenic compounds, which distribute widely in environment such as water, soil and air. Therefore, two water samples and two soil samples were collected as environmental samples. The chromatograms were shown in Fig. 5, and the results are listed in Table 2. As shown in Fig. 5a, FLU and B[b]FL are found in tap water after extraction. FLU, B[b]FL and B[a]PY are detected in lake water after MSPE. B[b]FL and B[a]PY are quantified and the contents are calculated to be 4.57 pg mL−1 and 0.86 pg mL−1, respectively. All six PAHs are detected in soil from lakeshore of East Lake and soil from roadside after MSPE, the contents are calculated to be 48.9–690.2 ng g−1 and 23.8–114.9 ng g−1, respectively. The results demonstrate that the soil was more seriously contaminated by PAHs than water. Although the amount of six PAHs detected in these environmental samples were under limit value, it is necessary to keep monitoring the accumulation of PAHs in environment in the future.
Recoveries in environmental samples were tested by spiking with six PAH standards at 50 pg mL−1 for two water samples, 80 ng g−1 and 40 ng g−1 for two soil samples. By calculating the mean value of three duplicates, spiked recoveries in water samples are in the range of 90.9–107.8%, recoveries in soil samples are in the range of 85.1–105.0%. The results of recovery show good accuracy of the method for determination of PAHs in environmental samples.
Conclusion
In this study, a novel COF-LZU1 functionalized magnetic nanoparticles (COF-LZU1@PEI@Fe3O4) was successfully synthesized and applied to magnetic solid phase extraction for the first time. The prepared COF-LZU1@PEI@Fe3O4 showed good stability and reusability. The COF-LZU1@PEI@Fe3O4 based MSPE exhibited high extraction efficiency towards PAHs, which was mainly attributed to strong π stacking and hydrophobic interaction. The MSPE coupled with HPLC method was also used for trace analysis of PAHs in environmental samples, such as tap water, lake water and soil. Good linearity, high sensitivity and great accuracy were obtained, demonstrating that the MSPE-HPLC method was rapid, efficient and sensitive for the determination of PAHs in real samples. The results of this study also suggest that COFs are promising adsorbent material and it is worth to explore the potential of other COFs for sample pretreatment.
References
Cote AP, Benin AI, Ockwig NW, O'Keeffe M, Matzger AJ, Yaghi OM (2005) Porous, crystalline, covalent organic frameworks. Science 310(5751):1166–1170
Feng X, Ding X, Jiang D (2012) Covalent organic frameworks. Chem Soc Rev 41(18):6010–6022
El-Kaderi HM, Hunt JR, Mendoza-Cortés JL, Côté AP, Taylor RE, O'Keeffe M, Yaghi OM (2007) Designed synthesis of 3D covalent organic frameworks. Science 316(5822):268–272
Ding S-Y, Wang W (2013) Covalent organic frameworks (COFs): from design to applications. Chem Soc Rev 42(2):548–568
Cote AP, El-Kaderi HM, Furukawa H, Hunt JR, Yaghi OM (2007) Reticular synthesis of microporous and mesoporous 2D covalent organic frameworks. J Am Chem Soc 129(43):12914–12915
Dalapati S, Jin S, Gao J, Xu Y, Nagai A, Jiang D (2013) An azine-linked covalent organic framework. J Am Chem Soc 135(46):17310–17313
Liu Y, Ma Y, Zhao Y, Sun X, Gándara F, Furukawa H, Liu Z, Zhu H, Zhu C, Suenaga K (2016) Weaving of organic threads into a crystalline covalent organic framework. Science 351(6271):365–369
Han SS, Furukawa H, Yaghi OM, Goddard Iii WA (2008) Covalent organic frameworks as exceptional hydrogen storage materials. J Am Chem Soc 130(35):11580–11581
Furukawa H, Yaghi OM (2009) Storage of hydrogen, methane, and carbon dioxide in highly porous covalent organic frameworks for clean energy applications. J Am Chem Soc 131(25):8875–8883
Rabbani MG, Sekizkardes AK, Kahveci Z, Reich TE, Ding R, El-Kaderi HM (2013) A 2D Mesoporous Imine-Linked Covalent Organic Framework for High Pressure Gas Storage Applications. Chem-Eur J 19(10):3324–3328
Keskin S (2012) Adsorption, diffusion, and separation of CH4/H2 mixtures in covalent organic frameworks: molecular simulations and theoretical predictions. J Phys Chem C 116(2):1772–1779
Huang N, Chen X, Krishna R, Jiang D (2015) Two-Dimensional Covalent Organic Frameworks for Carbon Dioxide Capture through Channel-Wall Functionalization. Angew Chem Int Ed 54(10):2986–2990
Wan S, Guo J, Kim J, Ihee H, Jiang D (2009) A Photoconductive Covalent Organic Framework: Self-Condensed Arene Cubes Composed of Eclipsed 2D Polypyrene Sheets for Photocurrent Generation. Angew Chem Int Ed 121(30):5547–5550
Chen L, Furukawa K, Gao J, Nagai A, Nakamura T, Dong Y, Jiang D (2014) Photoelectric covalent organic frameworks: converting open lattices into ordered donor–acceptor heterojunctions. J Am Chem Soc 136(28):9806–9809
Ding S-Y, Gao J, Wang Q, Zhang Y, Song W-G, Su C-Y, Wang W (2011) Construction of covalent organic framework for catalysis: Pd/COF-LZU1 in Suzuki–Miyaura coupling reaction. J Am Chem Soc 133(49):19816–19822
Fang Q, Gu S, Zheng J, Zhuang Z, Qiu S, Yan Y (2014) 3D Microporous Base-Functionalized Covalent Organic Frameworks for Size-Selective Catalysis. Angew Chem Int Ed 53(11):2878–2882
Yang C-X, Liu C, Cao Y-M, Yan X-P (2015) Facile room-temperature solution-phase synthesis of a spherical covalent organic framework for high-resolution chromatographic separation. Chem Commun 51(61):12254–12257
Bao T, Tang P, Kong D, Mao Z, Chen Z (2016) Polydopamine-supported immobilization of covalent-organic framework-5 in capillary as stationary phase for electrochromatographic separation. J Chromatogr A 1445:140–148
Niu X, Ding S, Wang W, Xu Y, Xu Y, Chen H, Chen X (2016) Separation of small organic molecules using covalent organic frameworks-LZU1 as stationary phase by open-tubular capillary electrochromatography. J Chromatogr A 1436:109–117
Zhang Z, Huang Y, Ding W, Li G (2014) Multilayer interparticle linking hybrid MOF-199 for noninvasive enrichment and analysis of plant hormone ethylene. Anal Chem 86(7):3533–3540
Xie L, Liu S, Han Z, Jiang R, Liu H, Zhu F, Zeng F, Su C, Ouyang G (2015) Preparation and characterization of metal-organic framework MIL-101 (Cr)-coated solid-phase microextraction fiber. Anal Chim Acta 853:303–310
Chang N, Gu Z-Y, Wang H-F, Yan X-P (2011) Metal–organic-framework-based tandem molecular sieves as a dual platform for selective microextraction and high-resolution gas chromatographic separation of n-alkanes in complex matrixes. Anal Chem 83(18):7094–7101
Zhang J, Zhang W, Bao T, Chen Z (2015) Polydopamine-based immobilization of zeolitic imidazolate framework-8 for in-tube solid-phase microextraction. J Chromatogr A 1388:9–16
Xu L, Qi X, Li X, Bai Y, Liu H (2016) Recent advances in applications of nanomaterials for sample preparation. Talanta 146:714–726
Wu M, Chen G, Liu P, Zhou W, Jia Q (2016) Polydopamine-based immobilization of a hydrazone covalent organic framework for headspace solid-phase microextraction of pyrethroids in vegetables and fruits. J Chromatogr A 1456:34–41
Wu M, Chen G, Ma J, Liu P, Jia Q (2016) Fabrication of cross-linked hydrazone covalent organic frameworks by click chemistry and application to solid phase microextraction. Talanta 161:350–358
Spietelun A, Marcinkowski Ł, de la Guardia M, Namieśnik J (2013) Recent developments and future trends in solid phase microextraction techniques towards green analytical chemistry. J Chromatogr A 1321:1–13
Mehdinia A, Aziz-Zanjani MO (2013) Advances for sensitive, rapid and selective extraction in different configurations of solid-phase microextraction. Trends Anal Chem 51:13–22
Wierucka M, Biziuk M (2014) Application of magnetic nanoparticles for magnetic solid-phase extraction in preparing biological, environmental and food samples. Trends Anal Chem 59:50–58
Goon IY, Lai LM, Lim M, Munroe P, Gooding JJ, Amal R (2009) Fabrication and dispersion of gold-shell-protected magnetite nanoparticles: systematic control using polyethyleneimine. Chem Mater 21(4):673–681
Bunkoed O, Kanatharana P (2015) Extraction of polycyclic aromatic hydrocarbons with a magnetic sorbent composed of alginate, magnetite nanoparticles and multiwalled carbon nanotubes. Microchim Acta 182:1519–1526
Mehdinia A, Khojasteh E, Kayyal TB, Jabbari A (2014) Magnetic solid phase extraction using gold immobilized magnetic mesoporous silica nanoparticles coupled with dispersive liquid–liquid microextraction for determination of polycyclic aromatic hydrocarbons. J Chromatogr A 1364:20–27
Zhang W, Zhang J, Bao T, Zhou W, Meng J, Chen Z (2013) Universal multilayer assemblies of graphene in chemically resistant microtubes for microextraction. Anal Chem 85(14):6846–6854
Amiri A, Baghayeri M, Kashmari M (2016) Magnetic nanoparticles modified with polyfuran for the extraction of polycyclic aromatic hydrocarbons prior to their determination by gas chromatography. Microchim Acta 183(1):149–156
Xue S-W, Tang M-Q, Xu L, Z-g S (2015) Magnetic nanoparticles with hydrophobicity and hydrophilicity for solid-phase extraction of polycyclic aromatic hydrocarbons from environmental water samples. J Chromatogr A 1411:9–16
Wang M, Cui S, Yang X, Bi W (2015) Synthesis of gC3N4/Fe3O4 nanocomposites and application as a new sorbent for solid phase extraction of polycyclic aromatic hydrocarbons in water samples. Talanta 132:922–928
Shi Y, Wu H, Wang C, Guo X, Du J, Du L (2016) Determination of polycyclic aromatic hydrocarbons in coffee and tea samples by magnetic solid-phase extraction coupled with HPLC–FLD. Food Chem 199:75–80
Zhang S, Yao W, Ying J, Zhao H (2016) Polydopamine-reinforced magnetization of zeolitic imidazolate framework ZIF-7 for magnetic solid-phase extraction of polycyclic aromatic hydrocarbons from the air-water environment. J Chromatogr A 1452:18–26
Acknowledgements
This work was supported by the National Natural Science Foundation of China (Grant No.81573384, 21375101 and 91417301), and Natural Science Foundation of Hubei Province (No. 2014CFA077).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The author(s) declare that they have no competing interests.
Electronic supplementary material
ESM 1
(DOCX 1243 kb)
Rights and permissions
About this article
Cite this article
Wang, R., Chen, Z. A covalent organic framework-based magnetic sorbent for solid phase extraction of polycyclic aromatic hydrocarbons, and its hyphenation to HPLC for quantitation. Microchim Acta 184, 3867–3874 (2017). https://doi.org/10.1007/s00604-017-2408-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00604-017-2408-8