Abstract
This review (with 144 refs.) focuses on the recent advances in the preparation and application of magnetic micro/nanoparticles. Specifically, it covers (a) methods for preparation (such as by coprecipitation, pyrolysis, hydrothermal, solvothermal, sol-gel, micro-emulsion, sonochemical, medium dispersing or emulsion polymerization methods), and (b) applications such as magnetic resonance imaging, magnetic separation of biomolecules (nucleic acids; proteins; cells), separation of metal ions and organic analytes, immobilization of enzymes, biological detection, magnetic catalysis and water treatment. Finally, the existing challenges and possible trends in the field are addressed.

This review focuses on the recent advances in the preparation and application of magnetic micro/nano particles. Finally, the existed problems and possible trends in the field were discussed.
a: Fe3O4@SiO2-PVAm: polyvinyl amine-coated Fe3O4@SiO2
b: CTS/MMT-Fe3O4 microsphere: chitosan/montmorillonite-Fe3O4 microsphere
c: MTAMs: magnetic targeted antibiotic microspheres
d: SM: the code of iron oxide-silica composite microspheres
e: PSt: poly styrene
f: gamma-PGA- PLA: poly(gamma-glutamic acid) and poly(lactide)
g: poly(-MMA–DVB–GMA) microspheres: poly(methylmethacrylate–divinylbenzene–glycidylmethacrylate) microspheres
h: AEAPS: N-(2-aminoethyl)-3-aminopropyltrimethoxysilane
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Magnetic micro/nanoparticles general mean Fe-, Co-, and Ni- based ferromagnetic elemental, alloy, oxide, or composite structures. According to the different properties, materials are divided into three types: paramagnetic, antimagnetic and ferromagnetic materials. Paramagnetic materials, whose magnetization intensity is proportional to exterior magnetic field and their susceptibility are positive value. Antimagnetic materials, whose magnetization intensity also is proportional to exterior magnetic field, but their susceptibility are negative value. The magnetization intensity of ferromagnetic materials increases with the strengthening of exterior magnetic field observably at the beginning, but when the exterior magnetic field intensity increases to a value, their magnetization intensity will not continue to increase, which is saturation phenomenon. Theoretically, various inorganic materials including Fe3O4, γ-Fe2O3, CoFe2O4, MnFe2O4, CoPt3 and FePt can be used in microparticles to provide magnetism, among which Fe3O4 nanoparticles are most commonly selected because of their easy availability and superparamagnetism. Ideal microparticles can be easily trapped by an external magnet but appear individually dispersed when the magnetic field is removed.
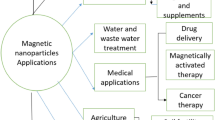
Ever since magnetic micro/nanoparticles are known, their preparation methods and applications have been the key point of research (Fig. 1). Their preparations can be divided into physical and chemical methods. The physical methods mainly are mechanical attrition method, magnetic materials being ground to a nanometer size, which is easy to operate but has long production cycle. Nowadays, the chemical methods primarily involve coprecipitation [1, 18], thermal decomposition [19, 20], hydrothermal [21–26], sol-gel [27–32], and micro-emulsion methods [33]. On the one hand, some of them are entirely different, such as coprecipitation method and micro-emulsion method, compared with the former one, the latter can avoid the products to aggregate effectively. On the other hand, the products prepared by those methods may have same properties. For example, the products prepared by pyrolysis and hydrothermally both have the advantages of tunable size and narrow diameter distribution [19].
Microparticles have good affinity [34, 35] and easy surface modification. They can combine kinds of functional groups and bioactive substances, such as enzymes, cells, antibodies, DNA and so on, by adsorption or covalent bonding. It is easy for them to separate from medium under external magnetic field due to their good magnetic conductivity, so they are widely used in the area of cell separation [7, 36–38], biological detection [39–44], enzyme immobilization [45, 46], solid-phase extraction and targeted drug delivery [8, 25, 47–49]. Moreover, they have good chemical stability, biocompatibility and biodegradability, which makes them safe in the clinical applications, so they also are used for MRI [50–53] and targeting drug delivery. For serious diseases, especially the premenstrual syndrome and effective treatment for cancer, microparticles have broad application prospect.
Although a few reviews about micro/nanoparticles have been reported up to now, those reporters did not focus on their current status and highlighted applications. Recent advances in magnetic micro/nanoparticles in this work, are reviewed detailed and timely, including preparation methods and related applications. We also speculate on their future and potential development for their use in biomedical field and sample treatment.
Preparations
Magnetic nanoparticles are usually embedded in some matrix or coated with a matrix layer. Generally speaking, the preparation includes two steps, the preparation of magnetic nano/μ particles and the combination of those particles and the matrix. Obviously, the nanoparticles prepared by kinds of methods have variable size (Figs. 2 and 3), which can be controlled by varying experimental parameters such as reaction time and temperature. Kim et al. [54] presented a rapid and reliable method using matrix-assisted laser desorption/ionization time of flight (MALDI-TOF) mass spectrometry MS in order to determine the sizes (Fig. 4).
Small magnetic nanoparticles prepared by (a) in situ solvothermal synthesis, (b) sol-gel method, (c) thermal decomposition and (d) microemulsion progress. Reprinted with permissions: (a, c) from [19, 55] copyright 2003, 2001 American Chemical Society; (b) from [29] copyright 2008 Elsevier; (d) from [56] copyright 2005 John Wiley and Sons
Large magnetic nanoparticles prepared by (a) one-pot hydrothermal process, (b) sol-gel method, (c) solvothermal method and subsequent calcining process and (d) self-assembly of nanocrystal in mixed solvents of ethylene glycol and water. Reprinted with permissions: (a, b, c, d) from [26, 31, 57, 58] copyright 2015, 2009, 2009, 2013 Elsevier
a Mass spectra of sample aliquots drawn from the reaction solution during heating. For clarity, the spectra in the mass ranges of <10.5 kDa (left) and >11 kDa (right) have been plotted using different scales. b TEM images of the NCs in the sample aliquots. c Schematics describing the formation mechanism proposed on the basis of the ex situ MS measurements. Reprinted with permission from [54]. Copyright (2013) American Chemical Society
Several methods (Table 1) have been proposed for the synthesis of naked magnetic particles, such as coprecipitation method, pyrolysis method, hydrothermal method, while other methods are actually on the purpose of binding magnetic particles with matrix, such as sol-gel method, and micro-emulsion method. It is worth nothing that magnetic micro / nanoparticles are usually coated with a long-chain hydrocarbon layer that provides steric repulsion for particles stabilization in solvents. The most common methods used for surface modification of magnetic micro / nanoparticles are ligand exchange, ligand addition, and hydrophilic SiO2 coating. Many reagents have been developed for protection/stabilization/functionalization, such as dopamine, poly (ethylene glycol) (PEG), 2,3-dimercaptosuccinic acid (DMSA), PEG-phospholipid copolymer and SiO2.
Coprecipitation method
Coprecipitation is a simple and efficient method for preparing iron oxide (Fe3O4, Fe2O3, etc.) and ferrite (Zn-Mn ferrite, Ni-Zn ferrite, Co-Zn ferrite) widely. In this method, alkaline solution (ammonia, sodium, hydroxide solution) is added into metallic salt solution as precipitant, making metal ions precipitate from the solution. This method has the advantages of shorter process, simple reaction conditions and higher product purity. But the agglomeration phenomenon of product will happen easily during washing, filtering and drying.
The coprecipitation method is most widely used to prepare ferroferric oxide magnetic microparticles:
Fe2+ with Fe3+ were mixed up according to certain proportion, and then excess ammonia water or sodium hydroxide solution was added as precipitant, as a result Fe3O4 magnetic microparticles were made. Kim et al. [1] added sodium oleate as surfactant to disperse precipitates in the process of preparing Fe3O4 magnetic microparticles by coprecipitation method, thus getting monodisperse Fe3O4 magnetic microparticles. Thapa et al. [18] prepared Fe3O4 magnetic microparticles by coprecipitation method, and they found out that when the particle size was 10 nm, the magnetic microparticles had best magnetism. When preparing ferrimagnetic microparticles, precursor powder was first prepared by coprecipitation method, and then the final product was fabricated after high temperature solid state reaction.
Pyrolysis method
Magnetic micro/nanoparticles can also be prepared by the thermal decomposition of precursors such as metal compounds ( Fe(acac)3, Fe(CO)5, Co2(CO)8 and Fe(Cup)3 (acac and Cup are acetyl acetone and cup ferrate respectively )) at high temperature and high pressure. Then they are further oxidized to magnetic metal oxide nanoparticles. The method has the advantages of high nanoparticle crystallinity, tunable size and narrow diameter distribution [19].
Under high temperature, Murray, Sun et al. [20] prepared ferrimagnetic nanoparticles, whose size ranged from few nanometers to tens of nanometers, by refluxing ferric acetylacetonate, long chain alcohol, oleic acid, lauryl amine and so on, with diphenyl oxide as medium.
Hydro-thermal method
The hydrothermal method is that under high temperature and pressure condition of autoclave, with water as reaction medium, those substances, which are usually sparingly soluble and insoluble, are dissolved and react, then recrystallize, thus getting ideal product. High temperature is beneficial to improve magnetism, and high pressure is good to improve product purity. Hydrothermal method can control the size and shape of particles effectively, and particles seldom agglomerate. Besides, the particles have a good dispersibility [24], and uniform size distribution [22]. The hydrothermal method can make substances react in static or dynamic closed environment, and the latter can speed up synthetic rate greatly due to the magnetic stirrer in autoclave.
Chen et al. [21] prepared Fe3O4 magnetic microparticles with different diameters in hydrothermal reaction vessel. Yao [23] et al. prepared magnetic carbonaceous (MC) microparticles, by two-step hydrothermal reactions.
At low temperature, Fe3O4 microparticles assembled by specific nanoparticles [24] are obtained via hydrothermal method, and the products still have good dispersibility. Combined with other synthetic processes, core–shell structured magnetic composite microparticles with ordered hexagonal mesopores, bright luminescence, and high magnetization saturation value are prepared [25]. This multifunctional system shows positive sustained properties by the surface modification, which can be potentially used as targeted drug delivery system. More recently, Liu et al. [26] prepared Water-dispersible monodisperse hollow Fe3O4 microparticles via a one-pot hydrothermal process which exhibited superparamagnetic properties with high saturation magnetization value of about 76.7 emu·g−1 at room temperature, and whose bet surface area was 50.04 m 2⋅ g−1.
The microwave hydrothermal synthesis method combines traditional hydrothermal method with microwave field. With microwave field as heat source, the reaction makes place in special reactor, through which microwave field can pass.
Solvent-thermal method
The solvent-thermal method is similar to hydro-thermal method, except the solvent of the former is nonaqueous solution.
Liang [57] et al. firstly prepared a kind of maghemite microparticles with such high surface area of 82.7 m2·g−1 by the solvothermal method and subsequent calcining process.
Recently, Lu et al. [58] synthesized sodium polyacrylate modified Fe3O4 magnetic microspheres (SPMFMs) with quasi-superparamagnetic behavior and high saturation magnetization by a solvothermal method. The loading capacity of the SPMFMs for bovine hemoglobin was 95 mg·g−1, and 33 % of the loaded bovine hemoglobin was released in a period of 75 h. The SPMFMs after the adsorption of the organic pollutants can be recycled and reused effectively with a slightly reduced adsorption capacity. Jiang et al. fabricated magnetically separable BiOBr/CoFe2O4 microparticles assembled from nanoparticles by a facile solvothermal method at 160 °C for 12 h. [59] Zhu et al. reported an immobilization of Candida rugosa lipase (CRL) onto PAMAM-dendrimer-grafted magnetic nanoparticles synthesized by a modified solvothermal reduction method. And this immobilized lipase exhibited excellent reusability [60].
Sol-gel method
The sol-gel method is frequently performed by preparing sol with metal organic compound solution or metal inorganic compound at first, and then the sol is dehydrated under certain conditions (heating) to get gel, at last nano-scale product is prepared with the gel after drying and roasting. This method has the advantages of mild reaction conditions, high product purity, accurate stoichiometry, simple process and a short reaction period, and usually used to prepare the core-shell SiO2 magnetic composites [27, 28, 31].
Liu [29] et al. prepared a new kind of magnetic luminescent nanocomposite (MLNC) particles by a combination of sol-gel process and electrostatic self-assembly techniques. Xu et al. [30] prepared Fe3O4 magnetic microparticles with different size after annealing treatment under the vacuum condition of 200 to 400 degrees Celsius. Shao [31] et al. first made a study on the separation of protein by submicron-size microparticles grafted with flexible polymer chains. Liu et al. successfully prepared Silica encapsulated core–shell structured carbonyl iron (CI) magnetic particles (CI@SiO2) via a facile sol–gel method based on the silane grafted CI particles, which shows enhanced property of heat-induced oxidation resistance with decreased particle density [32]. Xu et al. successfully fabricated novel core–shell structured magnetic Fe3O4/silica nanocomposite with gridlock-copolymer grafted on their surface (Fe3O4@SiO2@MDN) by sol–gel method and a seeded aqueous-phase radical copolymerization approach. [61] It has the excellent characteristics of the strong magnetic responsivity, outstanding hydrophilicity and abundant π-electron system.
Micro-emulsion method
The micro-emulsion method has been developed into an effective method of preparing magnetic microparticles. The micro-emulsions are transparent, isotropic and low viscosity thermodynamic stability system, which is made from oil (hydrocarbon), water (electrolyte aqueous solution) and surfactant (sometimes with alcohols as cosurfactant.). They are divided into water in oil type (W/O) and oil in water type (O/W). The droplet size of them is nanometer and the droplet separates from each other. The reaction space is limited to this micro-reactor – droplet. As an example, surfactant succinic acid-1-ethyl hexyl sodium sulfonate (AOT) dissolving in n-hexane can become water in oil type reversed-phase microemulsion system [33]. This method can make particles avoid agglomerating effectively, so it is easy to prepare magnetic microparticles with narrow particle size distribution, regular shape and good dispersion property.
Zhou et al. [62] prepared Fe3O4 magnetic microparticles with particle size of smaller than 10 nm, which had high coercivity, in the O/W microemulsion system with cyclohexylamine as oil phase, Fe2SO4 and Fe(NO3)3 as aqueous solution. Chen et al. [2] prepared chitosan/montmorillonite–Fe3O4(CTS/MMT–Fe3O4) microparticles, a magnetically separable adsorbent, by microemulsion process.
Sonochemical method
The ultrasonic vaporization bubble produced by ultrasonic wave [3] can make local high temperature and high pressure environment come into being and has micro-jet with strong impulsive force, which promotes oxidation reaction, reduction reaction, decomposition reaction, hydrolysis reaction and so on for preparing micro/nanoparticles. The application of ultrasonic technology, there not being special requirements to the properties of this system, only requests for liquid medium for energy transmission and has strong universality to all kinds of reaction medium. Compared with conventional stirring technology, ultrasonic cavitational effect produces shear action on agglomeration, which is beneficial to form small particles, and it makes the uniform mixing of medium easier to be realized, avoiding inhomogeneous local concentration, improving reaction rate.
Vijayakumar et al. [4] prepared superparamagnetic Fe3O4 magnetic microparticles with the particle size of 10 nm from acetic acid ferric salt solution at Ar atmosphere of 0.15 MPa in the high-intensity ultrasonic wave environment. Gedanken et al. [63] prepared Fe-Fe3C magnetic microparticles with stability in air and controllable particle size by this method. Most recently, Wu et al. [5] prepared magnetic targeted antibiotic microspheres (MTAMs)with ultrafine size, high biocompatibility, biodegradability, controlled-release, and antibiotic effect by a sonochemical method in the presence of hydrophobic Fe3O4 nanoparticles and tetracycline.
Medium dispersing method
The medium dispersing method is a new method for preparing magnetic micro/nanoparticles with superior thermal stability, great oxidation resistance, outstanding dispersity and small particle size. In this method, the usual mediums are nano-silica, meso-material and nano-ceramic.
Silica as carrier
Hong et al. [64] made ferric nitrate disperse in nano-silica by mechanical attrition method, then acetic acid was added to produce ferrite, then the solvent is removed via rotary evaporation following calcining, finally getting γ- Fe2O3/SiO2 composite magnetic microparticles with small particle size, monodispersity and excellent thermal stability.
Carbon nanotubes as carrier
Since discovered in 1991, carbon nanotubes have become the hotspot in many fields research such as physics, chemistry, biology, material science and etc. Its special one dimensional quantum nanostructure and remarkable properties make it widely applied in many areas such as machinery, medical treatment, and electronic industry. Many substances are coated on the carbon nanotubes or filled in it, endowing it with new functions. Among them, the composite material of carbon nanotubes and magnetic microparticles has the excellent properties of both perfect oxidation resistance and chemical stability, exhibiting much potential application value, such as drug delivery, cellular control, catalysis and water purification detection.
Jiang et al. [55] prepared carbon nanotubes complex coated with Fe3O4 magnetic microparticles with the particle size of 20 to 30 nm. Pu et al. [65] prepared the carbon nanotubes complex tightly coated with Fe3O4 magnetic microparticles. Kim et al. [66] prepared γ- Fe2O3 magnetic microparticles on the carbon nanotubes wall by medium dispersing method.
Other meso-material as carrier
The meso-material is the porous material with pore size distribution of 2 to 50 nm. This kind of material has the advantages of light density, high porosity, good gas permeability and good permselectivity. Zayat et al. [67] chose Vycor glass as mesoporous support, soaked it in saturated ferric salt solution for a certain time, and treated it by heating in air to get Fe2O3 particles, then sample was reduced for 3 h in H2 of 360 °C, at last it was heated for 3 h in air of 240 °C to prepare γ- Fe2O3/ Vycor composite magnetic microparticles.
Emulsion polymerization
Emulsion polymerization is that with mechanical stirring, monomers disperse in water to become emulsion under the effects of emulsifier, then polymerize initiated by initiator. At least, the emulsion polymerization system is composed of monomer, initiator, emulsifier and water.
With controllable particle size and narrow size distribution, water-soluble microparticles have been investigated for desirable application in biotechnology. Liu [68] et al. prepared magnetic polymer-coated microparticles, which were linked well with the avidin and fluorescein isothiocyanate (FITC) antibody. Yang [6] et al. prepared moderately uniform magnetic poly methl methacrylate-divinylbenzene-glycidyl methacrylate(MMA-DVB-GMA) microparticles with better capacity of protein adsorption by spraying suspension copolymerization. Lu [69] et al. prepared non-porous core-shell magnetic polymer microparticles with a narrow size distribution and high saturation magnetization with little remanence using a novel micro-suspension polymerization technique. Sun [34] et al. first made a research on a simple one-step method to prepare magnetic polymer microparticles that have both controllable morphologies and –NH2 groups located on their surface. Chen [9] et al. reported a new approach to preparing super-paramagnetic magnetite/polystyrene (PSt) composite particles by inverse emulsion polymerization.
Shao [10] et al. prepared monodispersed magnetite/silica composite microparticles by modified miniemulsion polymerization. Zhang [70] et al. firstly prepared magnetic composite microparticles by a modified dispersion polymerization process. And it was found that the microparticles contained high magnetite contents, which is much higher than others reported thus far. Zhang [71] et al. prepared magnetic polymer enhanced hybrid capsules (MPEHCs) from a novel Picking emulsion polymerization. Later, Zhang [72] et al. prepared submicron magnetic composite microparticles by a new surfactant free controlled radical polymerization. Salih et al. prepared streptavidin-modified magnetic monodispersed poly (2-hydroxyethyl methacrylate) (STV-mag.PHEMA) microparticles by multiple swelling polymerization and firstly adapted STV-mag.PHEMA microparticles as solid support in a DNA-based protocol [73]. Yuan et al. prepared superparamagnetic polymer composite microparticles Fe3O4/P(GMA-AA-MMA) by soap free emulsion polymerization, which were used to support Schiff base palladium complex. And the supported magnetic catalyst acted as a true heterogeneous catalyst in the Suzuki coupling reactions. Furthermore, the novel catalyst can be conveniently recovered and reused at least seven times without significant loss of its catalytic activity or Pd leaching [74].
From above, in a word, further surface modification is required to yield hydrophilic particles. There are some of the strategies followed to achieve this are summarized in Table 2.
The application of magnetic micro / nanoparticles
With the development of magnetic microparticles, they are not only applicated to be ferrofluids [75–77], their applications but also have been expanded to a wide range of fields such as magnetic resonance imaging, magnetic separation, immobilized enzymes, biological detection, magnetic catalysts and water treatment.
Magnetic resonance imaging
Fe2O3 magnetic micro/nanoparticles have superparamagnetism, they themselves have no magnetism—magnetism comes into being under the effect of external magnetic field, and it disappears with external magnetic field disappearing, so they are safe and easy to be controlled in body. The property can be used for distinguishing healthy tissues and lesion tissues by the MRI contrast agent. Superparamagnetic Fe3O4 microparticles are injected into body combine with plasma proteins and are identified by reticuloendothelial cells under the effect of opsonin, and then the phagocytes of healthy tissues take in them as foreign body. When imaging, the magnetic microparticles weaken the signal of corresponding area, making image dark. However, the tumor tissues cannot absorb magnetic microparticles because of having no healthy phagocytes, forming sparkling spots.
Magnetic micro/nanoparticles are mainly used in those tissues with rich reticuloendothelial cells in MRI, e.g. digestive tract, liver, spleen and lymph [50, 51]. Moreover, as a kind of blood-pool agent for perfusion imaging, Fe2O3 magnetic microparticles are also used to diagnose cerebral or myocardial ischemic patients [52, 53].
Azhdarzadeh et al. [78] engineered super-paramagnetic iron oxide nanoparticles (SPIONs) for MRI contrast enhancement of colon cancer cells. The cells treated with aptamer-Au@SPIONs exhibited a higher death rate compared to control cells upon exposure to near infrared light (NIR), so MUC1-aptamer targeted Au@SPIONs are better to be used as actively-targeted dual-purpose agents for MRI of colon cancer in a drug-free approach.2 FITC-dextran dye entrapped and silica coated Gd2O3 nanoparticles (NPs) were prepared by Kumar et al. [79] for dual purpose of optical and magnetic resonance imaging. Entrapment of dye imparts thermal stability to NPs and enhances their fluorescence in comparison to bare dye. Moreover, particles do not possess cytotoxic nature. Holbrook et al. [80] prepared Gd(III)-dithiolane gold nanoparticles contrast agent that accumulates in the pancreas and provides significant contrast enhancement by MRI to allow early detection of pancreatic adenocarcinoma. Significant contrast enhancement was observed allowing clear identification of the pancreas with contrast-to-noise ratios exceeding 35:1. Zhang et al. [81] reported NaHoF4 and NaDyF4 nanoparticles with modulated sizes (9–40 nm) and shapes (spherical-like, hexagonal prism, rod-like), which is suitable for high-field MRI. They found that the NaHoF4 and NaDyF4 nanoparticles have r 2 relaxivities of (274.0 ± 6.9) × 104 and (4767.3 ± 160.9) × 104 mMNP −1 s−1 per nanoparticle and high r 2 /r 1 ratio of 781 and 410 at a high magnetic field of 9.4 T, respectively, making them indeed good candidates for high-field (>3 T) T 2 imaging contrast agents. A multifunctional theranostic magnetic mesoporous silica nanoparticle (MMSN) with magnetic core was developed by Chen et al. [82]. Platinum(IV) prodrug in MMSNs would be restored to active platinum(II) drug after active-targeting endocytosis by cancer cells in response to the innative reducing microenvironment in cancer cells. With an external magnetic field, drug loaded MMSNs showed high contrast in MRI in vivo and exhibited magnetically enhanced accumulation in the cancer site. Ni et al. [83] firstly reported high-performance nano-contrast agents for ultra-high field MR and CT dual-modality imaging. They synthesized and explored NaHoF4 nanoparticles (NPs) with varied particle sizes as high-performance dual-modality contrast agents for ultra-high field MR and CT imaging. Chen et al. [84] made a novel multifunctional envelope-type mesoporous silica nanoparticle (MEMSN) system with the advantages of pH-responsiveness, non-toxicity and biological specificity, which is demonstrated for magnetic resonance imaging (MRI). Watcharin et al. [85] investigated the possibility of Gd-DTPA and rhodamine 123 (Gd-Rho-HSA-NPs) -conjugated human serum albumin nanoparticles for detecting hepatocellular carcinoma (HCC) by T1-weighted MRI. The new non-toxic biodegradable NP-based MRI contrast media may provide additional tools for the detection and differential diagnosis of liver lesions, because they have different pharmacokinetics compared with the currently used small molecule contrast media. Gd-Rho-HSA-NPs enable sensitive detection of HCC by T1-weighted MRI in mice. And poloxamine coating of the NPs delayed the tumor localization of the NPs.
Magnetic separation
Separation of metal ions and organic analytes
Magnetic beads are widely used for preconcentration of metal ions and organic analytes. Bunkoed et al. [86] prepared Fe3O4/MWCNTs/alginate composite sorbent. Respectively, magnetic NPs are good to simple and fast separation of the sorbent, the π–π interactions of the MWCNTs with the aromatic rings of PAHs and their large surface area facilitate strong adsorption obviously, and the hydrophilicity of the calcium alginate cage strengthens the dispersity of the sorbent in the water sample. So the composite sorbent can successfully applied to extract polycyclic aromatic hydrocarbons (PAHs) from (spiked) water samples, which has several attractive advantages of easily preparation and environmentally friendliness, a convenient and fast extraction procedure, and high extraction efficiency. And maybe this method can be used to extract non–polar molecule from water samples. Kifle et al. [87] used a commercial cyanomodified microparticle-based solid phase as a sorbent for the retention and elution of ions of the precious metals Au, Pd, Ir, Pt, Rh and Ru, sometimes in the presence of nonprecious elements ions. As a result, only Au and Pd (in the form of their chloro complexes) were retained, and Au has a much higher affinity for the sorbent than Pd. Obviously, the method can separate Au or both Au and Pd from hydrochloric acid solutions containing ions of other elements, which is proved to have effectiveness selectivity, high stability and good reusability. Wang et al. [88] used magnetic microparticles (m-MPs) as the separation substrate for immobilizing target brain natriuretic peptide (BNP), which provides a general strategy for screening of specific aptamers against various analytes. And with the help of BNP-m-MP as immobilization matrix by SELEX., oligonucleotides are bound to be separated directly, quickly, and with high efficiency. Yang et al. [89] synthesized a graphitic carbon nitride (g-C3N4) nanocomposite with magnetite as a sorbent for solid phase extraction of phenolic acids. In combination with the magnetism of Fe3O4, the high affinity of g-C3N4 for phenolic acids provides an efficient means for magnetic solid phase extraction. The method has the advantages of low limits of detection, good linearity, and high recovery, indicating that g- C3N4/Fe3O4 has a potential application in the separation of phenolic compounds, organic acids, and even other bioactive compounds containing carbon-based ring structures, from biological samples.
Separation of nucleic acids
Separating and purifying nucleic acids by magnetic separation technology follow three steps, adsorption, washing and desorption. Nucleic acids are adsorbed on the magnetic micro/nanoparticles surface in high concentration denaturant or PEG. Then those magnetic micro/nanoparticles are separated from sample solution under external magnetic field. After washing, purified nucleic acids are obtained through desorption in water. This method can simplify and quicken the progress of nucleic acid separation and purification, realizing the miniaturization, automation and parallelization of nucleic acid purification treatment. Oster et al. [12] separated DNA from 1 μL,10 μL,100 μL and 10 mL blood using M-PVA magnetic beads with 60 % Fe3O4 and low bonding rate non-specific protein, that had high yield, Among them, the DNA yield of 10 mL fresh blood was about 200 ~ 300 μg. Zhang [90] et al. prepared porous magnetic silica microparticles, which were used as solid-phase adsorbents for the extraction of genomic deoxyribonucleic acids (DNA) from biological samples. Zhang [91] et al. modified magnetic silica microparticles and compared with other magnetic silica microparticles bonded with epoxide, diol and carboxyl, respectively, the magnetic silica microparticles surface-modified with silanol groups produced the highest DNA recovery yields. Shao [10] et al. prepared monodispersed magnetite/silica composite microparticles (Fig. 5).
Schematic illustration of the procedure for synthesis of composite microparticles embedded with iron oxide nanoparticles. Reprinted with permission from [10] copyright 2008 Elsevier
The silica composite microparticles with high magnetite content and good uniformity can effectively separate the plasmid DNA from its solution under quickly magnetic manipulation. In 2009, solid-phase extraction has been widely reported for the preparation of DNA templates for polymerase chain reaction (PCR)-based analytical methods [92]. The purified DNA templates were amplified by PCR for screening of genetically modified organisms (GMOs). The magnetite-loaded silica microparticles encapsulated with silica shells served as great adsorbents in extraction and purification of DNA from raw and processed foodstuffs and the DNA templates obtained were amplifiable for rapid detection of GMOs in food. Later, Liu [93] et al. reported a facile approach to synthesizing high-magnetization γ-Fe2O3/alginate/silica microparticles. The composite microparticles with a typical average diameter of 4.4 m were spherical and superparamagnetic. The products were convenient to use, reliable, cheap, amenable to automation, and more importantly, time-saving, which will allow them to serve as ideal candidates for isolation of DNA, cancer diagnosis and treatment and drug delivery system. Gao [35] et al. demonstrated for the first time that the SA (streptavidin)-CBD (cellulose binding domain)-MCMS (micron-sized magnetic cellulose microparticles) created an effective affinity condition on paramagnetic particles that allows for a one-step isolation of mRNA from eukaryotic cells and tissues.
The basic principle of separating nucleic acids with specific base pair fragment by magnetic microparticles is that a length of primer chain is coupled with complementary base pair to target nucleic acid, and then nucleic acid is separated and purified with complementary base pairing effect between nucleic acid molecular. Compared with traditional one, this method has the advantages of simplicity, speediness and high selectivity.
Separation of protein
The separation of protein is that modifying magnetic microparticles surface with functional groups, which can recognize target proteins and have the ability of reversible binding of the functional group, then separating target protein directly by the operation of affinity adsorption, magnetic field separation, desorption, washing and etc. It is fast and has high purity and recovery rate (Table 3).
Obrien et al. [94] coupled imine iminodiacetic acid chelate copper ion on the surface of pass-free magnetic microparticles, whose adsorption capacity to cytochrome C and hemoglobin with much histidine was over 200 mg·g−1. Liu [13] et al. prepared magnetic silica nanospheres coupled with organosilane on the surface, which were treated by the amino-silane coupling agent. Because this kind of microparticles has active groups of –NH2 to be transformed to aldehyde groups, they can easily connect to proteins and enzymes via Schiff base linkage. Yang [6] et al. prepared moderately uniform magnetic poly (−MMA-DVB-GMA) microparticles modified by ethylene diamine (EDA) with amine groups which can be easily connected to proteins and enzymes. Shamim [95] et al. prepared a kind of thermosensitive polymer coated nanomagnetic adsorbents with high protein adsorption of 104 mg·g−1. And these thermosensitive nanoparticles were used as a bioseparation tool for the separation of BSA. The adsorption and desorption was done quickly due to their high adsorption capacity. Wang [96] et al. prepared amino-silane modified magnetic composite spheres, which are the effective supports for bioseparation, and the maximum bovine serum albumin immobilization capacity of them is up to 87.4 mg·g−1 in 0.1 mol·L−1 phosphate buffer at pH .0. Tsai [14] et al. found a method of protein detection based on a competitive immunoassay of magnetic separation in thin channels using functional magnetic nanoparticles (Fig. 6). Gasilova et al. chose C8-functionalized mesoporous magnetic microparticles as a sorbent, spatially confined with an applied magnetic field, which ensures a selective enrichment and analysis of large hydrophobic peptides (2.5–7 kDa) [97].
Schematic diagram of the competitive immunoassay for IgG detection. Reprinted with permission from [14] copyright 2009 Elsevier
The linear range of IgG detection was from 5.0 × 10−8 to 1.0 × 10−11 M, the detection limit was 3.69 × 10−12 M, and the running time was less than 10 min. In addition, selectivities were higher than 92 % and the relative errors were less than 7 %. The recoveries of IgG spiked in serum were found to be higher than 94 %. Above results all indicate that this method can provide simple, fast, and selective analysis for protein.
Separation of cells
Magnetic micro/nanoparticles with specific ligand (antibody) bonded on the surface are mixed and incubated with cell stock solution, then they are magnetic separated, finally polymer micro/nanoparticles are separated from them to realize the separation of cells. Magnetic polymer microparticles can be used to separate unwanted cells (negative voltage separation) and enrich needed cells (normal-phase separation) by choosing different surface specific antibody. Compared with fluorescent activated cell separation method and affinity cartridge separation method, immune magnetic cell separation method has the advantages of cheapness, simple operation, no effect on cell activity and easy amplification operating when needing to separate a certain kind of living cells. Traditional centrifugation and filtration methods for separating cells need a lot of shear stress and need breaking cells. This disadvantage can be overcome by magnetic separation method. The application of immune magnetic micro/nanoparticles is a revolution in the history of bioseparation, which is used widely, and it is one of the typical applications of magnetic micro/nanoparticles.
Poynton et al. [36] fabricated ligands (antibiotic protein, plant condensation and etc.) bonded on the surface of magnetic microparticles and separated T cells from bone marrow for treating leukemia. Wang et al. [37] made the cyclin express on the surface of breast tumor cells combine with anti cycle protein antibody specifically and separated MCF-7 breast tumor cells from serum solution by using supermagnetic γ- Fe2O3-CdSe-ZnS nucleus-shell composite magnetic microparticles with anti cyclin antibody. Chen [7] et al. prepared immunomagnetic nanoparticles. The silica-coated superparamagnetic nanoparticles modified with N-(2-aminoethyl)-3-aminopropyltrimethoxysilane (AEAPS) were covalently immobilized with anti-CD34+ monoclonal antibodies on the surface, which can rapidly and conveniently separate the CD34+ cells with high efficiency and specificity than normal ones.
Immobilized enzymes
Immobilized enzymes is a kind of technology of that the enzymes limited to a certain area by solid material still can go on its special catalytic reaction and they can be recycled. As immobilized enzymes, magnetic microparticles have the advantages of enhancing the activity and stability of enzyme, separating enzymes from the system simply and effectively by magnetic separation method, reducing cost, improving usage rate, decreasing the operation in reaction system and making them suited to large-scale continuous production. And the external magnetic field can control the motion mode and direction of magnetic material immobilized enzymes, improving the catalytic efficiency of immobilized enzymes. Demirel [45] et al. improved the functions of the popular adsorbing agent Duolite A568 by a simple process. The products have become magnetic with the g factor of 3.5. In addition, immobilization capacity for the enzyme was not changed and the model enzyme activity remains the same as that of free enzyme. After activated by thionyl chloride, the magnetic microparticles with carboxyl on the surface can make peptide bond with amino [46]. Lei et al. kept papain fixed on magnetic carrier directly using this reaction and the fixed enzymes had good catalytic activity and stability.
Sun et al. [101] used Aldehyde- and NHS-activated magnetic microparticles to immobilize trypsin (CHO-trypsin and NHS-trypsin), and NHS-trypsin provided greater sequence coverage and identified more peptides for the digestion of bovine serum albumin. A 1-min digestion at room temperature using the immobilized trypsin also identified more peptides (96 ± 6 vs. 48 ± 1) and produced higher sequence coverage (90 ± 2 % vs. 75 ± 2 %) than traditional free trypsin digestion for 12 h at 37 °C. Zhang et al. [15] prepared magnetic microparticles with carboxyl groups by copolymerization of vinyl acetate (VAC), acrylamide (AM), and acrylic acid (AA). They were applied as support to immobilize lipase via physical adsorption and covalent binding (Fig. 7). Microparticles with different hydrophobicities/hydrophilicities had different immobilized ratios and different activity recovery. Compared with microparticles having hydrophilic characteristics, that with hydrophobic characteristics had a much higher lipase binding efficiency.
Scheme of lipase immobilization onto support with moderate hydrophobicity/ hydrophilicity. Reprinted with permission from [15] copyright 2012 Elsevier
Biological detection
The application of magnetic microparticles mainly is the early diagnosis on the diseases of bacteria and virus and the detection to microorganism in water or food. The principle of detection is based on immunoreactions between antigen and antibody, radiolabelling, fluorescent labeling and so on. Besides, the combination of magnetic microparticles and biosensor can improve the detection sensitivity of biosensor effectively.
Hallier-Soulier et al. [39] enriched a kind of protozoa, which can cause enterogastric diseases in water environment by immunomagnetic microparticles, detected them by PCR detection method. And the sensitivity of this method was up to the level of detecting one oocyst for every 100 mL water. Perez et al. [40] can detect quite low concentrated herpes virus and adenovirus in serum with the help of MRI technology by using the recognition effect between antigen and antibody, under the influence of the magnetic microparticles coupled with antiviral antibody to hydrogen proton spin relaxation time of surrounding water molecule, leaving out the PCR operation and simplifying detection process effectively.
In tests of hemolysis, Chen et al. found that the MMPs displayed the weakest hemolytic activity [41], namely only about 6 % at the highest concentration (20 mg·mL−1). On the one hand, polymer-coated MMPs created less toxicity in red blood cells than uncoated Fe3O4 nanoparticles. On the other hand, the MMPs were shown to be less genotoxic than Fe3O4 nanoparticles by measuring the micronucleus (MN) frequency in CHO-K1 cells. Those evidence of low toxicity presented in the results is indicative of that the Fe3O4-poly(L-lactide)-poly(ethylene glycol)-poly(L-lactide) (Fe3O4–PLLA–PEG–PLLA) MMPs had great potential biomedical applications.
Chen et al. [42] used Fe3O4@MIL-100 core–shell magnetic microparticles, for the first time, as the sorbent for the magnetic solid-phase extraction (MSPE) of polychlorinated biphenyls at trace levels in environmental water samples, demonstrating that the Fe3O4 @MOF core–shell magnetic microparticles were promising sorbents in the MSPE of aromatic pollutants from environmental water samples.
The Helminthex™ method [43] is a very sensitive technique for detection of Schistosoma eggs and exhibits 100 % sensitivity at 1.3 eggs per gram of faeces, which is enough to detect even low-level infections. Further understanding the underlying egg-microparticle interactions would enable a targeted optimisation of egg-particle binding and may thus enable a significant improvement diagnostic sensitivity in areas with low infection rates.
In a further study, Plichta et al. [44] presented one opinion that a nanobiosensor based on the use of porous magnetic microparticles (PMM) as efficient capturing/preconcentration platform for detection of Alzheimer’s disease (AD) biomarkers.
These PMMs were prepared by a multistep swelling polymerization. Novel properties of PMMs in terms of high functionality and high active area available for enhanced catalytic activity of the captured AuNPs electrocatalytic tags were exploited for the first time. What is more, the optimized and characterized PMMs are also applied for the first time for the detection of beta amyloid and ApoE at clinical relevant levels in cerebrospinal fluid (CSF), serum and plasma samples of patients suffering from AD.
Orlov et al. developed dry-reagent immunomagnetic (DRIM) biosensing platform, which combines the advantages of immune chromatography with highly sensitive quantification of 200-nm magnetic nanoparticles (MP) from the entire volume of lateral flow membranes, for rapid high-precision quantitative analyses for simple, rapid and sensitive quantification of protein biomarkers for in vitro diagnostics both in laboratory and near-patient conditions, for food analysis, environmental monitoring, security, and safety applications [102].
Magnetic catalysts
Semiconductor photocatalysts, especially TiO2, attract wide attention of human because of their potential application for removing all kinds of pollutants in water and air. However, due to beset by the difficulties of recycling nanoscale TiO2 microparticles from water, their application still remains a challenge to a great extent now. In order to overcome the difficulty of separating catalyst, the engineered photocatalysts, with TiO2 coated on carriers (glass beads [103], glass fiber [104], zeolite [105]) have been reported. However, the small specific surface area of those carriers used by photocatalysts reduces their own photocatalyst activity. Because magnetic nano-particles have the advantages of high specific surface area and being recycled quickly and conveniently in the external magnetic field, they are used to be catalyst carrier, making the prepared composite TiO2 photocatalysts have the good activity of powdery nanoscale TiO2 and easy to be recycled by external magnetic field, as a result, they are getting more and more attention.
Beydoun et al. [106] reported the study on the preparation of magnetically separable photocatalysts with TiO2 carried on Fe3O4. Chung et al. [16] prepared the photocatalysts with NiFe2O4 as carrier at high temperature by multistep ultrasonic injection method. Specifically, a simple and green method for the deposition of gold nanoparticles (Au NPs) on the surface of polydopamine (PDA)-encapsulated Fe3O4 nanoparticles was proposed to fabricate a core–shell Fe3O4@PDA–Au nanocatalysts (Fig. 8) [107]. Both the size of Au NPs and the thickness of PDA layer were tunable to load more Au NPs with appropriate size on the PDA coating. The Au content on Fe3O4@PDA–Au nanocomposites endowed the nanocatalyst with high catalytic performance in the reduction of o-nitroaniline to benzenediamine by NaBH 4 (with a conversion of 99 % in 7 min).
Schematic diagram of the synthetic strategy, possible mechanism, and application of Fe3O4@PDA–Au nanocomposites. Reprinted with permission from [107] copyright 2013 Elsevier
Most crucially, the catalyst can be easily recycled by using an external magnetic field due to the high magnetization (39.6 emu·g −1) and showed excellent reusability (8 cycles with a conversion of >98 % for o-nitroaniline).The catalyst also showed good activity for the reduction of other nitrobenzene analogs. These were good to the practical application of the catalyst in reduction of nitroaromatic compounds.
Yang et al. [108] made l-4-Hydroxyproline successfully grafted onto the core–shell structural silica magnetic microparticles. They found that the synthesized catalyst can be rapidly separated from the reaction mixture through an external magnetic field and reused up to five runs without any obvious loss of activity, indicating its easy-separated property and excellent recyclability. Polyvinyl amine coated Fe3O4@SiO2 composite microparticles with a core-shell structure were prepared and employed as a magnetic catalyst for Knoevenagel condensation under mild conditions [17]. The catalyst can be readily recovered using a magnet and reused several times without loss in activity or selectivity. The performance of the magnetic base catalyst was compared with that of polyvinyl amine functionalized mesoporous SBA-15, which showed that the magnetic nanoparticles gave improved reaction rate and yield. (PW12 –TH) n multilayer films (PW12 = PW12O3− 40, TH = thionine) were deposited successfully on core–shell structured Fe3O4@SiO2 magnetic microparticles through layer-by-layer (LbL) self-assembly method. The microparticles exhibit better photocatalytic activity toward the degradation of methyl orange (MO) under visible light irradiation than the quartz slides support. In addition, the use of magnetic support guarantees facile, clean, fast, and efficient separation of the photocatalyst after the degradation of MO. Such catalysts can be reused several times and display good reproducibility by magnetic separation [109]. Jiang et al. prepared novel magnetically MIL-53(Al)@SiO2@Fe3O4 catalysts with different MIL-53(Al) contents through an in situ method. The results prove that the catalysts showed the excellent recycle rate and reusability. And the recovery catalyst can be reused for five times and the recycle rate remained above 99 % all the time [110]. Li et al. prepared successfully a ternary magnetic composite of Fe3O4@TiO2/SiO2 aerogel with good photocatalytic activity by combining sol-gel and simple hydrothermal methods, which meant that this material can serve as an efficient and recyclable multifunctional photocatalyst for the degradation of hazardous organic dyes in wastewater [111]. Zhang et al. first prepared Fe3O4/TiO2 mircospheres by the solvothermal method, and then Ag nanoparticles were anchored onto the out-layer of TiO2 by the tyrosine-reduced method. The magnetic Fe3O4/TiO2/Ag composite mircospheres were used as photocatalysis for the photocatalytic degradation of methylene blue [112].
Water treatment
The increasing contamination of surface and ground water by a wide variety of inorganic and organic pollutants is one of the major challenges faced by humanity at the beginning of the twenty-first century. What lead to the emissions of these pollutants, which tend to accumulate in the trophic chain, having a detrimental impact on ecosystem and our health, are the development of agriculture, industry and domestical activities. Magnetic hollow silica microparticles (MHSM) have potential application for adsorbent of heavy metal ions or organic pollutants. MHSM were successfully synthesized by combining a sol–gel technology with a low temperature drying by Shen et al. [113].
Treatment of heavy metal
Up to now, an army of work concerning wastewater treatment by magnetic micro/nanoparticles has been reported. For heavy metals, with great adsorption and reusability (Table 4), the microparticles are usually used to remove Cr(VI) or U(VI) from an aqueous solution [13, 88–92].
Podzus [114] et al. successfully prepared magnetic chitosan cross-linked microparticles with mechanical stability. The application of this magnetic chitosan for heavy metal ions removal appears to be technically feasible and efficient. Combined with reusability, this material seems to be a good candidate for wastewater treatment. Ozcan [115] et al. prepared functionalized superparamagnetic particles, which were an effective extractant for Cr(VI). Chen et al. [27] found that the extraction quantity of CTS/MMT–Fe3O4 microparticles for Cr(VI) decreased with the MMT content, which had high adsorption capacity and uniform desorption efficiency during the consecutive three-time adsorption–desorption processes of CTS/MMT–Fe3O4 microparticles, implying that the microparticle can be used as promising adsorbents for the removal of Cr(VI) from wastewater. Following that, Sun et al. [116] used polyethylenimine-functionalized poly(glycidyl methacrylate) magnetic microparticles, whose maximum adsorption capacity was evaluated to be 492.61 mg·g−1, for the removal of Cr(VI) in batch experiments. And this material can be reused without significant loss of adsorption efficiency. In conclusion, this material should be a promising adsorbent for the removal of Cr(VI) from waste water. Most recently, Zhao et al. [117] applied the prepared Fe3O4@SiO2-AO to adsorb U(VI) from aqueous solutions, which exhibited enhanced sorption capacity for U(VI) in comparison with Fe3O4 coated raw silica. Sun et al. [118] also generated a novel adsorbent, polyethylenimine-functionalized poly(vinyl alcohol) (PVA-PEI) magnetic microparticles, and used them as adsorbent to remove Cr(VI) from an aqueous solution. Owing to the large magnetic content, relatively high adsorption capacity and rapid adsorption rate, the PVA-PEI magnetic microparticles should be a promising adsorbent in the removal of Cr(VI) from wastewater. Sun et al. firstly developed a novel magnetic composite bio-adsorbent, graphene oxide and magnetic chitosan-ionic liquids (GOMCS-ILs) for removing Pb(II) from water, which demonstrated the potential applications of GOMCS-ILs microparticles in efficient removal of Pb(II) from wastewater and deep-purification of polluted water [119].
Treatment of organic pollutants
Compared with the heavy metal wastewater pollution mentioned above, Organic wastewater pollution is more common in the water environment. And organic contaminants such as phenol and chlorophenols in water have long been a serious issue to the environment even at a low level due to their resistance to natural degradation. Microparticles have been widely used for water treatment [57, 58, 129], because their advantages of high surface area, magnetic separation and reusability.
For organic pollutants, Liang [57] et al. prepared a kind of maghemite (γ-Fe2O3) with large surface area, which can remove the organic pollutants in water efficiently and be recycled simply by magnetic separation and regenerated by ethanol desorption. Li et al. [130] synthesized surface-imprinted core-shell magnetic beads, which showed outstanding affinity and selectivity towards bisphenol A (BPA) over structurally related compounds (Fig. 9). Lu et al. [58] prepared sodium polyacrylate modified magnetic Fe3O4 microparticles (SPMFMs). After the adsorption of the organic pollutants, SPMFMs can be quickly separated from the solution by a magnet in less than 1 min, and they can be recycled and reused effectively with a slightly reduced adsorption capacity, implying that the SPMFMs are promising for waste water treatment. Shi et al. found that, compared with the conventional drinking water treatment, the treatment with quaternized magnetic microparticles (NDMP) evidently reduced the concentrations of dissolved organic carbon, adsorbable organic halogens (AOX), bromide and disinfection by-products. As it can effectively reduce pollutant levels and the toxicities of drinking water, NDMP might be widely used for drinking water treatment in future [131]. Moreover, Zhou et al. [132] presented that the Fe3O4 coated SiO2 decorated multi-walled carbon nanotubes (Fe3O4@SiO2–MWCNTs) adsorbent was a potential low-cost effective material for pentachlorophenol (PCP) removal from contaminated wastewater. And Ma et al. [129] prepared chitosan/kaolin/Fe3O4 microparticles and used them as adsorbents for the removal of ciprofloxacin from aqueous solution. The adsorption capacity of the material showed no obvious deterioration in four adsorption–desorption cycles at least. In addition, the prepared microparticles had advantages of good adsorption capacity, regeneration property, and can be easily and rapidly separated (removed) from the solution phase with magnetic force, which improved their potential application in wastewater treatment. Lin et al. immobilized laccase onto Cu(II)- and Mn(II)-chelated magnetic microparticles and successfully applied them to remove bisphenol A (BPA) from water, which showed that metal-ion-chelated magnetic microparticles have great potential for industrial applications [133].
Synthesis route of surface-imprinted core-shell magnetic beads and their application for removal of BPA with the help of an applied magnetic field. Reprinted with permission from [130] copyright 2010 Elsevier
Conclusion and prospects
This paper has provided an overview of the synthetic approaches and highlighted applications of magnetic micro/nanoparticles. In this review, much work has been performed to resolve the existing challenges hindering the development of magnetic micro/nanoparticles.
However, there are still substantial development challenges and potential applications waiting for exploiting. Although nano-sized magnetic particles provide a large surface area, they suffer from low magnetic response in dispersed state. Considering the limited magnetic susceptibility, particle size should be increased in a reasonable range to obtained high magnetic response. Great reduction of microparticles surface area is avoidable if mesoporous materials are taken into consideration. Endeavors are also continually needed to provide low-cost, biocompatible and marketable magnetic micro/nanoparticles prepared by simple and cheap procedures. However, when it comes to the preparation of microparticles with uniform and controlled particle size, good dispersity, strong magnetic response and large specific surface area, the method itself and its reproducibility deserves equal attention and focus.
Multifunctional magnetic composites are interesting newcomers to the world of magnetic micro/nanoparticles. For example, magnetic carbon nanotubes have broad application prospects in the area of ultra-high density magnetic storage, environment pollutant treatment [134] and microwave absorption. Although the research on the physicochemical properties of magnetic carbon nanotubes is extensive, there still has a host of problems need to be deal with, such as the micromechanism, formation mechanism and magnetic properties of the composites. Developing more accurate qualitative and quantitative characterization technology and preparing magnetic carbon nanotubes arrays with uniform performance will become increasingly crucial. Moreover, magnetic micro/nanoparticles can be used for the magnetic graphene oxide composites preparation, making graphene functional effectively, and giving it new characteristics and broadening its applications, such as magnetic targeted drug delivery [135], MRI [135], solid-phase extraction [136] and waste water treatment [137]. In addition, microparticles have been applied to metal-organic framework preparation [138], which is used for magnetic resonance bimodal imaging, and magnetic nanoparticle composite [139] for controlled drug release.
The magnetic nanoparticles are also widely used in biomedical applications, such as magnetic targeted drug delivery system and magnetic resonance imaging, which brings much gospel to patients. Up to now, MRI has been usually applicated to diagnose a certain disease in those tissues with rich reticuloendothelial cells or cerebral or myocardial ischemic patients as a safe and controllable technology. Magnetic targeted drug delivery system promises to be a breakthrough point of tumor treatment. At the moment, the system is staying on the stage from animal experiment to clinical application. Magnetic targeting treatment used in the area of clinical treatment has been reported in German and America in succession [140]. There is still a series of obstacles before magnetic drug is applied to patients, for example, how to enhance the active targeting properties of carriers by changing their surface properties, how to protect magnetic drug from being phagocytosed by reticuloendothelial system (RES) system after drug delivery through blood vessel, how to avoid adverse reaction resulting from the accumulation of paramagnetic substance in blood system and how to track biological metabolic processes of magnetic particles and subcellular localization.
Magnetic micro/nanoparticles have brought sample treatment including wastewater and biological products some sunshine up to now. Compared to other processing techniques, microparticles have the advantages with faster analysis in large volumes of sample treatment [141] and smaller loss in solid-liquid mixture treatment. But the concerning applications should not be only connected to their nonspecific physical adsorption. The research focus in the future may be the modification of magnetic micro/nanoparticles. For example, molecularly imprinted polymer on the surface of magnetic micro/nanoparticles makes them have specific adsorption to different chemical substances. Therefore, they can remove pollutants from water samples more selectively and effectively.
Magnetic catalysts, which can be applied in the field of green chemistry, organic synthesis and sustainable and benign chemical transformations, may replace traditional catalysts in the future. Magnetic materials in this field began only in around 2008 and continue growing increasingly year in and year out. Magnetic catalysts can be easily, fast and cost-effectively separated from the reaction medium. And the recyclability and highly stability are the salient features for the popularity and sustainable applications of magnetic catalysts [142]. To produce magnetic catalysts endowed with both magnetic and catalytic properties, magnetic nanoparticles are often combined with metal catalysts or enzymes, among which, surface functionalization of magnetic nanoparticles is a subtly designed way to bridge the gap between heterogeneous and homogeneous catalysis. However, they suffer from unwanted aggregation and high iron leaching which generates undesired iron sludge and limits their reusability after the treatment [143]. The former question has been improved by transplanting catalyst onto pre-synthesized magnetic nanoparticles, as well as by the functionalization and modification of magnetic nanoparticles, and coating or encapsulating with stabilizing materials such as polymers, ionic liquids and carbon. But it is challenging whether their persistent activity is maintained over a period of time due to the poisoning or leaching of the catalysts in the reactions. Thus, the development of new magnetic catalysts demands further research to overcome these problems [144].
In conclusion, although there have been many exciting practical and/or potential applications of magnetic micro/nanoparticles, considerable challenges and issues remain unsolved. Nevertheless, the whole trend undoubtedly is that further utilization of magnetic micro/nanoparticles would potentially lead to a most attractive and promising research area all over the world.
References
Kim DK, Zhang Y, Voit W, Rao KV, Muhammed M (2001) Synthesis and characterization of surfactant-coated superparamagnetic monodispersed iron oxide nanoparticles. J Magn Magn Mater 225(1–2):30–36
Chen D, Li W, Wu Y, Zhu Q, Lu Z, Du G (2013) Preparation and characterization of chitosan/montmorillonite magnetic microspheres and its application for the removal of Cr (VI). Chem Eng J 221:8–15
Gedanken A (2004) Using sonochemistry for the fabrication of nanomaterials. Ultrason Sonochem 11(2):47–55
Vijayakumar R, Koltypin Y, Felner I, Gedanken A (2000) Sonochemical synthesis and characterization of pure nanometer-sized Fe3O4 particles. Mat Sci Eng A-Struct 286(1):101–105
Wu S, Jiang W, Zhang X, Sun H, Zhang W, Dai J, Liu L, Chen X, Li F (2012) A sonochemical route for the encapsulation of drug in magnetic microspheres. J Magn Magn Mater 324(2):124–127
Yang CL, Liu HZ, Guan YP, Xing JM, Liu JG, Shan GB (2005) Preparation of magnetic poly(methylmethacrylate-divinylbenzene-glycidylmethacrylate) microspheres by spraying suspension polymerization and their use for protein adsorption. J Magn Magn Mater 293(1):187–192
Chen W, Shen H, Li X, Jia N, Xu J (2006) Synthesis of immunomagnetic nanoparticles and their application in the separation and purification of CD34(+) hematopoietic stem cells. Appl Surf Sci 253(4):1762–1769
Liang HF, Yang TF, Huang CT, Chen MC, Sung HW (2005) Preparation of nanoparticles composed of poly(gamma-glutamic acid)-poly(lactide) block copolymers and evaluation of their uptake by HepG2 cells. J Control Release 105(3):213–225
Chen Y, Qian Z, Zhang Z (2008) Novel preparation of magnetite/polystyrene composite particles via inverse emulsion polymerization. Colloids Surf A Physicochem Eng Asp 312(2–3):209–213
Shao D, Xia A, Hu J, Wang C, Yu W (2008) Monodispersed magnetite/silica composite microspheres: preparation and application for plasmid DNA purification. Colloids Surf A Physicochem Eng Asp 322(1–3):61–65
Liu P, Zhong Y, Luo Y (2014) Preparation of monodisperse biodegradable magnetic microspheres using a T-shaped microchannel reactor. Mater Lett 117:37–40
Oster J, Parker J, Brassard LA (2001) Polyvinyl-alcohol-based magnetic beads for rapid and efficient separation of specific or unspecific nucleic acid sequences. J Magn Magn Mater 225(1–2):145–150
Liu XQ, Ma ZY, Xing JM, Liu HZ (2004) Preparation and characterization of amino-silane modified superparamagnetic silica nanospheres. J Magn Magn Mater 270(1–2):1–6
Tsai HY, Jian SJ, Huang ST, Fuh CB (2009) Competitive magnetic immunoassay for protein detection in thin channels. J Chromatogr A 1216(44):7493–7496
Zhang D-H, Yuwen L-X, Xie Y-L, Li W, Li X-B (2012) Improving immobilization of lipase onto magnetic microspheres with moderate hydrophobicity/hydrophilicity. Colloids Surf B: Biointerfaces 89:73–78
Chung YS, Park SB, Kang DW (2004) Magnetically separable titania-coated nickel ferrite photocatalyst. Mater Chem Phys 86(2–3):375–381
Zamani F, Izadi E (2014) Polyvinyl amine coated Fe3O4@SiO2 magnetic microspheres for Knoevenagel condensation. Chin J Catal 35(1):21–27
Thapa D, Palkar VR, Kurup MB, Malik SK (2004) Properties of magnetite nanoparticles synthesized through a novel chemical route. Mater Lett 58(21):2692–2694
Hyeon T, Lee SS, Park J, Chung Y, Bin Na H (2001) Synthesis of highly crystalline and monodisperse maghemite nanocrystallites without a size-selection process. J Am Chem Soc 123(51):12798–12801
Sun SH, Murray CB (1999) Synthesis of monodisperse cobalt nanocrystals and their assembly into magnetic superlattices (invited). J Appl Phys 85(8):4325–4330
Chen D, Xu R (1998) Hydrothermal synthesis and characterization of nanocrystalline Fe3O4 powders. Mater Res Bull 33(7):1015–1021
Mishra D, Anand S, Panda RK, Das RP (2004) Studies on characterization, microstructures and magnetic properties of nano-size barium hexa-ferrite prepared through a hydrothermal precipitation-calcination route. Mater Chem Phys 86(1):132–136
Yao G, Qi D, Deng C, Zhang X (2008) Functionalized magnetic carbonaceous microspheres for trypsin immobilization and the application to fast proteolysis. J Chromatogr A 1215(1–2):82–91
Lv Y, Wang H, Wang X, Bai J (2009) Synthesis, characterization and growing mechanism of monodisperse Fe3O4 microspheres. J Cryst Growth 311(13):3445–3450
Yang P, Quan Z, Hou Z, Li C, Kang X, Cheng Z, Lin J (2009) A magnetic, luminescent and mesoporous core-shell structured composite material as drug carrier. Biomaterials 30(27):4786–4795
Liu Y, Li C, Zhang H, Fan X, Liu Y, Zhang Q (2015) One-pot hydrothermal synthesis of highly monodisperse water-dispersible hollow magnetic microspheres and construction of photonic crystals. Chem Eng J 259:779–786
Lou MY, Wang DP, Huang WH, Chen D, Liu B (2006) Effect of silane-coupling agents on synthesis and character of core-shell SiO2 magnetic microspheres. J Magn Magn Mater 305(1):83–90
Xu H, Tong N, Cui L, Lu Y, Gu H (2007) Preparation of hydrophilic magnetic nanospheres with high saturation magnetization. J Magn Magn Mater 311(1):125–130
Liu B, Xie W, Wang D, Huang W, Yu M, Yao A (2008) Preparation and characterization of magnetic luminescent nanocomposite particles. Mater Lett 62(17–18):3014–3017
Xu J, Yang H, Fu W, Du K, Sui Y, Chen J, Zeng Y, Li M, Zou G (2007) Preparation and magnetic properties of magnetite nanoparticles by sol-gel method. J Magn Magn Mater 309(2):307–311
Shao D, Xu K, Song X, Hu J, Yang W, Wang C (2009) Effective adsorption and separation of lysozyme with PAA-modified Fe3O4@silica core/shell microspheres. J Colloid Interface Sci 336(2):526–532
Liu YD, Choi HJ, Choi S-B (2012) Controllable fabrication of silica encapsulated soft magnetic microspheres with enhanced oxidation-resistance and their rheology under magnetic field. Colloids Surf A Physicochem Eng Asp 403:133–138
Gupta AK, Gupta M (2005) Synthesis and surface engineering of iron oxide nanoparticles for biomedical applications. Biomaterials 26(18):3995–4021
Sun Y, Wang B, Wang H, Jiang J (2007) Controllable preparation of magnetic polymer microspheres with different morphologies by miniemulsion polymerization. J Colloid Interface Sci 308(2):332–336
Gao Z, Zhang Q, Cao Y, Pan P, Bai F, Bai G (2009) Preparation of novel magnetic cellulose microspheres via cellulose binding domain-streptavidin linkage and use for mRNA isolation from eukaryotic cells and tissues. J Chromatogr A 1216(45):7670–7676
Fegan C, Poynton CH, Whittaker JA (1990) The gut mucosal barrier in bone marrow transplantation. Bone Marrow Transplant 5(6):373–377
Wang DS, He JB, Rosenzweig N, Rosenzweig Z (2004) Superparamagnetic Fe2O3 Beads-CdSe/ZnS quantum dots core-shell nanocomposite particles for cell separation. Nano Lett 4(3):409–413
Chung T-H, Chang J-Y, Lee W-C (2009) Application of magnetic poly(styrene-glycidyl methacrylate) microspheres for immunomagnetic separation of bone marrow cells. J Magn Magn Mater 321(10):1635–1638
Hallier-Soulier S, Guillot E (1999) An immunomagnetic separation polymerase chain reaction assay for rapid and ultra-sensitive detection of Cryptosporidium parvum in drinking water. FEMS Microbiol Lett 176(2):285–289
Perez JM, Simeone FJ, Saeki Y, Josephson L, Weissleder R (2003) Viral-induced self-assembly of magnetic nanoparticles allows the detection of viral particles in biological media. J Am Chem Soc 125(34):10192–10193
Chen A-Z, Lin X-F, Wang S-B, Li L, Liu Y-G, Ye L, Wang G-Y (2012) Biological evaluation of Fe3O4-poly(L-lactide)-poly(ethylene glycol)-poly(L-lactide) magnetic microspheres prepared in supercritical CO2. Toxicol Lett 212(1):75–82
Chen X, Ding N, Zang H, Yeung H, Zhao R-S, Cheng C, Liu J, Chan TWD (2013) Fe3O4@MOF core-shell magnetic microspheres for magnetic solid-phase extraction of polychlorinated biphenyls from environmental water samples. J Chromatogr A 1304:241–245
Candido RRF, Favero V, Duke M, Karl S, Gutierrez L, Woodward RC, Graeff-Teixeira C, Jones MK, St Pierre TG (2015) The affinity of magnetic microspheres for Schistosoma eggs. Int J Parasitol 45(1):43–50
de la Escosura-Muniz A, Plichta Z, Horak D, Merkoci A (2015) Alzheimer’s disease biomarkers detection in human samples by efficient capturing through porous magnetic microspheres and labelling with electrocatalytic gold nanoparticles. Biosens Bioelectron 67:162–169
Demirel D, Ozdural AR, Mutlu M (2004) Preparation and characterization of magnetic duolite-polystyrene composite particles for enzyme immobilization. J Food Eng 62(3):203–208
Lei H, Wang W, Chen LL, Li XC, Yi B, Deng L (2004) The preparation and catalytically active characterization of papain immobilized on magnetic composite microspheres. Enzym Microb Technol 35(1):15–21
Hafeli U, Pauer G, Failing S, Tapolsky G (2001) Radiolabeling of magnetic particles with rhenium-188 for cancer therapy. J Magn Magn Mater 225(1–2):73–78
Lubbe AS, Bergemann C, Riess H, Schriever F, Reichardt P, Possinger K, Matthias M, Dorken B, Herrmann F, Gurtler R, Hohenberger P, Haas N, Sohr R, Sander B, Lemke AJ, Ohlendorf D, Huhnt W, Huhn D (1996) Clinical experiences with magnetic drag targeting: a phase I study with 4’-epidoxorubicin in 14 patients with advanced solid tumors. Cancer Res 56(20):4686–4693
Ren J, Hong H, Ren T, Teng X (2006) Preparation and characterization of magnetic PLA-PEG composite nanoparticles for drug targeting. React Funct Polym 66(9):944–951
Fahlvik AK, Holtz E, Klaveness J (1990) Relaxation efficacy of paramagnetic and superparamagnetic microspheres in liver and spleen. Magn Reson Imaging 8(4):363–369
Gellissen J, Axmann C, Prescher A, Bohndorf K, Lodemann KP (1999) Extra- and intracellular accumulation of ultrasmall superparamagnetic iron oxides (USPIO) in experimentally induced abscesses of the peripheral soft tissues and their effects on magnetic resonance imaging. Magn Reson Imaging 17(4):557–567
Muhler A, Zhang X, Wang H, Lawaczeck R, Weinmann HJ (1995) Investigation of mechanisms influencing the accumulation of ultrasmall superparamagnetic iron oxide particles in lymph nodes. Investig Radiol 30(2):98–103
Moghimi SM, Hunter AC, Murray JC (2001) Long-circulating and target-specific nanoparticles: theory to practice. Pharmacol Rev 53(2):283–318
Kim BH, Shin K, Kwon SG, Jang Y, Lee H-S, Lee H, Jun SW, Lee J, Han SY, Yim Y-H, Kim D-H, Hyeon T (2013) Sizing by weighing: characterizing sizes of ultrasmall-sized iron oxide nanocrystals using MALDI-TOF mass spectrometry. J Am Chem Soc 135(7):2407–2410
Jiang LQ, Gao L (2003) Carbon nanotubes-magnetite nanocomposites from solvothermal processes: formation, characterization, and enhanced electrical properties. Chem Mater 15(14):2848–2853
Lee Y, Lee J, Bae CJ, Park JG, Noh HJ, Park JH, Hyeon T (2005) Large-scale synthesis of uniform and crystalline magnetite nanoparticles using reverse micelles as nanoreactors under reflux conditions. Adv Funct Mater 15(3):503–509
Liang X, Xi B, Xiong S, Zhu Y, Xue F, Qian Y (2009) Porous soft magnetic material: the maghemite microsphere with hierarchical nanoarchitecture and its application in water purification. Mater Res Bull 44(12):2233–2239
Lu B-Q, Zhu Y-J, Zhao X-Y, Cheng G-F, Ruan Y-J (2013) Sodium polyacrylate modified Fe3O4 magnetic microspheres formed by self-assembly of nanocrystals and their applications. Mater Res Bull 48(2):895–900
Jiang R, Zhu HY, Li JB, Fu FQ, Yao J, Jiang ST, Zeng GM (2016) Fabrication of novel magnetically separable BiOBr/CoFe2O4 microspheres and its application in the efficient removal of dye from aqueous phase by an environment-friendly and economical approach. Appl Surf Sci 364:604–612
Zhu W, Zhang Y, Hou C, Pan D, He J, Zhu H (2016) Covalent immobilization of lipases on monodisperse magnetic microspheres modified with PAMAM-dendrimer. J Nanopart Res 18(2):32
Xu M, Liu M, Sun M, Chen K, Cao X, Hu Y (2016) Magnetic solid-phase extraction of phthalate esters (PAEs) in apparel textile by core shell structured Fe3O4@silica@triblock-copolymer magnetic microspheres. Talanta 150:125–134
Zhou ZH, Wang J, Liu X, Chan HSO (2001) Synthesis of Fe3O4 nanoparticles from emulsions. J Mater Chem 11(6):1704–1709
Sivakumar M, Gedanken A, Zhong W, Du YW, Bhattacharya D, Yeshurun Y, Felner I (2004) Nanophase formation of strontium hexaferrite fine powder by the sonochemical method using Fe(CO)(5). J Magn Magn Mater 268(1–2):95–104
Hong RY, Fu HP, Di GQ, Zheng Y, Wei DG (2008) Facile route to gamma-Fe2O3/SiO2 nanocomposite used as a precursor of magnetic fluid. Mater Chem Phys 108(1):132–141
Pu HT, Jiang FJ (2005) Towards high sedimentation stability: magnetorheological fluids based on CNT/Fe3O4 nanocomposites. Nanotechnology 16(9):1486–1489
Kim IT, Nunnery GA, Jacob K, Schwartz J, Liu X, Tannenbaum R (2010) Synthesis, characterization, and alignment of magnetic carbon nanotubes tethered with maghemite nanoparticles. J Phys Chem C 114(15):6944–6951
Zayat MZ, del Monte F, Morales MD, Rosa G, Guerrero H, Serna CJ, Levy D (2003) Highly transparent gamma-Fe2O3/Vycor-glass magnetic nanocomposites exhibiting faraday rotation. Adv Mater 15(21):1809–1812
Liu ZL, Yang XB, Yao KL, Du GH, Liu ZS (2006) Preparation and characterization of magnetic P(St-co-MAA-co-AM) microspheres. J Magn Magn Mater 302(2):529–535
Lu M, Bai S, Yang K, Sun Y (2007) Synthesis and characterization of magnetic polymer microspheres with a core-shell structure. China Particuology 5(1–2):180–185
Zhang J, Yu D, Chen W, Xie Y, Wan W, Liang H, Min C (2009) Preparation of poly(styrene-glucidylmethacrylate)/Fe3O4 composite microspheres with high magnetite contents. J Magn Magn Mater 321(6):572–577
Zhang K, Wu W, Guo K, Chen JF, Zhang PY (2009) Magnetic polymer enhanced hybrid capsules prepared from a novel pickering emulsion polymerization and their application in controlled drug release. Colloids Surf A Physicochem Eng Asp 349(1–3):110–116
Zhang H, Zhang Q, Zhang B, Guo F (2009) Preparation of magnetic composite microspheres by surfactant free controlled radical polymerization: preparation and characteristics. J Magn Magn Mater 321(23):3921–3925
Salih T, Ahlford A, Nilsson M, Plichta Z, Horak D (2016) Streptavidin-modified monodispersed magnetic poly(2-hydroxyethyl methacrylate) microspheres as solid support in DNA-based molecular protocols. Mat Sci Eng C-Mater 61:362–367
Yuan D, Chen L, Yuan L, Liao S, Yang M, Zhang Q (2016) Superparamagnetic polymer composite microspheres supported Schiff base palladium complex: an efficient and reusable catalyst for the Suzuki coupling reactions. Chem Eng J 287:241–251
Salgueirino-Maceira V, Liz-Marzan LM, Farle M (2004) Water-based ferrofluids from FexPt1-x nanoparticles synthesized in organic media. Langmuir 20(16):6946–6950
Lopez-Lopez MT, Duran JDG, Delgado A, Gonzalez-Caballero F (2005) Stability and magnetic characterization of oleate-covered magnetite ferrofluids in different nonpolar carriers. J Colloid Interface Sci 291(1):144–151
Liong M, Shao H, Haun JB, Lee H, Weissleder R (2010) Carboxymethylated polyvinyl alcohol stabilizes doped ferrofluids for biological applications. Adv Mater 22(45):5168-+
Azhdarzadeh M, Atyabi F, Saei AA, Varnamkhasti BS, Omidi Y, Fateh M, Ghavami M, Shanehsazzadeh S, Dinarvand R (2016) Theranostic MUC-1 aptamer targeted gold coated superparamagnetic iron oxide nanoparticles for magnetic resonance imaging and photothermal therapy of colon cancer. Colloids Surf B: Biointerfaces 143:224–232
Kumar S, Meena VK, Hazari PP, Sharma RK (2016) FITC-dextran entrapped and silica coated gadolinium oxide nanoparticles for synchronous optical and magnetic resonance imaging applications. Int J Pharm 506(1–2):242–252
Holbrook RJ, Rammohan N, Rotz MW, MacRenaris KW, Preslar AT, Meade TJ (2016) Gd(III)-dithiolane gold nanoparticles for T-1-weighted magnetic resonance imaging of the pancreas. Nano Lett 16(5):3202–3209
Zhang X, Blasiak B, Marenco AJ, Trudel S, Tomanek B, van Veggel FCJM (2016) Design and regulation of NaHoF4 and NaDyF4 nanoparticles for high-field magnetic resonance imaging. Chem Mater 28(9):3060–3072
Chen W-H, Luo G-F, Lei Q, Cao F-Y, Fan J-X, Qiu W-X, Jia H-Z, Hong S, Fang F, Zeng X, Zhuo R-X, Zhang X-Z (2016) Rational design of multifunctional magnetic mesoporous silica nanoparticle for tumor-targeted magnetic resonance imaging and precise therapy. Biomaterials 76:87–101
Ni D, Zhang J, Bu W, Zhang C, Yao Z, Xing H, Wang J, Duan F, Liu Y, Fan W, Feng X, Shi J (2016) PEGylated NaHoF4 nanoparticles as contrast agents for both X-ray computed tomography and ultra-high field magnetic resonance imaging. Biomaterials 76:218–225
Chen Y, Ai K, Liu J, Sun G, Yin Q, Lu L (2015) Multifunctional envelope-type mesoporous silica nanoparticles for pH-responsive drug delivery and magnetic resonance imaging. Biomaterials 60:111–120
Watcharin W, Schmithals C, Pleli T, Koeberle V, Korkusuz H, Huebner F, Waidmann O, Zeuzem S, Korf H-W, Terfort A, Gelperina S, Vogl TJ, Kreuter J, Piiper A (2015) Detection of hepatocellular carcinoma in transgenic mice by Gd-DTPA- and rhodamine 123-conjugated human serum albumin nanoparticles in T1 magnetic resonance imaging. J Control Release 199:63–71
Bunkoed O, Kanatharana P (2015) Extraction of polycyclic aromatic hydrocarbons with a magnetic sorbent composed of alginate, magnetite nanoparticles and multiwalled carbon nanotubes. Microchim Acta 182(7–8):1519–1526
Kifle D, Wibetoe G (2013) Retention and elution of precious metals on cyano-modified solid phase microparticle sorbent. Microchim Acta 180(11–12):981–987
Wang Y, Wu J, Chen Y, Xue F, Teng J, Cao J, Lu C, Chen W (2015) Magnetic microparticle-based SELEX process for the identification of highly specific aptamers of heart marker--brain natriuretic peptide. Microchim Acta 182(1–2):331–339
Yang J, Si L, Cui S, Bi W (2015) Synthesis of a graphitic carbon nitride nanocomposite with magnetite as a sorbent for solid phase extraction of phenolic acids. Microchim Acta 182(3–4):737–744
Zhang L, Zhang Z, Wan Q (2006) Preparation of porous magnetic silica microspheres and their application for genomic deoxyribonucleic acid extraction. Chin J Anal Chem 34(7):923–926
Zhang Z-C, Cui Y, Wan Q-H (2007) Surface modification of magnetic silica microspheres and its application to the isolation of plant genomic nucleic acids. Chin J Anal Chem 35(1):31–36
Shi R, Wang Y, Hu Y, Chen L, Wan Q-H (2009) Preparation of magnetite-loaded silica microspheres for solid-phase extraction of genomic DNA from soy-based foodstuffs. J Chromatogr A 1216(36):6382–6386
Liu JW, Zhang Y, Chen D, Yang T, Chen ZP, Pan SY, Gu N (2009) Facile synthesis of high-magnetization gamma-Fe2O3/alginate/silica microspheres for isolation of plasma DNA. Colloids Surf A Physicochem Eng Asp 341(1–3):33–39
Obrien SM, Thomas ORT, Dunnill P (1996) Non-porous magnetic chelator supports for protein recovery by immobilised metal affinity adsorption. J Biotechnol 50(1):13–25
Shamim N, Hong L, Hidajat K, Uddin MS (2007) Thermosensitive polymer (N-isopropylacrylamide) coated nanomagnetic particles: preparation and characterization. Colloids Surf B: Biointerfaces 55(1):51–58
Wang P, J-q Z, Z-j J, Y-c L, S-m L (2009) Preparation of magnetic iron/mesoporous silica composite spheres and their use in protein immobilization. Trans Nonferrous Metals Soc China 19:S605–S610
Gasilova N, Srzentic K, Qiao L, Liu B, Beck A, Tsybin YO, Girault HH (2016) On-Chip mesoporous functionalized magnetic microspheres for protein sequencing by extended bottom-up mass spectrometry. Anal Chem 88(3):1775–1784
Yan X, Kong J, Yang C, Fu G (2015) Facile synthesis of hairy core-shell structured magnetic polymer submicrospheres and their adsorption of bovine serum albumin. J Colloid Interface Sci 445:9–15
Ding C, Ma X, Yao X, Jia L (2015) Facile synthesis of copper(II)-decorated magnetic particles for selective removal of hemoglobin from blood samples. J Chromatogr A 1424:18–26
Zheng J, Lin Z, Zhang L, Yang H (2015) Polydopamine-mediated immobilization of phenylboronic acid on magnetic microspheres for selective enrichment of glycoproteins and glycopeptides. Sci China Chem 58(6):1056–1064
Sun L, Li Y, Yang P, Zhu G, Dovichi NJ (2012) High efficiency and quantitatively reproducible protein digestion by trypsin-immobilized magnetic microspheres. J Chromatogr A 1220:68–74
Orlov AV, Bragina VA, Nikitin MP, Nikitin PI (2016) Rapid dry-reagent immunomagnetic biosensing platform based on volumetric detection of nanoparticles on 3D structures. Biosens Bioelectron 79:423–429
Yamazaki S, Matsunaga S, Hori K (2001) Photocatalytic degradation of trichloroethylene in water using TiO(2) pellets. Water Res 35(4):1022–1028
Horikoshi S, Watanabe N, Onishi H, Hidaka H, Serpone N (2002) Photodecomposition of a nonylphenol polyethoxylate surfactant in a cylindrical photoreactor with TiO(2) immobilized fiberglass cloth. Appl Catal B Environ 37(2):117–129
Anpo M, Zhang SG, Mishima H, Matsuoka M, Yamashita H (1997) Design of photocatalysts encapsulated within the zeolite framework and cavities for the decomposition of NO into N-2 and O-2 at normal temperature. Catal Today 39(3):159–168
Beydoun D, Amal R, Low GKC, McEvoy S (2000) Novel photocatalyst: titania-coated magnetite. Activity and photodissolution. J Phys Chem B 104(18):4387–4396
Zeng T, X-l Z, H-y N, Ma Y-r, W-h L, Y-q C (2013) In situ growth of gold nanoparticles onto polydopamine-encapsulated magnetic microspheres for catalytic reduction of nitrobenzene. Appl Catal B Environ 134:26–33
Yang H, Li S, Wang X, Zhang F, Zhong X, Dong Z, Ma J (2012) Core-shell silica magnetic microspheres supported proline as a recyclable organocatalyst for the asymmetric aldol reaction. J Mol Catal A Chem 363:404–410
Li H, Gao S, Cao M, Cao R (2013) Self-assembly of polyoxometalate-thionine multilayer films on magnetic microspheres as photocatalyst for methyl orange degradation under visible light irradiation. J Colloid Interface Sci 394:434–440
Jiang S, Yan J, Habimana F, Ji S (2016) Preparation of magnetically recyclable MIL-53(Al)@SiO2@Fe3O4 catalysts and their catalytic performance for Friedel-crafts acylation reaction. Catal Today 264:83–90
Li Z-D, Wang H-L, Wei X-N, Liu X-Y, Yang Y-F, Jiang W-F (2016) Preparation and photocatalytic performance of magnetic Fe3O4@TiO2 core-shell microspheres supported by silica aerogels from industrial fly ash. J Alloys Compd 659:240–247
Zhang L, Wu Z, Chen L, Zhang L, Li X, Xu H, Wang H, Zhu G (2016) Preparation of magnetic Fe3O4/TiO2/Ag composite microspheres with enhanced photocatalytic activity. Solid State Sci 52:42–48
Shen S-L, Wu W, Guo K, Meng H, Chen J-F (2007) A novel process to synthesize magnetic hollow silica microspheres. Colloids Surf A Physicochem Eng Asp 311(1–3):99–105
Podzus PE, Daraio ME, Jacobo SE (2009) Chitosan magnetic microspheres for technological applications: preparation and characterization. Phys B Condens Matter 404(18):2710–2712
Ozcan F, Ersoz M, Yilmaz M (2009) Preparation and application of calix 4 arene-grafted magnetite nanoparticles for removal of dichromate anions. Mater Sci Eng C 29(8):2378–2383
Sun X, Yang L, Xing H, Zhao J, Li X, Huang Y, Liu H (2013) Synthesis of polyethylenimine-functionalized poly(glycidyl methacrylate) magnetic microspheres and their excellent Cr(VI) ion removal properties. Chem Eng J 234:338–345
Zhao Y, Li J, Zhao L, Zhang S, Huang Y, Wu X, Wang X (2014) Synthesis of amidoxime-functionalized Fe3O4@SiO2 core-shell magnetic microspheres for highly efficient sorption of U(VI). Chem Eng J 235:275–283
Sun X, Yang L, Li Q, Liu Z, Dong T, Liu H (2015) Polyethylenimine-functionalized poly(vinyl alcohol) magnetic microspheres as a novel adsorbent for rapid removal of Cr(VI) from aqueous solution. Chem Eng J 262:101–108
Sun W, Li L, Luo C, Fan L (2016) Synthesis of magnetic graphene nanocomposites decorated with ionic liquids for fast lead ion removal. Int J Biol Macromol 85:246–251
Chang YC, Chen DH (2005) Preparation and adsorption properties of monodisperse chitosan-bound Fe3O4 magnetic nanoparticles for removal of Cu(II) ions. J Colloid Interface Sci 283(2):446–451
Huang C, Hu B (2008) Silica-coated magnetic nanoparticles modified with gamma-mercaptopropyltrimethoxysilane for fast and selective solid phase extraction of trace amounts of Cd, CuHg, and Pb in environmental and biological samples prior to their determination by inductively coupled plasma mass spectrometry. Spectrochim Acta B At Spectrosc 63(3):437–444
Hao Y-M, Chen M, Hu Z-B (2010) Effective removal of Cu (II) ions from aqueous solution by amino-functionalized magnetic nanoparticles. J Hazard Mater 184(1–3):392–399
Ge F, Li M-M, Ye H, Zhao B-X (2012) Effective removal of heavy metal ions Cd2+, Zn2+, Pb2+, Cu2+ from aqueous solution by polymer-modified magnetic nanoparticles. J Hazard Mater 211:366–372
Zargoosh K, Abedini H, Abdolmaleki A, Molavian MR (2013) Effective Removal of Heavy Metal Ions from Industrial Wastes Using Thiosalicylhydrazide-Modified Magnetic Nanoparticles. Ind Eng Chem Res 52(42):14944–14954
Zhou X, You S-J, Wang X-H, Gan Y, Zhong Y-J, Ren N-Q (2014) Hydrothermal synthesis of magnetic carbon microspheres for effective adsorption of Cd(II) in water. J Chem Technol Biotechnol 89(7):1051–1059
Chou C-M, Lien H-L (2011) Dendrimer-conjugated magnetic nanoparticles for removal of zinc (II) from aqueous solutions. J Nanopart Res 13(5):2099–2107
Ngomsik A-F, Bee A, Talbot D, Cote G (2012) Magnetic solid-liquid extraction of Eu(III), La(III), Ni(II) and Co(II) with maghemite nanoparticles. Sep Purif Technol 86:1–8
Giraldo L, Erto A, Carlos Moreno-Pirajan J (2013) Magnetite nanoparticles for removal of heavy metals from aqueous solutions: synthesis and characterization. Adsorpt-J Int Adsorpt Soc 19(2–4):465–474
Ma W, Dai J, Dai X, Yan Y (2014) Preparation and Characterization of Chitosan/Kaolin/Fe3O4 Magnetic Microspheres and Their Application for the Removal of Ciprofloxacin. Adsorpt Sci Technol 32(10):775–790
Li Y, Li X, Chu J, Dong C, Qi J, Yuan Y (2010) Synthesis of core-shell magnetic molecular imprinted polymer by the surface RAFT polymerization for the fast and selective removal of endocrine disrupting chemicals from aqueous solutions. Environ Pollut 158(6):2317–2323
Shi P, Ma R, Zhou Q, Li A, Wu B, Miao Y, Chen X, Zhang X (2015) Chemical and bioanalytical assessments on drinking water treatments by quaternized magnetic microspheres. J Hazard Mater 285:53–60
Zhou L, Pan S, Chen X, Zhao Y, Zou B, Jin M (2014) Kinetics and thermodynamics studies of pentachlorophenol adsorption on covalently functionalized Fe3O4@SiO2-MWCNTs core-shell magnetic microspheres. Chem Eng J 257:10–19
Lin J, Liu Y, Chen S, Le X, Zhou X, Zhao Z, Ou Y, Yang J (2016) Reversible immobilization of laccase onto metal-ion-chelated magnetic microspheres for bisphenol a removal. Int J Biol Macromol 84:189–199
Lu S, Chen L, Dong Y, Chen Y (2011) Adsorption of Eu(III) on iron oxide/multiwalled carbon nanotube magnetic composites. J Radioanal Nucl Chem 288(2):587–593
Ma X, Tao H, Yang K, Feng L, Cheng L, Shi X, Li Y, Guo L, Liu Z (2012) A functionalized graphene oxide-iron oxide nanocomposite for magnetically targeted drug delivery, photothermal therapy, and magnetic resonance imaging. Nano Res 5(3):199–212
Han Q, Wang Z, Xia J, Chen S, Zhang X, Ding M (2012) Facile and tunable fabrication of Fe3O4/graphene oxide nanocomposites and their application in the magnetic solid-phase extraction of polycyclic aromatic hydrocarbons from environmental water samples. Talanta 101:388–395
Bai S, Shen X, Zhong X, Liu Y, Zhu G, Xu X, Chen K (2012) One-pot solvothermal preparation of magnetic reduced graphene oxide-ferrite hybrids for organic dye removal. Carbon 50(6):2337–2346
Tian C, Zhu L, Lin F, Boyes SG (2015) Poly(acrylic acid) Bridged Gadolinium Metal-Organic Framework-Gold Nanoparticle Composites as Contrast Agents for Computed Tomography and Magnetic Resonance Bimodal Imaging. ACS Appl Mater Interfaces 7(32):17765–17775
Yang F, Zhang X, Song L, Cui H, Myers JN, Bai T, Zhou Y, Chen Z, Gu N (2015) Controlled Drug Release and Hydrolysis Mechanism of Polymer-Magnetic Nanoparticle Composite. ACS Appl Mater Interfaces 7(18):9410–9419
Josephson L, Tung CH, Moore A, Weissleder R (1999) High-efficiency intracellular magnetic labeling with novel superparamagnetic-tat peptide conjugates. Bioconjug Chem 10(2):186–191
Zong P, Wang S, Zhao Y, Wang H, Pan H, He C (2013) Synthesis and application of magnetic graphene/iron oxides composite for the removal of U(VI) from aqueous solutions. Chem Eng J 220:45–52
Gawande MB, Branco PS, Varma RS (2013) Nano-magnetite (Fe3O4) as a support for recyclable catalysts in the development of sustainable methodologies. Chem Soc Rev 42(8):3371–3393
Munoz M, de Pedro ZM, Casas JA, Rodriguez JJ (2015) Preparation of magnetite-based catalysts and their application in heterogeneous Fenton oxidation - a review. Appl Catal B Environ 176:249–265
Baig RBN, Nadagouda MN, Varma RS (2015) Magnetically retrievable catalysts for asymmetric synthesis. Coord Chem Rev 287:137–156
Acknowledgments
This work was supported by the National Natural Science Foundation of China (Grant No. 81402899) and Shandong Provincial Natural Science Foundation, China (No. ZR2014HP020).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The authors declare that they have no conflict of interest. All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. This article does not contain any studies with animals performed by any of the authors. Informed consent was obtained from all individual participants included in the study.
Additional information
Deli Xiao and Ting Lu equally contributed to this work and should be considered co-first authors.
Rights and permissions
About this article
Cite this article
Xiao, D., Lu, T., Zeng, R. et al. Preparation and highlighted applications of magnetic microparticles and nanoparticles: a review on recent advances. Microchim Acta 183, 2655–2675 (2016). https://doi.org/10.1007/s00604-016-1928-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00604-016-1928-y