Abstract
The authors describe an immunoassay for the determination of carcinoembryonic antigen (CEA) tumor markers by depositing a polydopamine-Pb(II) nanocomposite on the surface of a glassy carbon electrode. The nanocomposite acts as a redox system that displays a large specific surface and provides a strong current signal at −0.464 V (vs. Ag/AgCl). After the deposition of PDA-Pb2+ on glassy carbon electrode, the electrode was additionally coated with a chitosan-gold nanocomposite. The immunoassay platform was obtained by immobilization of antibodies against carcinoembryonic antigens by using glutaraldehyde and blocking with bovine albumin. Owing to its large surface, good electrical conductivity and powerful current response, the immunoassay has a wide linear range that extends from 1 fg·mL−1 to 100 ng·mL−1, with a detection limit as low as 0.26 fg·mL−1. The results obtained with this immunoassay when determining CEAs in human serum were found to be consistent with those obtained by ELISAs.

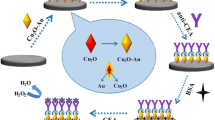
Schematic of an ultrasensitive electrochemical immunosensor for the carcinoembryonic antigen. It is based on a glassy carbon electrode modified with a polydopamine-Pb(II) nanocomposite acting as a signal-inherent substrate.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Early diagnosis and medical treatments of cancers are of great importance for prolonging the survival rate [1]. Owing to the ultratrace level of biomarker at the early stage of cancer, the development of high sensitive immunoassay becomes very urgent [2]. Up to now, many immunoassays have been developed, such as fluorescent, luminescent, colorimetric, electrochemical and photoelectrochemical assays [3–8]. Among these methods, amperometric immunoassay is attractive due to its advantages of easy fabrication, rapid detection, low cost and high sensitivity, especially in label-free immunoassay.
The sensitivity of amperometric immunoassay is significantly affected by the specific surface area and conductivity of the sensing surface [9]. Conventionally, conductive nanomaterials were commonly employed to enhance the sensitivity [10, 11]. While the current responses for tumor markers are generally at tens of microamperes level, indicating that the ultrasensitive immunoassay is still a challenge. Besides, the electrochemical activity of redox species is key to the current signal of the immunosensor [12]. However, very few redox materials are available, resulting in a pressing need of new redox materials for electrochemical immunoassay.
Heavy metal ions as redox species, which are relatively stable and can offer good current responses even at an ultralow quantity, have shown great potential in electrochemical immunoassay [13, 14]. There are four main kinds of redox species composed of metal ions. (1) Poor conductivity compounds. In order to produce electrochemical signal, these insoluble complexes must be dissolved by using strong acid. For example, when CdSeTe@CdS quantum dot was implemented as redox species, its Cd2+ signal was observed after treatment with HNO3 [15]. The application of this method was seriously limited due to the rigorous conditions of this method. (2) Conductive metal complex salts. These complex salts such as Prussian blue, Cd3[Co(CN)6]2 and Cu3[Co(CN)6]2 commonly possessed good conductivity and can be used directly for providing electrochemical signals [16, 17]. However, relatively complicated fabrication procedures were employed to synthesize these homogeneous complexes. (3) Composites formed by electrostatic adsorption. These composites were fabricated generally by using nanomaterials to adsorb the oppositely charged metal ions or coordinating metal ions [18, 19]. Although these redox composites were easy to acquire, there were such issues to be solved that the aggregation easily occurred for the formation and storage of such composites. (4) Composites formed by the coordination of metal ions with polymers. This strategy was developed by using of the carboxylated and aminated materials to fix metal ions [20, 21]. For example, alginate was successfully used to fix Cu2+, Cd2+ and Pb2+ by coordination [21]. However, the conductivity of such materials was commonly poor, which might make the immunosensor become insensitive to the biomarker. Considering such situations, developing a universal and simple protocol for synthesizing a new metal ions-based redox material is necessary. And such conductive and stable metal ions-based redox species are of great significance for realizing the ultrasensitive detection of tumor markers.
Herein, polydopamine-Pb2+ (PDA-Pb2+) nanocomposite was implemented as a novel redox species. Owing to the excellent conductivity of PDA and its fantastic adsorption capacity for Pb2+, PDA-Pb2+ can provide a strong current signal at −0.464 V (vs. Ag/AgCl) [22]. An ultrasensitive label-free amperometric immunoassay for carcinoembryonic antigens (CEA) was fabricated by using PDA-Pb2+ and chitosan-Au (Chit-Au) nanocomposites to fabricate the substrate. After antibodies against carcinoembryonic antigens (anti-CEA) were fixed on the substrate, the immunosensor was obtained by blocking with bovine albumin. The immunosensor exhibited a wide linear range from 1 fg·mL−1 to 100 ng·mL−1 for CEA. For human serum samples, the results of the presented method were good accordance with them of enzyme linked immunosorbent assay (ELISA), indicating the good reliability of this method.
Experimental section
Materials
Ascorbic acid (AA, 99.7%), HAuCl4·3H2O (99.99%), uric acid (UA), D-(+)-glucose (GC) and dopamine hydrochloride (DA) were commercially obtained from Alfa Aesar (Beijing, China, http://www.inno-chem.com.cn/). Pb(NO3)2 was purchased from Beijing Chemical Reagents Company (Beijing, China, http://www.reagent.com.cn/). Chitosan was purchased from TCI (Tokyo, Japan). CEA, alpha fetoprotein antigen (AFP) and anti-CEA were brought from Linc-Bio Science Co., Ltd. (Shanghai, China, http://www.linc-bio.com/). Bovine albumin (BSA, standard grade) was bought from Xinjingke Biotechnology Company (Beijing, China, http://www.bjxjk.com/). Clinical human serum samples were provided by Capital Normal University Hospital (Beijing, China, http://xyy.cnu.edu.cn/). All the chemical reagents were analytically pure grade.
Instruments
Ultrapure water was purified through an Olst ultrapure K8 apparatus (Olst, Ltd., resistivity >18 MΩ cm, http://www.olst.cn/). Microwave reaction instrument (CEM, USA, http://cem.com/) was used to synthesize Chit-Au. Transmission electron microscopy (TEM) measurements were conduct on a Hitachi (H7650, 80 kV, http://www.hitachi.com.cn/) transmission electron microscope. X-ray photoelectron spectroscopy (XPS) analysis was performed on an escalab 250 X-ray photoelectron spectroscope (Thermofisher, American, http://xpssimplified.com/) using an Al (mono) Kα radiation. Fourier transform infrared (FTIR) spectroscopy measurements were performed on a VECTORTM 22 spectrometer (Bruker, https://www.bruker.com/). Zeta potential measurements were performed on a Zetasizer Nano ZS (Malvern Instruments Co., UK, http://www.malvern.com/en/). Electrochemical measurements were performed on a CHI832 electrochemical workstation (Shanghai Chenhua Instruments Co., LTD, China, http://www.chinstr.com/lxwm) with a three-electrode system: a platinum wire as the auxiliary electrode, an Ag/AgCl electrode (saturated KCl) as the reference electrode and a modified glassy carbon electrode (GCE, Φ = 4 mm) as the working electrode.
Preparation of PDA-Pb2+
PDA nanoparticles were synthesized according to literature with a little modification [23]. In brief, 18 mg DA was dissolved into 9 mL water at 50 °C, and then NaOH (1 M, 76 μL) was quickly injected under vigorous stirring for 5 h. Then, PDA nanoparticles were centrifuged, thoroughly washed with water, and diluted with water to 5 mL. After 2 mL PDA was mixed with Pb(NO3)2 (0.1 M, 2 mL) under vigorous stirring for 6 h, PDA-Pb2+ was collected by centrifugation.
Preparation of chit-Au
A homogenous solution was prepared by mixing 5 mL 0.2% chitosan (wt. 1% CH3COOH) with HAuCl4 (10 mM, 100 μL). Then the microwave-assisted reaction was performed at 110 °C for 10 min with stirring. After that, the red Chit-Au solution was obtained and stored at 4 °C before use.
Fabrication of the immunosensor
GCE was carefully polished with 0.05 μm alumina slurries and sonication washed with deionized water. 10 μL PDA-Pb2+ was dropped on GCE and dried at 37 °C. Then 5 μL Chit-Au were coated on PDA-Pb2+/GCE. After being treated with glutaraldehyde (1%, 20 μL) for 30 min, Chit-Au/PDA-Pb2+/GCE was incubated with 100 μg mL−1 anti-CEA at 4 °C for 12 h. The immunosensor for CEA was obtained when anti-CEA/Chit-Au/PDA-Pb2+/GCE was blocked by 0.25% BSA for 1 h at 37 °C. Among each step, the modified GCE was carefully washed by water. The BSA/anti-CEA/Chit-Au/PDA-Pb2+/GCE was stored at 4 °C before use.
Measurement procedure
80 μL CEA or human serum sample was incubated on the BSA/anti-CEA/Chit-Au/PDA-Pb2+/GCE for 1 h at 37 °C, followed by washing with water. Then, the square wave voltammetry (SWV) measurement, from −1.3 V to 0 V (vs. Ag/AgCl), was performed in 0.2 M acetate buffer (pH 5.5).
Results and discussion
Principle of this electrochemical immunoassay
The fabrication of the electrode and the detection scheme of the immunoassay are illustrated in Scheme 1. The substrate was fabricated by orderly dropping PDA-Pb2+ and Chit-Au on GCE. PDA enriching amine, imine, phenol, and ο-diphenol groups possessed good adsorption capacity for Pb2+ through electrostatic adsorption and coordination effect. Therefore, the substrate based on PDA-Pb2+ provided a strong current signal in electrochemical measurements. Meanwhile, Chit-Au played important roles in the immunoassay: amplifying the current signal and immobilizing anti-CEA. After anti-CEA and BSA were subsequently incubated on Chit-Au/PDA-Pb2+/GCE, the immunosensor for CEA was obtained. In this case, CEA can be determined.
Characterization of PDA-Pb2+ and chit-Au
PDA as an adsorbent with excellent conductivity was commonly employed to fabricate stable, hydrophilic and modifiable interfaces [24, 25]. PDA-Pb2+ was approximately 120 nm in size and exhibited good monodispersion (Fig. 1a). Gold nanoparticles (AuNPs) about 20 nm in diameter were uniformly distributed in Chit-Au (Fig. 1b).
Further, the detailed compositional analysis of PDA and PDA-Pb2+ was characterized by XPS spectroscopy analysis (Fig. 1c, d and Fig. S1). C1s, N1 s and O1s peaks were mainly derived from PDA (Fig. S2a-c). The peaks of Pb4f at 139.16 eV and 144.15 eV were consistent with Pb2+ state, indicating that Pb2+ was adsorbed by PDA (Fig. S1d). Differ from the peaks shift of C1s and N1 s, O1s peak was shifting to low binding energy after mixing PDA with Pb2+ (Fig. S1a-d). This phenomenon might be owing to the coordination reaction between PDA as a polyphenolic polymer and Pb2+ [22, 26–31].
Zeta potentials of PDA and PDA-Pb2+ were measured. After mixing with Pb2+, the zeta potential of PDA was significantly changed from −26 mV to −5 mV (Fig. 2a), indicating that Pb2+ was adsorbed by PDA through electrostatic adsorption. Detailed structural analysis of dopamine, PDA and PDA-Pb2+ was characterized by FTIR spectroscopy measurements (Fig. 2b). The results showed agreement with those of literature [25]. The peak at 3436 cm−1 was assigned to the stretching vibration of O-H and N-H, and the peak at 1628 cm−1 corresponded to the stretching vibration of aromatic ring and bending vibration of N-H. For dopamine, peaks at 1395 cm−1 and 1291 cm−1 were attributed to the bending and stretching vibration of phenolic C-OH. In contrast, these two peaks disappeared in PDA, demonstrating that dopamine was successfully polymerized. Compared with PDA, the peak of benzene ring in PDA-Pb2+ was obviously red shift from 1628 cm−1 to 1637 cm−1, owing to the adsorbed Pb2+ connecting with phenolic hydroxyl group through electrostatic interaction.
Stepwise construction procedures of the immunosensor
To investigate the conductivity and stability of PDA/GCE and Chit-Au/GCE, cyclic voltammetry (CV) measurements were performed in 5 mM [Fe(CN)6]3−/4- containing 0.1 M KCl, respectively. 20 μL PDA and 20 μL Chit-Au were coated on GCEs, respectively. Both of the current responses of PDA/GCE and Chit-Au/GCE were obviously larger than that of bare GCE (Fig. S2a). These phenomena possibly resulted from the excellent conductivity and permeability of PDA and Chit-Au. In addition, no obvious change was observed after CV with 50 cycles, suggesting that PDA and Chit-Au film had a good stability (Fig. S2b). Then, SWV measurements were performed to investigate the electrochemical activity of PDA-Pb2+ (Fig. S2c). For PDA/GCE, no current signal peak was observed. Conversely, a strong current signal from PDA-Pb2+/GCE (−0.464 V (vs. Ag/AgCl), 371 μA) was observed, indicating that PDA-Pb2+ was an excellent redox species.
Further, the fabrication procedures of the immunosensor were monitored by SWV measurements in pH 5.5 buffer (Fig. 3). No current peak was observed for the bare electrode (Fig. 3a). After PDA-Pb2+ was coated on GCE, a strong current signal was observed (Fig. 3b). When Chit-Au was coated on PDA-Pb2+/GCE, a stronger electrochemical signal was observed (Fig. 3c), resulting from the good conductivity of Chit-Au. When the electrode was incubated with anti-CEA, BSA and CEA, the electrochemical signal gradually decreased. These phenomena were caused by the protein hindering electron transfer, indicating that the immunosensor was obtained and CEA was immobilized on BSA/anti-CEA/Chit-Au/PDA-Pb2+/GCE (Fig. 3d-f).
Optimization of pH and analytical performance of the immunosensor
The current difference between immunosensor treated with and without CEA was defined as ΔI. Obviously, a large ΔI represented the high sensitivity of the immunosensor. Since pH of electrolyte can significantly affect the conductivity of this immunosensor and the adsorption ability of PDA for Pb2+ [22], pH of acetate buffer was optimized. The ΔI increased from pH 4.5 to 5.5 and then gradually decreased from 5.5 to 6.5 (Fig. S3). Hence, pH 5.5 buffer was chosen during the following experiments.
CEA, as a conventional tumour marker relating to many kinds of cancers such as gastric, pancreatic, liver, lung and breast cancers, was used as a model target in this immunoassay [30, 32]. The analytical performance of the immunosensor for CEA was evaluated by investigating different concentrations of CEA samples in pH 5.5 buffer (Fig. 4a). The linear equation was “ΔI (μA) = 9.95log10C (ng·mL-1) +75” (Fig. 4b). The linear range (LOR) of the immunosensor for CEA was from 1 fg·mL−1 to 100 ng·mL−1. The limit of detection (LOD) reached an ultralow level of 0.26 fg·mL−1 at a signal-to-noise ratio of 3σ (σ is the standard deviation of signal of the immunosensor incubated without CEA). In comparison to some previous works (Table S1), the linearity range of the method for CEA was two orders of magnitude wider and the limit of detection was one order of magnitude lower. Compared with other nanomaterial-based methods for CEA, the present method possessed following merits (Table 1). (1) PDA-Pb2+ and chitosan-Au were cheap and easily obtained. (2) This substrate can be readily fabricated by drop coating method. (3) This method was more sensitive and exhibited a wider LOR and a lower LOD. Additionally, it is simpler since no complicated signal amplification procedures are involved.
Repeatability, stability, specificity and reliability of the immunoassay
Three parallel experiments were conducted in pH 5.5 buffer. The signal differences among ΔI (84.74 μA, 85.58 μA, and 85.50 μA) of the triple measurements for 10 ng·mL−1 CEA were negligible, demonstrating the good repeatability of the immunosensor. To address the specificity, 10 ng·mL−1 CEA samples containing interfering substance (AFP, AA, GC and UA) were detected, respectively [39]. The relative standard deviations were less than 10% (Fig. 5), suggesting that the immunosensor possessed good specificity. To investigate the stability of this immunosensor, some freshly prepared BSA/anti-CEA/Chit-Au/PDA-Pb2+/GCEs were stored at 4 °C. After 21 days, 95% of the initial current responses were remained in comparison to the freshly prepared immunosensor, indicating the good stability of the immunosensor.
The widely applied ELISA was employed as a standard method to investigate the reliability of the immunoassay. Clinical serum samples were determined by this immunosensor and ELISA method, respectively. The results of the method for CEA showed a good agreement with these of ELISA with relative error less than 10%, indicating that the immunoassay possessed good reliability and was promising in clinical diagnosis (Table S2).
Conclusion
In summary, this method showed some advantages: (1) PDA-Pb2+ as a novel metal ions-based redox species was easily synthesized with a strong current signal at −0.464 V (vs. Ag/AgCl). (2) PDA-Pb2+ and Chit-Au were feasible to acquire. (3) An ultrasensitive label-free amperometric immunosensor for carcinoembryonic antigens was developed by using PDA-Pb2+ and Chit-Au nanocomposites. In addition, this method was simpler, more sensitive, wider LOR and lower limit of detection. This method can be extended to detect other tumor markers.
References
Torre LA, Bray F, Siegel RL, Ferlay J, Lortet-Tieulent J, Jemal A (2015) Global cancer statistics, 2012. CA Cancer J Clin 65:87–108
Tingen MS, Weinrich SP, Heydt DD, Boyd MD, Weinrich MC (1998) Perceived benefits: a predictor of participation in prostate cancer screening. Cancer Nurs 21:349–345
Wang C, Zhai W, Wang Y, Yu P, Mao LQ (2015) MnO2 nanosheets based fluorescent sensing platform with organic dyes as a probe with excellent analytical properties. Analyst 140:4021–4029
Li C, Fu Z, Li Z, Wang Z, Wei W (2011) Cross-talk-free multiplexed immunoassay using a disposable electrochemiluminescent immunosensor array coupled with a non-array detector. Biosens Bioelectron 27:141–147
Xianyu Y, Chen Y, Jiang X (2015) Horseradish peroxidase-mediated, iodide-catalyzed cascade reaction for plasmonic immunoassays. Anal Chem 87:10688–10692
Zhou J, Du L, Zou L, Zou Y, Hu N, Wang P (2014) An ultrasensitive electrochemical immunosensor for carcinoembryonic antigen detection based on staphylococcal protein A-Au nanoparticle modified gold electrode. Sensors Actuators B Chem 197:220–227
Wang GL, Shu JX, Dong YM, Wu XM, Li ZJ (2015) An ultrasensitive and universal photoelectrochemical immunoassay based on enzyme mimetics enhanced signal amplification. Biosens Bioelectron 66:283–289
Serafín V, Úbeda N, Agüí L, Yáñez-Sedeño P, Pingarrón JM (2012) Ultrasensitive determination of human growth hormone (hGH) with a disposable electrochemical magneto-immunosensor. Anal Bioanal Chem 403:939–946
Cheng MS, Toh CS (2013) Novel biosensing methodologies for ultrasensitive detection of viruses. Analyst 138:6219–6229
Peng J, Zhu YD, Li XH et al (2014) Electrochemical immunoassay for the prostate specific antigen using ceria mesoporous nanospheres. Microchim Acta 181:1505–1512
Tang J, Tang D, Niessner R, Knopp D (2011) A novel strategy for ultra-sensitive electrochemical immunoassay of biomarkers by coupling multifunctional iridium oxide (IrOx) nanospheres with catalytic recycling of self-produced reactants. Anal Bioanal Chem 400:2041–2051
Shan J, Ma ZF (2016) Simultaneous detection of five biomarkers of lung cancer by electrochemical immunoassay. Microchim Acta 183:2889–2897
Yantasee W, Hongsirikarn K, Warner CL et al (2008) Direct detection of Pb in urine and Cd, Pb, Cu, and Ag in natural waters using electrochemical sensors immobilized with DMSA functionalized magnetic nanoparticles. Analyst 133:348–355
Shiddiky MJ, Rauf S, Kithva PH, Trau M (2012) Graphene/quantum dot bionanoconjugates as signal amplifiers in stripping voltammetric detection of EpCAM biomarkers. Biosens Bioelectron 35:251–257
Zhou S, Wang Y, Zhu J (2016) Simultaneous detection of tumor cell apoptosis regulators Bcl-2 and Bax through a dual-signal-marked electrochemical immunosensor. ACS Appl Mater Interfaces 8:7674–7682
Liu Z, Yuan R, Chai Y, Zhuo Y, Hong C, Yang X (2008) Highly sensitive, reagentless amperometric immunosensor based on a novel redox-active organic–inorganic composite film. Sensors Actuators B Chem 134:625–631
Wang ZF, Chen X, Ma ZF (2014) Chitosan coated copper and cadmium hexacyanocobaltate nanocubes as immunosensing probes for the construction of multiple analytes platform. Biosens Bioelectron 61:562–568
Xu T, Jia XL, Chen X, Ma ZF (2014) Simultaneous electrochemical detection of multiple tumor markers using metal ions tagged immunocolloidal gold. Biosens Bioelectron 56:174–179
Shi WT, Ma ZF (2011) A novel label-free amperometric immunosensor for carcinoembryonic antigen based on redox membrane. Biosens Bioelectron 26:3068–3071
Zhang S, Du B, Li H, Xin X, Ma H, Wu D, Yan LG, Wei Q (2014) Metal ions-based immunosensor for simultaneous determination of estradiol and diethylstilbestrol. Biosens Bioelectron 52:225–231
Wang ZF, Liu N, Feng F, Ma ZF (2015) Synthesis of cadmium, lead and copper alginate nanobeads as immunosensing probes for the detection of AFP, CEA and PSA. Biosens Bioelectron 70:98–105
Liu Y, Ai K, Lu L (2014) Polydopamine and its derivative materials: synthesis and promising applications in energy, environmental, and biomedical fields. Chem Rev 114:5057–5115
Ju KY, Lee Y, Lee S, Park SB, Lee JK (2011) Bioinspired polymerization of dopamine to generate melanin-like nanoparticles having an excellent free-radical-scavenging property. Biomacromolecules 12:625–632
He Y, Sun J, Wang X, Wang L (2015) Detection of human leptin in serum using chemiluminescence immunosensor: signal amplification by hemin/G-quadruplex DNAzymes and protein carriers by Fe3O4/polydopamine/Au nanocomposites. Sensors Actuators B Chem 221:792–798
Lee H, Dellatore SM, Miller WM, Messersmith PB (2007) Mussel-inspired surface chemistry for multifunctional coatings. Science 318:426–430
Hong L, Simon JD (2005) Physical and chemical characterization of iris and choroid melanosomes isolated from newborn and mature cows. Photochem Photobiol 81:517–523
Nalwa HS (1991) Optical and X-ray photoelectron spectro-scopic studies of electrically conducting benzimidazobenzophenanthroline type ladder polymers. Polymer 32:802–807
Xu X, Friend CM (1989) The role of coverage in determining adsorbate stability: phenol reactivity on rhodium(111). J Phys Chem 93:8072–8080
d'Ischia M, Napolitano A, Pezzella A, Meredith P, Sarna T (2009) Chemical and structural diversity in eumelanins: unexplored bio-optoelectronic materials. Angew Chem Int Ed 48:3914–3921
Uchida N, Fukino S, Kodama W, Tamai N, Hiroe T, Fukata T (2007) Large-cell carcinoma of the lung with a remarkable preoperative elevation of serum carcinoembryonic antigen level. Gen Thorac Cardiovasc Surg 55:217–221
Zou CE, Yang BB, Bin D, Wang J, Li SM, Yang PG, Wang CQ, Shiraishi Y, Du YK (2017) Electrochemical synthesis of gold nanoparticles decorated flower-like graphene for high sensitivity detection of nitrite. J Colloid Interf Sci 488:135–141
Gao X, Zhang Y, Chen H, Chen Z, Lin X (2011) Amperometric immunosensor for carcinoembryonic antigen detection with carbon nanotube-based film decorated with gold nanoclusters. Anal Biochem 414:70–76
Li F, Jiang L, Han J, Liu Q, Dong Y, Yy L, Wei Q (2015) A label-free amperometric immunosensor for the detection of carcinoembryonic antigen based on novel magnetic carbon and gold nanocomposites. RSC Adv 5:19961–19969
Lin J, Zhang H, Niu S (2015) Simultaneous determination of carcinoembryonic antigen and α-fetoprotein using an ITO immunoelectrode modified with gold nanoparticles and mesoporous silica. Microchim Acta 182:719–726
Zhao D, Wang Y, Nie G (2016) Electrochemical immunosensor for the carcinoembryonic antigen based on a nanocomposite consisting of reduced graphene oxide, gold nanoparticles and poly (indole-6-carboxylic acid). Microchim Acta 183:2925–2932
He SJ, Wang QY, Yu YY, Shia QJ, Zhang L, Chen ZG (2015) One-step synthesis of potassium ferricyanide-doped polyaniline nanoparticles for label-free immunosensor. Biosens Bioelectron 68:462–467
Zhang Y, Lu F, Yan Z, Wu D, Ma H, Du B, Wei Q (2015) Electrochemiluminescence immunosensing strategy based on the use of Au@ Ag nanorods as a peroxidase mimic and NH4CoPO4 as a supercapacitive supporter: application to the determination of carcinoembryonic antigen. Microchim Acta 182:1421–1429
Xu TS, Li XY, Xie ZH, Li XG, Zhang HY (2015) Poly(o-phenylenediamine) nanosphere-conjugated capture antibody immobilized on a glassy carbon electrode for electrochemical immunoassay of carcinoembryonic antigen. Microchim Acta 182:2541–2549
Wang CQ, Du J, Wang HW, Zou CE, Jiang FX, Yang P, Du YK (2014) A facile electrochemical sensor based on reduced graphene oxide and Au nanoplates modified glassy carbon electrode for simultaneous detection of ascorbic acid, dopamine and uric acid. Sensors Actuators B Chem 204:302–309
Acknowledgements
This research was financed by grants from the National Natural Science Foundation of China (21273153, 21673143), Natural Science Foundation of Beijing Municipality (2132008), and the Project of the Construction of Scientific Research Base by the Beijing Municipal Education Commission.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The author(s) declare that they have no competing interests.
Electronic supplementary material
ESM 1
(DOCX 1514 kb)
Rights and permissions
About this article
Cite this article
Tang, Z., Ma, Z. Ultrasensitive amperometric immunoassay for carcinoembryonic antigens by using a glassy carbon electrode coated with a polydopamine-Pb(II) redox system and a chitosan-gold nanocomposite. Microchim Acta 184, 1135–1142 (2017). https://doi.org/10.1007/s00604-017-2117-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00604-017-2117-3