Abstract
The authors describe an voltammetric catechol (CC) assay based on the use of a glassy carbon electrode (GCE) modified with a composite consisting of graphene oxide and polymelamine (GO/PM). The modified GCE was characterized by field emission scanning electron microscopy, elemental analysis, Raman spectroscopy and FTIR. Cyclic voltammetry reveals a well-defined response to CC, with an oxidation peak current that is distinctly enhanced compared to electrodes modified with GO or PM only. The combined synergetic activity of GO and PM in the composite also results in a lower oxidation potential. Differential pulse voltammetry (DPV) shows a response that is linear in the 0.03 to 138 μM CC concentration range. The detection limit is 8 nM, and the sensitivity is 0.537 μA⋅μM−1⋅cm−2. The sensor is selective for CC even in the presence of potentially interfering compounds including hydroquinone, resorcinol and dopamine. The modified GCE is highly reproducible, stable, sensitive, and shows an excellent practicability for detection of CC in water samples.

Voltammetric sensing of catechol at polymelamine entrapped graphene oxide composite.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Catechol (CC) is an ortho-isomer of benzenediols, has been common used as a building block in organic synthesis [1]. It has also been widely used in variety of applications including production of pesticides and precursor to perfumes and pharmaceuticals [2, 3]. In addition, CC has classified as a periodic environmental pollutant and Group 2B human carcinogen due to its toxicity and low degradability in the ecological environment [4, 5]. Continues exposure of CC can result in the prolonged rise of blood pressure and depression of central nervous system in animals [6]. Therefore, considerable attention has been paid for the fabrication of sensitive devices for low level detection of CC in environmental samples. Various analytical methods have been used for the sensitive detection of CC such as high performance liquid chromatography, gas chromatography, mass spectrometry, spectrophotometry, chemiluminescence, flow injection analysis and electrochemical methods [7–12]. The electrochemical methods are found simple and cost effective than other available aforementioned analytical methods [12]. The modified electrodes have played an important role in the fabrication of sensitive CC sensors due to their high selectivity, low oxidation potential and high sensitivity. On the other hand, the unmodified electrodes displayed a poor selectivity and sensitivity along with fouling of signals towards detection of CC [12, 13].
Graphene oxide (GO) has attached much attention in the scientific community owing to its extreme hydrophilicity and presence of abundant functional groups [14, 15]. In addition, GO has been widely used for surface modification with different molecules due to the presence of different functional groups on the surface [15]. Instead, polymelamine (PM) is known as a class conducting polymer, has been widely used for electrode modification owing to its high stability, strong adherence to electrode surface and presence of abundant nitrogen and amine groups [16, 17]. Furthermore, the PM modified electrodes have been used for electrochemical sensing of different analytes such as gallic acid, dihydroxybenzene isomers and neurotransmitters [16–20]. Considering the aforementioned properties of PM and GO, one may have assumed that an electrode modified with PM combined with GO can provide good electrocatalytic activity. However, only limited reports are available for PM based electrochemical sensors [16–21]. To the best of our knowledge, the GO/PM composite modified electrode has not been used yet for the electrochemical determination of CC.
In this paper, we report the fabrication of a simple and sensitive CC electrochemical sensor based on GO/PM composite modified glassy carbon electrode (GCE). The GO/PM composite modified electrode shows an enhanced catalytic activity for oxidation of CC than PM and GO modified electrodes. The sensitivity, selectivity, stability and practicality of the sensor was evaluated and discussed in detail.
Experimental
Materials and method
Natural graphite was purchased from Sigma Aldrich and used as received (http://www.sigmaaldrich.com/taiwan.html). All chemicals including melamine and catechol were received from Sigma Aldrich (http://www.sigmaaldrich.com/taiwan.html). The supporting electrolyte, 0.1 M phosphate buffer pH 7 was prepared using 0.1 M Na2PO4 and 0.1 M NaH2PO4 with doubly distilled water. The other pH (pH 3, 5, 7 and 9) were prepared by adjusting the phosphate buffer with diluted H2SO4 and NaOH. Ultrapure doubly distilled water (resistivity >18.2 MΩ⋅cm at 25 °C) from a LOTUN Ultrapure Water System was used for the preparation of all chemicals.
Cyclic voltammetry and differential pulse voltammetry (DPV) measurements were carried out using CHI1205B and CHI750A electrochemical work stations (http://www.chinstruments.com/). Typical three-electrode configuration was used for electrochemical experiments. The modified GCE with an apparent surface area about 0.079 cm2 was used as a working electrode for the electrochemical experiments. Saturated Ag/AgCl and a Pt wire were used as reference and counter electrodes, respectively. Scanning electron microscopic (SEM) image of the composite modified electrode was taken using Hitachi S-3000H Scanning Electron Microscope (SEM). The elemental analysis and elemental mapping of the composite modified electrode were performed using HORIBA EMAX X-ACT attached Hitachi S-3000 H SEM charge-coupled detector. Fourier transform infrared (FTIR) spectra were analyzed using the Thermo SCIENTIFIC Nicolet iS10 instrument.
Fabrication of GO/PM composite modified electrode
The GO and GO dispersion (5 mg mL−1 in double distilled water) were prepared by our previously reported methods [31]. To prepare GO@PM composite modified electrode, about 10 μL (optimum) of GO dispersion was coated on pre-cleaned GCE, and dried in an air oven. The resulting GO modified electrode was immersed in the electrochemical cell containing 1 mM melamine and 0.01 M HCl, and performed 10 constitutive cycles in the potential ranging from 0 to 1.5 V at a scan rate of 50 mV s−1 [18]. Finally, GO@PM composite was fabricated on the electrode surface and dried in an air oven and stored under dry conditions when not in use. The GO and PM modified electrodes were prepared by similar method without melamine and GO. The schematic representation for the fabrication of GO@PM composite modified electrode is shown in Scheme 1. All electrochemical measurements were performed in the presence of high purity nitrogen gas.
Results and discussion
Choice of materials
The fabrication of simple and robust sensors for the determination of CC is of interest to the analytical chemists. CC is highly electro-active on carbon modified electrodes, but they often show poor selectivity, sensitivity and reproducibility. In the present work, we have chosen the GO and PM composite for sensitive and selective determination of CC due to the unique properties of GO and PM. The abundant oxygen functional groups of GO are more favourable to form the stable composite with PM. In addition, the abundant nitrogen and amine groups of PM can possibly interact with more number of CC molecules and result into the enhanced sensitivity, lower oxidation potential and low detection limit of CC on GO/PM composite. In addition, the sensor is more simple, inexpensive and has appropriate analytical features towards CC than previously reported carbon nanomaterial based CC sensors (Table 1).
Characterizations
Figure 1a shows the SEM image of GO/PM composite modified electrode, and can be seen that uniform rectangle type structures of the PM film were uniformly distributed throughout the surface of GO nanosheets. It also noted that the PM is interlinked with one another and strongly enfolded by GO nanosheets, which is possibly due to the strong interaction of amino groups of PM with OH group of GO. The unique structure of GO/PM composite modified electrode provides more electroactive surface when compared to GO and PM. The observed surface morphology of GO/PM composite is similar to that of the obtained surface morphology of PM (Fig. S1A) and GO (Fig. S1B). The EDX and corresponding elemental mapping analysis were further used to confirm the presence of PM on GO/PM composite and results are displayed in Fig. 1b–e. The EDX and elemental mapping of GO/PM composite showed specific regions of carbon, nitrogen and oxygen, which confirmed the presence of GO and PM in the composite modified electrode. It is also noted that the surface morphology of PM on GO is similar to previous reports [20, 21]. The FTIR (Fig. S2) and Raman spectra (Fig. S3) findings also confirmed the formation of GO/PM composite and the detailed discussions can be found in electronic supplementary material (ESM).
Electrochemical behavior of CC on different modified electrodes
The cyclic voltammetry was used to investigate the electrochemical behavior of CC at different modified electrodes. Figure 2 shows the cyclic voltammetry response of bare (a), GO (b), PM (c) and GO/PM composite (d) modified electrodes in 100 μM CC containing pH 7 at a scan rate of 50 mV s−1. The unmodified electrode exhibited a weak oxidation peak current response to CC at 0.305 V, with the peak to peak separation (ΔEp) of 0.257 V. The weak oxidation peak is due to the oxidation of CC to corresponding quinone, as shown in Scheme 1. The GO modified electrode shows the oxidation peak of CC at quite less potential (0.234 V) than unmodified electrode. In addition, the ΔEp of CC was 84 mV lower than unmodified electrode, and the anodic peak current was lower than unmodified electrode. It addition, the GO widely known as an insulating material and has poor electrocatalytic activity.
Cyclic voltammetric response of bare (a), GO (b), PM (c) and GO/PM composite (d) modified GCEs in 100 μM CC containing pH 7 at a scan rate of 50 mV s−1. Inset shows the catalytic activity (oxidation peak current response and peak to peak separation) of different modified electrodes towards CC. Error bar relative to five measurements
The PM modified electrode shows an enhanced oxidation peak current to CC than bare and GO modified electrodes, which indicates the high adsorption ability of PM towards CC. The observed ΔEp of CC was 153 and 69 mV lower than those observed at bare and GO modified electrodes. However, the GO/PM composite modified electrode shows a well-defined redox couple with 3-fold higher oxidation peak current response for CC than PM modified electrode. The oxidation peak potential of CC was appeared at 0.223 V. As shown in Fig. 3 inset, the ΔEp of CC was 64, 133 and 217 mV lower than PM, GO and bare modified electrodes. The result indicates that the electrochemical behavior of CC is greatly enhanced on GO/PM composite modified electrode than other modified electrodes. The combined unique properties of GO and PM are resulting into the enhanced catalytic activity towards oxidation CC. The strong interaction between PM and GO with CC via hydrogen bonding is the possible reason for enhanced electrocatalytic activity of the composite. The possible electro-oxidation mechanism of CC at GO/PM composite modified electrode is shown in Scheme 1. The effect of scan rate (Fig. S4A) and pH studies (Fig. S4B) and their corresponding discussions on the electrochemical behavior of CC at GO/PM modified electrode can be found ESM.
a Differential pulse voltammograms for GO/PM composite modified electrode for the absence and presence of different concentration (0.03–147.5 μM) of CC in pH 7. Inset shows the calibration plot for current response vs. [CC]. b The effect of 100 μM addition of dopamine (a), ascorbic acid (b), uric acid (c), resorcinol (d), hydroquinone (e) and 150 μM (f) and 300 μM (g) addition of hydroquinone in 1 μM CC containing pH 7. The DPV working conditions are amplitude =0.05 V, pulse width = 0.05 s, sampling width = 0.0167 s and pulse period =0.2 s
Determination of CC
DPV was used to determine the CC using GO/PM composite modified electrode due to its high sensitivity than other voltammetric methods. Figure 3a shows the typical DPV response of GO/PM modified electrode for the absence and presence of different concentration of CC (0.03–147.5 μM) into the pH 7 . In the absence of CC, the GO/PM modified electrode did not show any obvious response, which indicates that the composite modified electrode is electrochemically inactive in this particular potential window. A sharp DPV response was observed at GO/PM modified electrode for the addition of 0.03, 0.1 and 0.5 μM of CC into the pH 7. In addition, the response current of CC linearly increases with the increasing the concentration of CC. As shown in Fig. 3a inset, the response current of CC was linear over the concentration ranging from 0.03 to 138.0 μM with the LOD of 8 nM (S/N = 3). The linear regression equation was expressed as (μA) = 0.1112 (μM) + 0.4275 and R2 = 0.9925. The sensitivity of the sensor was estimated to be 0.537 ± 0.008 μAμM−1 cm−2, where the electrochemically active surface area of GO/PM composite modified electrode is 0.207 cm2.
To evaluate the novelty and superiority of the sensor, the analytical features of the sensor were compared with previously reported carbon nanomaterials and polymers based CC sensors. The comparative results are shown in Table 1. It is noted that the LOD of the sensor was much lower than previously reported GR and RGO based composites based CC sensors, as shown in Table 1. For instance, the LOD was lower than carbon nanocages-reduced graphene oxide (CNCs/RGO) [22], GR [23], graphene/polydopamine (GR/PDA) [24], single-walled carbon nanotubes (SWCNT) [25], graphene/chitosan (GR/CHI) [26], RGO/ZrO2/Pt [27], nafion/SWCNT/cyclodextrin (Nf/SWCNT/CD) [28], GR/TiO2 [29], CHI/MWCNT/PDA/AuNPs [30] and RGO/Cu-NPs [31] modified GCEs for the detection of CC. In addition, the analytical features such as linear response range and sensitivity of the sensor were comparable with previously reported CC sensors [22–31]. Hence, the fabricated GO/PM composite can be used as a sensitive probe for the determination of CC.
Selectivity
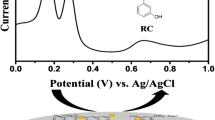
The selectivity of sensor is more crucial for practical applications, hence we have investigated the selectivity of sensor in the presence of range of potentially active compounds such as hydroquinone (HQ), resorcinol (RC), dopamine (DA), ascorbic acid (AA) and uric acid (UA). These compounds can possibly interfere with CC due to an oxidation potential close to CC [31]. Figure 3b shows the DPV response of GO/PM modified electrode for 100 μM each addition of DA (a), AA (b), UA (c), RC (d), HQ (e) and 150 (f) and 300 μM (g) additions of HQ into the 1 μM CC containing pH 7. The other experimental conditions are same as of in Fig. 3a. It can be seen that the 100 μM addition of DA (a), AA (b), UA (c), RC (d), HQ (e) and 150 μM (f) addition of HQ did not show any obvious response on the composite modified electrode. It is worthy to note that the DPV response aforementioned electroactive compounds are minimum and had no impact on the oxidation signal of CC. However, 300 μM addition of HQ (g) shows a sharp DPV response at the potential of 0.186 V, and a small change in the oxidation peak current response of CC was observed. The reason is possibly due to the similar chemical and structural features of HQ with CC. However, the oxidation peak potential of CC was not affected by aforementioned potentially interfering compounds including the addition of 300 μM of HQ. The results clearly indicate that the interference effect caused by the aforementioned species are minimum for CC on GO/PM modified electrode, and can be used for selective detection of CC.
Determination of CC in water samples
To verify the practical applicability of the sensor, we have determined the CC in different water samples by GO/PM modified electrode. DPV method was used to detect the CC and the experimental conditions are similar as of in Fig. 3a. The recovery of CC in water samples were calculated using the standard addition method [31]. The modified electrode does not show appropriate signal for the ground and tap water samples, which shows that CC was absent in the water samples. Then, a known concentration of CC containing ground and tap water samples were spiked into the pH 7 and the recoveries were calculated. The recovery values for CC in the ground and tap water samples are summarized in Table S1. The average recoveries about 95.5 and 94.2% of CC were found in ground and tap water samples using GO/PM composite modified electrode. In addition, the practical ability of the composite modified electrode is comparable with results obtained from spectrophotometric method. The results revealed that the fabricated sensor can be used for the determination of CC in water samples.
The repeatability and reproducibility of the sensor (not shown) were investigated using cyclic voltammetry, and the experimental conditions are similar to Fig. 3. Five different independently prepared GO/PM composite modified electrodes show the relative standard deviation (RSD) about 4.5% for the detection of 100 μM CC. On the other hand, a single GO/PM composite modified electrode for detection of 100 μM CC containing 8 set of pH 7.0 shows the RSD of 3.9%. The result validates that the GO/PM composite modified electrode has appropriate reproducibility and repeatability for the detection of CC. We have also investigated the storage stability (not shown) of the fabricated GO/PM composite modified electrode for detection of 100 μM CC by CC up to 4 weeks by CV. The experimental conditions are similar to Fig. 2. The sensor retains 90.1% of initial oxidation peak current response to CC after the 4-week storage, which indicates the high stability and reusability of the composite modified electrode for the detection of CC. Hence, the result proves that the fabricated sensor is highly stable for long time use.
Conclusions
In summary, a sensitive and selective CC sensor has been developed using an electrochemically derived GO/PM composite modified electrode. The sensor exhibited a low LOD (8 nM) with an appropriate analytical features (sensitivity and linear response range) than previously reported nanomaterials based CC sensors. As a proof of concept, the sensor was also successfully applied for the detection of CC in different water samples, and the recoveries of CC were highly satisfactory with the standard method. The sensor showed a high selectivity towards CC in the presence of potentially active interfering compounds. However, the sensor also has some limitations, such as selectivity in the presence of high concentrations of HQ. As a future perspective, the GO/PM composite can be used for accurate detection of CC in environmental samples.
References
Barner AB, Bongat AFG, Demchenko AV (2004) “Catechol” in encyclopedia of reagents for organic synthesis. J Wiley & Sons, New York
Zhao DM, Zhang XH, Feng LJ, Jia L, Wang SF (2009) Simultaneous determination of hydroquinone and catechol at PASA/MWNTs composite film modified glassy carbon electrode. Colloids Surf B 74:317–321
Chen HL, Yao Y, Wang F, Choi MMF, Bramanti E, Zaray G (2009) Study on the toxic effects of diphenol compounds on soil microbial activity by a combination of methods. J Hazard Mater 167:846–851
Feng SQ, Zhang YY, Zhong YM, Li YC, Li SX (2014) Simultaneous determination of hydroquinone and catechol using covalent layer-by-layer self-assembly of carboxylated-MWNTs. J Electroanal Chem 733:1–5
Si WM, Lei W, Han Z, Hao QL, Zhang YH, Xia MZ (2014) Selective sensing of catechol and hydroquinone based on poly(3,4-ethylenedioxythiophene)/nitrogen-doped graphene composites. Sensors Actuators B Chem 199:154–160
Huang YH, Chen JH, Sun X, Su ZB, Xing HT, Hu SR, Weng W, Guo HX, Wu WB, He YS (2015) One-pot hydrothermal synthesis carbon nanocages-reduced graphene oxide composites for simultaneous electrochemical detection of catechol and hydroquinone. Sensors Actuators B Chem 212:165–173
Marrubini G, Calleri E, Coccini T, Castoldi AF, Manzo L (2005) Direct analysis of phenol, catechol and hydroquinone in human urine by coupled-column HPLC with fluorimetric detection. Chromatographia 62:25–31
Moldoveanu SC, Kiser M (2007) Gas chromatography/mass spectrometry versus liquid chromatography/fluorescence detection in the analysis of phenols in mainstream cigarette smoke. J Chromatogr A 1141:90–97
Nagaraja P, Vasantha RA, Sunitha KR (2001) A sensitive and selective spectrophotometric estimation of catechol derivatives in pharmaceutical preparations. Talanta 55:1039–1046
Cui H, He C, Zhao G (1999) Determination of polyphenols by high-performance liquid chromatography with inhibited chemiluminescence detection. J Chromatogr A 855:171–179
Mesa JAG, Mateos R (2007) Direct automatic determination of bitterness and Total phenolic compounds in virgin olive oil using a pH-based flow-injection analysis system. J Agric Food Chem 55:3863–3868
He JH, Qiu R, Li WP, Xing SH, Song ZR, Li Q, Zhang ST (2014) A voltammetric sensor based on eosin Y film modified glassy carbon electrode for simultaneous determination of hydroquinone and catechol. Anal Methods 6:6494–6503
Li SJ, Xing Y, Deng DH, Shi MM, Guan PP (2015) A comparative study of different types of reduced graphene oxides as electrochemical sensing platforms for hydroquinone and catechol. J Solid State Electrochem 19:861–870
Roy S, Soin N, Bajpai R, Misra DS, McLaughlin JA, Roy SS (2011) Graphene oxide for electrochemical sensing applications. J Mater Chem 21:14725–14731
Li F, Jiang X, Zhao J, Zhang S (2015) Graphene oxide: a promising nanomaterial for energy and environmental applications. Nano Energy 16:488–515
He S, Chen Z, Yu Y, Shi L (2014) A novel non-enzymatic hydrogen peroxide sensor based on poly-melamine film modified with platinum nanoparticles. RSC Adv 4:45185–45190
Dorraji PS, Jalaliz F (2015) A nanocomposite of poly(melamine) and electrochemically reduced graphene oxide decorated with Cu nanoparticles: application to simultaneous determination of hydroquinone and catechol. J Electrochem Soc 162:B237–B244
Su YL, Cheng SH (2015) Sensitive and selective determination of gallic acid in green tea samples based on an electrochemical platform of poly(melamine) film. Anal Chim Acta 901:41–50
Peng J, Feng Y, Han XX, Gao ZN (2016) Simultaneous determination of bisphenol a and hydroquinone using a poly(melamine) coated graphene doped carbon paste electrode. Microchim Acta 183(7):2289–2296
Liu X, Luo L, Ding Y, Wu Q, Wei Y, Ye D (2012) A highly sensitive method for determination of guanine, adenine and epinephrine using poly-melamine film modified glassy carbon electrode. J Electroanal Chem 675:47–53
Rosy R, Goyal RN (2015) Gold nanoparticles decorated poly-melamine modified glassy carbon sensor for the voltammetric estimation of domperidone in pharmaceuticals and biological fluids. Talanta 141:53–59
Huang YH, Chen JH, Sun X, Su ZB, Xing HT, Hu SR, Weng W, Guo HX, Wu WB, He YS (2015) One-pot hydrothermal synthesis carbon nanocages-reduced graphene oxide composites for simultaneous electrochemical detection of catechol and hydroquinone. Sen Actuators B 212:165–173
Du H, Ye J, Zhang J, Huang X, Yu C (2011) A voltammetric sensor based on graphene-modified electrode for simultaneous determination of catechol and hydroquinone. J Electroanal Chem 650:209–213
Wang L, Zhang Y, Du Y, Lu D, Zhang Y, Wang C (2012) Simultaneous determination of catechol and hydroquinone based on poly (diallyldimethylammonium chloride) functionalized graphene-modified glassy carbon electrode. J Solid State Electrochem 16:1323–1331
Wang Z, Li S, Lv Q (2007) Simultaneous determination of dihydroxybenzene isomers at single-wall carbon nanotube electrode. Sen Actuators B 127:420–425
Yin H, Zhang Q, Zhou Y, Ma Q, Liu T, Zhu L, Ai S (2011) Electrochemical behavior of catechol, resorcinol and hydroquinone at graphene–chitosan composite film modified glassy carbon electrode and their simultaneous determination in water samples. Electrochim Acta 56:2748–2753
Ezhil Vilian AT, Chen SM, Huang LH, Ali MA, Al-Hemaid FMA (2014) Simultaneous determination of catechol and hydroquinone using a Pt/ZrO2-RGO/GCE composite modified glassy carbon electrode. Electrochim Acta 125:503–509
Wei C, Huang Q, Hu S, Zhang H, Zhang W, Wang Z, Zhu M, Dai P, Huang L (2014) Simultaneous electrochemical determination of hydroquinone, catechol and resorcinol at nafion/multi-walled carbon nanotubes/carbon dots/multi-walled carbon nanotubes modified glassy carbon electrode. Electrochim Acta 149:237–244
Zhang Y, Xiao S, Xie J, Yang Z, Pang P, Gao Y (2014) Simultaneous electrochemical determination of catechol and hydroquinone based on graphene–TiO2 nanocomposite modified glassy carbon electrode. Sen Actuators B 204:102–108
Wang Y, Xiong Y, Qu J, Qu J, Li S (2016) Selective sensing of hydroquinone and catechol based on multiwalled carbon nanotubes/polydopamine/gold nanoparticles composites. Sen Actuators B 223:501–508
Palanisamy S, Karuppiah C, Chen SM, Yang CY, Periakaruppan P (2014) Simultaneous and selective electrochemical determination of dihydroxybenzene isomers at a reduced graphene oxide and copper nanoparticles composite modified glassy carbon electrode. Anal Methods 6:4271–4278
Acknowledgements
The project was supported by the Ministry of Science and Technology (MOST), Taiwan.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
The authors declare that they have no competing interests.
Electronic supplementary material
ESM 1
(DOCX 4.15 mb)
Rights and permissions
About this article
Cite this article
Palanisamy, S., Ramaraj, S.K., Chen, SM. et al. Voltammetric determination of catechol based on a glassy carbon electrode modified with a composite consisting of graphene oxide and polymelamine. Microchim Acta 184, 1051–1057 (2017). https://doi.org/10.1007/s00604-017-2073-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00604-017-2073-y