Abstract
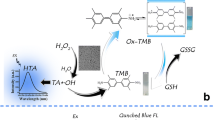
Nitrogen- and iron-containing carbon dots (N,Fe-CDs) are synthesized by hydrothermal treatment of branched polyethylenimine (BPEI) and hemin at 180 °C. The N,Fe-CDs are mainly doped with nitrogen and trace amounts of iron(III). The N,Fe-CDs also display intrinsic fluorescence with excitation/emission maxima at 365/452 nm and a quantum yield of 27 %. The nanodots are shown to act as peroxidase mimics by catalyzing the oxidation of tetramethylbenzidine (TMB) by hydrogen peroxide to form a blue product whose quantity can be determined by photometry at 652 nm. This was exploited to design colorimetric and fluorometric assays for dopamine (DA). The colorimetric assay is based on the oxidation of DA by H2O2 in presence of the N,Fe-CDs and TMB. It has an instrumental detection limit of 40 nM (at an S/N ratio of 3), and a visual detection limit of 0.4 μM. The fluorometric assay is based on an inner filter effect that is caused by the formation of oxidized TMB which overlaps (and absorbs) the emission of the N,Fe-CDs located at 452 nm. The fluorometric detection limit is as low as 20 nM (at an S/N ratio of 3).

Carbon dots containing nitrogen and iron (N,Fe-CDs) were synthesized by hydrothermal treatment of branched polyethylenimine and hemin. The N,Fe-CDs display excellent fluorescent properties, peroxidase-like activity and potential application in colorimetric and fluorometric detection of dopamine.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Carbon dots (CDs) have been widely studied in optoelectronics, photocatalysis, medical diagnosis, and bioassay due to their superior optical properties, and good chemical stability [1, 2]. A variety of CDs (such as phosphorus, and nitrogen co-doped carbon dots, nitrogen-doped carbon dots, nitrogen and sulfur co-doped carbon dots) have been reported to be utilized in analytical measurements [2–4]. The catalytical activity of carbon dots attracted considerable attention due to their potentials in the applications of analytical assay and photo/electrocatalysis [1, 5, 6]. However, there were only few species of catalytically active CDs have been reported which relatively limited their development and applications.
Most of catalytically active CDs were synthesized by the oxidation of various carbon materials (such as graphite, carbon rod, or candle soot, etc. [5–7]) to obtain the graphene-like structure and oxygen functional groups [8] according to previous reports. It was suggested that ketonic carbonyl groups (−C = O) in CDs acted as catalytically active sites while the carboxylic groups (O = C–O–) served as substrate binding sites. In addition, the nitrogen-doped C-dots (N-CDs) prepared from refluxing carbonized β-cyclodextrin in nitric acid also possessed catalytic ability because of the existence of graphitic carbon in the CDs [9].
Hemin (Fe(III)-protoporphyrin IX), as the catalytic center of metalloprotein, has attracted extensive attention in biomimetic catalysis of simulated enzymes [10, 11]; However, it was difficult to directly employ hemin as a mimic catalyst due to its poor water solubility. Hemin molecules were prone to aggregation in aqueous environment and lost its catalytic activity [12]. Thus a variety of supporting materials (graphene, polymers, nanofibers and nanotubes etc.) were employed to prevent hemin from aggregation and maintain the catalytic activity of hemin [13–15].
Due to the properties discussed above, hemin was expected to serve as the precursor to produce CDs with good catalytic activity. However, the poor water solubility of hemin would seriously limit the yield of the CDs. Our results indicated that the solubility of hemin would be improved in the presence of -NH2 rich polymer branched polyethylenimine (BPEI). Also most N-CDs display high fluorescent quantum yields (QY) due to the nitrogen-containing functional groups [16, 17]. Therefore, it was expected that the employment of BPEI (Fig. S1) together with hemin to serve as co-precursors would produce catalytically active CDs with high production yield and QY.
DA is an important neurotransmitter which plays quite important roles in mammalians brain and body. Dysfunctions of the DA secretion cause serious diseases such as Parkinson’s diseases, senile dementia, schizophrenia, and drug addiction etc. [18–20]. Thus the detection of DA is significantly important in biomedical research and disease diagnose. Various methods, such as electrochemistry, fluorescence, colorimetry and liquid chromatography − mass spectrometry etc., were investigated for the detection of DA [21–24]. However, the sensitivity of these methods still required to be improved and the utilisation of these methods in clinical application was still a great challenge.
We reported the fabrication of catalytically active nitrogen and iron-containing carbon dots (N,Fe-CDs) by the simple hydrothermal treatment with BPEI and hemin as co-precursors. The N,Fe-CDs successfully combined the fluorescent feature of BPEI based carbon dots with the peroxidase like activity from hemin thus showed great potentials in the applications of fluorescent and catalytic assay. In addition, the N,Fe-CDs exhibited excellent water solubility. Thus, they may be utilized in aqueous environment directly in comparison with hemin. It was found that these N,Fe-CDs can initialize the H2O2 mediated catalytic oxidation of TMB. As DA inhibits the oxidation of TMB, a colorimetric and fluorescent assay was developed to detect DA with the N,Fe-CDs. This assay was verified to be effective in the detection of DA in human serum samples. The method provided a strategy for the detection of DA and the potential to be utilised in practical application.
Experimental section
Materials and apparatus
Hemin, dopamine hydrochloride (DA), branched polyethylenimine (BPEI, Mw = 600), 3, 3′, 5, 5′-tetramethylbenzidine (TMB), and 5-Hydroxyindole-3-acetic acid (5-HIAA) were purchased from Sigma-Aldrich (Shanghai, China; http://www.sigmaaldrich.com/china-mainland.html). Protoporphyrin IX, Dopa, resorcinol, hydroquinone, Abacavir, Acarbose Hydrate and Hydrogen peroxide were purchased from Aladdin Biochemical Technologies Inc. (http://www.aladdin-e.com/). Deionized water (18.2 MΩ.cm at 25 °C) prepared by a Milli-Q water system was used throughout all experiments. All the reagents were analytical grade and used as received.
UV–vis absorption spectra were recorded using a UV2550 spectrophotometer (Shimadzu, Japan) with 10 mm quartz cells. Fourier Transform infrared (FTIR) spectra were performed by a Thermo-Fisher FTIR spectrophotometer (Nicolet 6700). Transmission electron microscopy (TEM, JEOL, JEM-2100) measurements were carried out using an accelerating voltage of 200 kV. The samples for TEM characterization were prepared by coating N,Fe-CDs dispersion on a carbon-coated copper grid, and then dried in vacuum oven at 50 °C for 24 h. The X-ray photoelectron spectroscopy (XPS) data were obtained by using a spectrometer (Escalab 250xi, Thermo Scientific). Fluorescent emission spectra of the N,Fe-CDs were recorded by an FL spectrophotometer (Shimadzu, RF-5301PC).
Synthesis of N,Fe-CDs
N,Fe-CDs were synthesized via a hydrothermal method using BPEI and hemin as carbon precursors. In a typical synthesis, 2.0 g BPEI and 50.0 mg hemin were dispersed in 10 mL DI water and transferred into a 25 mL Teflon-lined autoclave and heated at 180 °C for 10 h. Then the suspension was obtained via centrifugation at 6800 rcf for 15 min and further dialyzed with 1000 Da dialysis tube for 24 h. The resulting dark brown solution was diluted to different concentration with deionized water and stored at 4 °C when not in use.
Measurement of fluorescence quantum yields
The QY of N,Fe-CDs was determined using quinine sulfate as the standard (QY: 0.54 at 360 nm in 0.1 M H2SO4). During the measurement, absorbance of these samples in the 10 mm fluorescence cuvette was kept under 0.05 at the excitation wavelength (360 nm) to minimize the re-absorption effects. The QY of the N,Fe-CDs was calculated by the following equation:
Where Q denotes the QY, I represents the measured integrated emission intensity, n is the refractive index of the solvent, and A refers to the absorbance. The subscript R refers to the corresponding parameter of known fluorescent standard.
DA detection
In a typical process, variable amounts of freshly prepared DA aqueous solution (0 ~ 2 μM,) H2O2 (0.8 mM), TMB (10 μg mL−1) and N, N,Fe-CDs (10 μM) were introduced into 2 mL acetate buffer solution (0.2 M, pH 4.5). The absorption spectra of the reaction solutions were measured after incubating for 15 min at room temperature. In order to investigate the selectivity of our strategy for DA detection, the same experiments were carried out using other related specimens, such as urea, ascorbic acid, histidine, lysine, glutamic acid, phenol, dichlorophenol, glycerol, dopa, resorcinol, hydroquinone, 5-HIAA, abacavir, and acarbose to replace DA. All determinations were repeated at least three times. Fluorescence determination of DA was performed in the same way.
Results and discussion
Physical characterizations
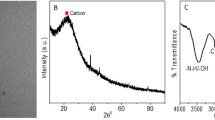
The morphologies of the N,Fe-CDs nanodots were firstly characterized using TEM. Figure 1a and b showed that the nanodots were well dispersed, and the corresponding particle size ranged from 2 to7 nm with an average diameter of 4.3 nm.
FTIR spectrum of the N,Fe–CDs indicated the organic functional groups on N,Fe-CDs (as shown in Fig. 1c). The absorption bands at 3437 cm−1 represent O–H and N–H stretching vibrations, which would be the possible reason of the improved water solubility of N,Fe-CDs [24]. The peaks at 2955 and 2845 cm−1 can be ascribed to the C-H stretching vibrations while the peaks at 1580 cm−1 and 1648 cm−1 are corresponding to N–H bending vibrations and C = O group [16, 25, 26]. The peak 1456 cm−1 is assigned to symmetric stretching vibrations of C–O–C [27]. The peak at 1306 cm−1 refers to C–N bond, and the absorption bands at 1125–1021 cm−1 are attributed to C–H bending vibrations [25].
The elements and the chemical composition of the N,Fe-CDs were characterized by X-ray photoelectron spectroscopy (XPS). As shown in Fig. 2a, the N,Fe-CDs were mainly composed of three elements including carbon (67.8 %), nitrogen (21.7 %) and oxygen (10.4 %) with binding energy of 285, 398.5 and 531 eV, respectively. Only trace amount of iron coming from hemin residues (0.08 %) can be observed in Fig. 2a. In the high-resolution C1s XPS spectrum of the N,Fe-CDs (Fig. 2b), three main binding energy peaks at 284.5, 285.5 and 287.5 eV can be seen that are attributed to C-C, C-O/C-N and C = O, respectively [28]. The high-resolution spectrum of N1s exhibited three main peaks at 399.5, 400.3 and 401.1 eV, indicating the presence of C–N–C, N–(C)3, and N–H bands, respectively (Fig. 2c). [29] In the high-resolution spectrum of Fe2p (Fig. 2d), the presence of peaks at 711.8, 724.4 eV and the satellite peak at about 718.2 eV confirmed the existence of Fe2O3 [30].
Optical properties
The optical properties of the N,Fe-CDs were explored using UV–Vis absorption and emission spectra at room temperature. Figure 3a showed that N,Fe-CDs in aqueous solution had a strong absorption peak at 338.8 nm. Under UV light (365 nm) excitation, bright blue luminescence can be observed (inset in Fig. 3a). The emission spectrum with the maximum emission wavelength of 452 nm was obtained at excitation wavelength of 365 nm. The QY of N,Fe-CDs was calculated to be about 26.6 % with the quinine sulfate as the standard.
Most of the reported CDs displayed excitation-dependent fluorescent behaviors due to the different sized particles in the samples and the different surface states distribution [31]. However, the emission spectra of our N,Fe-CDs was nearly excitation-independent from 320 nm to 390 nm (Fig 3b), which agreed well with previously reported BPEI-based CDs [16]. The excitation-independent fluorescence behavior indicated that the N,Fe-CDs possess uniform surface states and the fluorescence properties of the N,Fe-CDs were determined by their surface states rather than their morphology [32].
The peroxidase-like property of the N,Fe-CDs
To explore the catalytic activity of this N,Fe-CDs, the oxidation of 3, 3′, 5, 5′-tetramethylbenzidine (TMB, a well-known peroxidase substrate) in the presence of H2O2 was performed at room temperature [9]. Figure 4a shows that the N,Fe-CDs converted colorless TMB to its blue oxidized state with an absorbance peak at 652 nm, which originates from the oxidation of TMB [33].
a Absorption spectra of the N,Fe-CDs (a), N,Fe-CDs + H2O2 (b), TMB + N,Fe-CDs (c), TMB+ H2O2 + N,Fe-CDs (d) in pH 4.0 acetate buffer. b UV-Vis spectra of TMB/H2O2 in the presence of BPEI-CDs (e), protoporphyrin-CDs (f), FeCl3-CDs (g), hemin-CDs (h) and N,Fe-CDs (i) in pH 4.0 acetate buffer. (TMB, 0.1 mM; H2O2, 13 mM; and CDs, 0.05 mg mL−1). Inset: The corresponding photographs
According to previous reports, the mechanism of this peroxidase-like activity is attributed to the Fe species from hemin in N,Fe-CDs, which acted like Fenton’s reagent. The Fe species catalyzed the decomposition of H2O2, producing highly reactive hydroxyl species (·OH), which actually oxidized TMB to its blue oxidized state (as shown in Scheme 1) [34, 35].
In order to study the catalytic activity of the nanodots, several other carbon dots were synthesized by the hydrothermal treatments of BPEI, protoporphyrin IX, BPEI + FeCl3, and hemin, respectively. The catalytic activities of these carbon dots were also explored by TMB/H2O2 system. As shown in Fig 4b, CDs made from BPEI (BPEI-CDs) did not demonstrate obvious catalytic property and CDs prepared from protoporphyrin IX (protoporphyrin-CDs) displayed very weak catalytic activity. However, CDs prepared from BPEI + FeCl3 (FeCl3-CDs), hemin (hemin-CDs) and N,Fe-CDs (synthesized from BPEI and hemin) displayed high peroxidase-like activity, indicating that peroxidase-like carbon dots can be prepared from various iron containing compounds. Although hemin-CDs also possessed peroxidase-like activity, the yield of hemin-CDs was very low (about 3.2 %) due to the poor solubility of hemin in water, and QY of hemin-CDs was not high (about 5.6 %) as well. By contrast, the QY of BPEI-CDs can achieve as high as 31.5 % due to the presence of nitrogen. The CDs prepared with FeCl3 displayed good catalytic activity, but their QY was lower than 5 % as Fe3+ can quench the fluorescence. The balance between catalytic activity and QY was achieved by using hemin and BPEI as co-precursors. The reason we used hemin as precursor together with BPEI was that hemin can be better dissolved in BPEI aqueous solution than in water before hydrothermal treatment and BPEI can improve the fluorescence of N,Fe-CDs by introducing amino group into carbon dots as shown in Fig. 4b. The QY of N,Fe-CDs was calculated to be around 27 %, indicating that hemin is a better iron source than FeCl3 to maintain the high QY while introducing catalytic ability into N,Fe-CDs. The FTIR data in Fig. S2 also demonstrated that N,Fe-CDs possess more hydrophilic organic groups which come from BPEI.
Colorimetric detection of DA
The H2O2 mediated catalytic oxidation of TMB was often utilized in chemical sensing through the convenient colorimetric reactions [36]. As shown in Fig. 5a, the oxidation of TMB catalysed by N,Fe-CDs in the presence of H2O2 led to the increase in the intensity of the absorbance peak at 652 nm and changed the colour of reaction system from colourlesscolorless to blue. However, with the addition of DA, the blue color of oxidized TMB disappeared gradually due to the reducing property of DA. DA was added into TMB/H2O2 system before and after the addition of N,Fe-CDs to identify the probable mechanism. The results show that a final equilibrium is established by both methods but adding DA afterwards takes longer to achieve this. It was assumed that oxidized TMB was reduced by DA and transferred to colorless state under this situation. If, however, DA is added into the system before the N,Fe-CDs, DA reacts with H2O2 or ·OH generated from H2O2 directly thus prevent the oxidation of TMB. These results demonstrate that DA inhibits the oxidation of TMB, and this agrees well with previous reports [37].
a UV/Vis absorption spectra of the N,Fe-CDs + TMB (a), N,Fe-CDs + TMB + H2O2 (b), and N,Fe-CDs + TMB + H2O2 + DA (c) and corresponding photograph (inset). b UV/Vis absorption spectra of the N,Fe-CDs/H2O2/TMB system in the presence of different concentration of DA (inset: photographs of the system with DA concentration of 0.00, 0.20, 0.30, 0.40, 0.60, 0.80, 1.00, 2.00, 4.00, and 6.00 μM, respectively. c Calibration curves for DA determination. d The effect of urea (a), ascorbic acid (b), histidine (c), glutamic acid (d), phenol (e), dichlorophenol (f), glycerol (g), dopa (h), resorcinol (i), hydroquinone (j), 5-HIAA (k), abacavir (l), and acarbose (m) on the detection of DA (Inset: The corresponding photographs). All measurements were performed in 0.1 M acetate buffer solutions (pH 4.5) containing 0.8 mM H2O2, 0.1 mM TMB, and 10 μg mL−1 N,Fe-CDs. (Error bars represent the standard deviation for three measurements)
In order to obtain the best efficiency, some experimental conditions were optimized. Firstly, the synthesis of N,Fe-CDs was explored with different hemin concentration. As shown in Fig S3, when the concentration of hemin in the co-precursors increased, the catalytic activity of N,Fe-CDs increased accordingly until the content of hemin reached 50 mg in 10 mL water containing 2 g BPEI. Then buffer pH, reaction time, TMB concentration, and H2O2 concentration were also optimized and results were exhibited in Fig. S4. The results suggested that the best UV-Vis absorption response signal to DA was obtained at pH 4.5. Fig. S4B showed that the absorbance values at 652 nm increased gradually after these materials were mixed together and reached equilibrium after 15 min. Fig. S4C indicated that the presence of 100 μM of TMB and 800 μM of H2O2 would lead to the best determination efficiency.
DA was detected by the H2O2 mediated TMB oxidation under optimized condition. It was added into TMB/H2O2 system before the addition of N,Fe-CDs. As shown in Fig. 5b, with the increase of DA concentration, the UV-Vis absorption decreased gradually and the absorbance intensity at 652 nm was proportional to the concentration of DA in the range of 0.05 ~ 0.5 μM (Fig. 5c). The regression equation was A = 0.555C + 0.745 with a correlation coefficient of 0.995 and a low detection limit of 0.03 μM (S/N = 3) for DA sensing. The inset in Fig. 5d shows that a 0.4 μM concentration of DA causes a detectable color change (Sample 3). In comparison with most of the reported methods, the colorimetric strategy exhibited higher sensitivity (as shown in Table S1) [37–44]. This result suggested that the synthesized N,Fe-CDs can be used as colorimetric method to detect DA.
Selectivity of DA determination
To investigate the selectivity of the sensing strategy, we have performed the TMB oxidation study in the presence of other possible interferences, such as urea, ascorbic acid, histidine, glutamic acid, phenol, dichlorophenol, glycerol, dopa, resorcinol, hydroquinone, 5-HIAA, abacavir, and acarbose. From the results in Fig. 5d, it is clear that DA induced a dramatic change in the absorption intensity, whereas little changes were observed in the presence of other interference substances. The results indicated that the method showcased high selectivity toward DA. It is worth noting that the interferences with strong reducing ability, such as phenol, dopa, resorcinol and hydroquinone, would cause higher optical interference. So when this strategy was applied in practical samples containing high concentration of reducing agents, the results may be affected.
Fluorescent determination of DA
As shown in Fig. 6a, the N,Fe-CDs displayed strong fluorescence at excitation wavelength of 360 nm. When TMB was mixed with N,Fe-CDs, their fluorescence did not display significant change (Fig. 6a curve a and c). When TMB/H2O2 were added into the N,Fe-CDs solution (0.1 M pH 4.5 acetate buffer solution), and the fluorescence was quenched with the quenching efficiency of 82.3 %. That was probably attributed to the oxidation products of TMB (oxTMB). The absorption of oxTMB has a peak at 460 nm (Fig. 4b, curve c), which overlapped with the emission peak of the N,Fe-CDs. Thus the fluorescence may be quenched attributed to the fluorescence inner-filter effect of oxTMB on N,Fe-CDs. Figure 6a, plot e shows that quenching is inhibited in the presence of 200 nM DA because it prevents the oxidation of TMB.
a Emission spectra of N,Fe-CDs (a), DA (b), N,Fe-CDs + TMB (c), H2O2 + TMB + N,Fe-CDs (d), and DA + H2O2 + N,Fe-CDs + TMB (e) (λex = 360 nm); Inset, the photographs of (e) and (f) under UV (365 nm) light. b Emission spectra of DA/H2O2/TMB/N,Fe-CDs with different concentration of DA. c The calibration curves for DA determination. d The effect of urea (a), ascorbic acid (b), histidine (c), glutamic acid (d), phenol (e), dichlorophenol (f), glycerol (g), dopa (h), resorcinol (i), hydroquinone (j), 5-HIAA (k), abacavir (l), and acarbose (m) on the detection of DA. All measurements were performed in 0.1 M acetate buffer solutions (pH 4.5) containing 0.2 mM H2O2, 0.05 mM TMB, and 5 μg mL−1 N,Fe-CDs. (Error bars represent the standard deviation for three measurements)
Figure 6b showed the emission spectra of H2O2/TMB/N,Fe-CDs in the presence of different concentrations of DA. Fluorescence intensity of the system increased gradually with the increase of DA concentration. In the concentration range of 0.10 ~ 0.65 μM, the fluorescence response displayed linear relationship with the concentration of DA with a correlation coefficient of 0.995. The limit of detection was calculated to be about 20 nM (S/N = 3), which was lower than most of previously reported methods (as shown in Table S1) [37–44].
The selectivity of the sensing strategy was displayed in Fig. 5d. From the results we can see that DA caused dramatic increase in the fluorescent intensity, while the presence of all the possible interferences only brought little changes. The influence of urea acid, ascorbic acid, histidine, glutamic acid, phenol, dichlorophenol, glycerol, dopa, resorcinol, hydroquinone, 5-HIAA, abacavir and acarbose hydrate on the detection of dopamine was negligible. The results indicated that the method possessed high selectivity towards DA. Likewise, these reducing interferences caused higher effect on DA detection than others.
DA determination in human serum samples
To further evaluate the applicability of the colorimetric and fluorescent detection method, DA detection in human serum were performed using the standard addition method. Human serum samples were diluted with 0.1 M acetate buffer solution (pH 4.5) for 10-fold, then the known quantities of DA, 0.8 mM H2O2, 0.1 mM TMB, and 10 μg mL−1 N,Fe-CDs were added and mixed for 15 min. The absorbance values at 652 nm were recorded to calculate the concentration of DA. The assay results in Table 1 indicated that the recovery was in the range of 93.5–105.3 %, and the RSD value is less than 4.3 %, demonstrating that the method exhibited good accuracy for determination of DA in clinical samples.
Conclusion
In summary, nitrogen and iron-containing carbon dots were successfully synthesized with the mixture of BPEI and hemin as co-precursors. The N,Fe-CDs displayed strong fluorescence and high peroxidase-like activity towards catalyzing the oxidization of TMB in the presence of H2O2. The colorimetric and fluormetric assay for DA detection was developed based on DA inhibiting the oxidation of TMB. The method exhibited high sensitivity, selectivity and good feasibility in the analysis of DA in the presence of multiple interferences as well as in practical samples (human serum). The main limitation of this method lies in the possibility that the detection may be affected in the presence of high concentration of strong reducing agents. Importantly, we report a method to synthesize peroxidase-like activity carbon dots and their applications as colorimetric and fluorometric assay in DA detection.
References
Baker SN, Baker GA (2010) Luminescent carbon nanodots: emergent Nanolights. Angew Chem Int Ed 49:6726–6744
Lim SY, Shen W, Gao Z (2015) Carbon quantum dots and their applications. Chem Soc Rev 44:362–381
Gong Y, Yu B, Yang W, Zhang X (2016) Phosphorus, and nitrogen co-doped carbon dots as a fluorescent probe for real-time measurement of reactive oxygen and nitrogen species inside macrophages. Biosens Bioelectron 79:822–828
Zuo P, Lu X, Sun Z, Guo Y, He H (2016) A review on syntheses, properties, characterization and bioanalytical applications of fluorescent carbon dots. Microchim Acta 183:519–542
Li H, He X, Kang Z, Huang H, Liu Y, Liu J, Lian S, Tsang CHA, Yang X, Lee S (2010) Water-soluble fluorescent carbon quantum dots and Photocatalyst design. Angew Chem Int Ed 49:4430–4434
Shi W, Wang Q, Long Y, Cheng Z, Chen S, Zheng H, Huang Y (2011) Carbon nanodots as peroxidase mimetics and their applications to glucose detection. Chem Commun 47:6695–6697
Zhuo S, Shao M, Lee ST (2012) Upconversion and Downconversion fluorescent graphene quantum dots: ultrasonic preparation and photocatalysis. ACS Nano 6:1059–1064
Sun H, Zhao A, Gao N, Li K, Ren J, Qu X (2015) Deciphering a Nanocarbon-based artificial peroxidase: chemical identification of the catalytically active and substrate-binding sites on graphene quantum dots. Angew Chem Int Ed 54:7176–7180
Zhu W, Zhang J, Jiang Z, Wang W, Liu X (2014) High-quality carbon dots: synthesis, peroxidase-like activity and their application in the detection of H2O2, Ag+ and Fe3+. RSC Adv 4:17387–17392
Zhang G, Dasgupta PK (1992) Hematin as a peroxidase substitute in hydrogen peroxide determinations. Anal Chem 64:517–522
Ge C, Luo Q, Wang D, Zhao S, Liang X, Yu L, Xing X, Zeng L (2014) Colorimetric detection of copper(II) ion using click chemistry and hemin/G-Quadruplex horseradish peroxidase-mimicking DNAzyme. Anal Chem 86:6387–6392
Bruice TC (1991) Reactions of hydroperoxides with metallotetraphenyl- porphyrins in aqueous solutions. Acc Chem Res 24:243–249
Li Y, Huang X, Li Y, Xu Y, Wang Y, Zhu E, Duan X, Huang Y (2013) Graphene-hemin hybrid material as effective catalyst for selective oxidation of primary C-H bond in toluene. Sci Rep 3:1787
Wang Q, Xu N, Gui Z, Lei J, Ju H, Yan F (2015) Strand displacement activated peroxidase activity of hemin for fluorescent DNA sensing. Analyst 140:6532–6537
Nakagaki S, Wypych F (2007) Nanofibrous and nanotubular supports for the immobilization of metalloporphyrins as oxidation catalysts. J Colloid Interface Sci 315:142–157
Dong Y, Wang R, Li H, Shao J, Chi Y, Lin X, Chen G (2012) Polyamine-functionalized carbon quantum dots for chemical sensing. Carbon 50:2810–2815
Hsu PC, Chang HT (2012) Synthesis of high-quality carbon nanodots from hydrophilic compounds: role of functional groups. Chem Commun 48:3984–3986
Zhang A, Neumeyer J, Baldessarini RJ (2007) Recent progress in development of dopamine receptor subtype-selective agents: potential therapeutics for neurological and psychiatric disorders. Chem Rev 107:274–302
Dawson TM, Dawson VL (2003) Molecular pathways of neurodegeneration in Parkinson’s disease. Science 302:819–822
Hong C, Liu H, Liu T, Liao D, Tsai SJ (2005) Association studies of the adenosine A2a receptor (1976 T > C) genetic polymorphism in Parkinson’s disease and schizophrenia. J Neural Transm 112:1503–1510
Sanghavi BJ, Wolfbeis OS, Hirsch T, Swami NS (2015) Nanomaterial-based electrochemical sensing of neurological drugs and neurotransmitters. Microchim Acta 182:1–41
Yusoff N, Pandikumar A, Ramaraj R, Lim HN, Huang NM (2015) Gold nanoparticle based optical and electrochemical sensing of dopamine. Microchim Acta 182:2091–2114
Palanisamy S, Sakthinathan S, Chen S, Thirumalraj B, Wu T, Lou B, Liu X (2016) Preparation of β-cyclodextrin entrapped graphite composite for sensitive detection of dopamine. Carbohydr Polym 135:267–273
Guo Z, Seol M, Kim M, Ahn J, Huang X (2013) Sensitive and selective electrochemical detection of dopamine using an electrode modified with carboxylated carbonaceous spheres. Analyst 138:2683–2690
Yang Y, Cui J, Zheng M, Hu C, Tan S, Xiao Y, Yang Q, Liu Y (2012) One-step synthesis of amino-functionalized fluorescent carbon nanoparticles by hydrothermal carbonization of chitosan. Chem Commun 48:380–382
Dong Y, Zhou N, Lin X, Lin J, Chi Y, Chen G (2010) Extraction of Electrochemiluminescent oxidized carbon quantum dots from activated carbon. Chem Mater 22:5895–5899
Zhu H, Wang X, Li Y, Wang Z, Yang F, Yang X (2009) Microwave synthesis of fluorescent carbon nanoparticles with electrochemiluminescence properties. Chem Commun 45:5118–5120
Liu S, Tian J, Wang L, Zhang Y, Qin X, Luo Y, Asiri AM, Al-Youbi AO, Sun X (2012) Hydrothermal treatment of grass: a low-cost, green route to nitrogen-doped, carbon-rich, photoluminescent polymer nanodots as an effective fluorescent sensing platform for label-free detection of Cu(II) ions. Adv Mater 24:2037–2041
Fleutot S, Dupin JC, Renaudin G, Martinez H (2011) Intercalation and grafting of benzene derivatives into zinc–aluminum and copper–chromium layered double hydroxide hosts: an XPS monitoring studyw. Phys Chem Chem Phys 13:17564–17578
Yamashita T, Hayes P (2008) Analysis of XPS spectra of Fe2+ and Fe3+ ions in oxide materials. Appl Surf Sci 254:2441–2449
Sun Y, Zhou B, Lin Y, Wang W, Fernando KAS, Pathak P, Meziani MJ, Harruff BA, Wang X, Wang H, Luo PG, Yang H, Kose ME, Chen B, Veca LM, Xie S (2006) Quantum-sized carbon dots for bright and colorful photoluminescence. J Am Chem Soc 128:7756–7757
Dong Y, Pang H, Yang H, Guo C, Shao J, Chi Y, Li C, Yu T (2013) Carbon-based dots Co-doped with nitrogen and sulfur for high quantum yield and excitation-independent emission. Angew Chem Int Ed 52:7800–7804
Gao L, Wu J, Gao D (2011) Enzyme-controlled self-assembly and transformation of nanostructures in a tetramethylbenzidine/horseradish peroxidase/H2O2 system. ACS Nano 5:6736–6742
Shete MD, Fernandes JB (2015) A simple one step solid state synthesis of nanocrystalline ferromagnetic α-Fe2O3 with high surface area and catalytic activity. Mater Chem Phys 165:113–118
Cao S, Kang F, Li P, Chen R, Liu H, Wei Y (2015) Photoassisted hetero-Fenton degradation mechanism of acid blue 74 by a γ-Fe2O3 catalyst. RSC Adv 5:66231–66238
Nirala NR, Abraham S, Kumar V, Bansal A, Srivastava A, Saxen PS (2015) Colorimetric detection of cholesterol based on highly efficient peroxidase mimetic activity of graphene quantum dots. Sensors Actuators B 218:42–50
Dutta S, Ray C, Mallick S, Sarkar S, Sahoo R, Negishi Y, Pal T (2015) A gel-based approach to design hierarchical CuS decorated reduced graphene oxide Nanosheets for enhanced peroxidase-like activity leading to colorimetric detection of dopamine. J Phys Chem C 119:23790–23800
Chen Z, Zhang C, Zhou T, Ma H (2015) Gold nanoparticle based colorimetric probe for dopamine detection based on the interaction between dopamine and melamine. Microchim Acta182: 1003–1008
Liu J, Wang X, Cui M, Lin L, Jiang S, Jiao L, Zhang L (2013) A promising non-aggregation colorimetric sensor of AuNRs–Ag+ for determination of dopamine. Sensors Actuators B Chem 176:97–102
Yang A, Xue Y, Zhang Y, Zhang X, Zhao H, Li X, He Y, Yuan Z (2013) A simple one-pot synthesis of graphene nanosheet/SnO2 nanoparticle hybrid nanocomposites and their application for selective and sensitive electrochemical detection of dopamine. J Mater Chem B 1:1804–1811
Li H, Liu J, Yang M, Kong W, Huang H, Liu Y (2014) RSC Adv 4:46437–46443
Li H, Yang M, Liu J, Zhang Y, Yang Y, Huang H, Liu Y, Kang Z (2015) A practical and highly sensitive C3N4-TYR fluorescent probe for convenient detection of dopamine. Nanoscale 7:12068–12075
Teng Y, Jia X, Li J, Wang E (2015) Ratiometric fluorescence detection of Tyrosinase activity and dopamine using thiolate-protected gold nanoclusters. Anal Chem 87:4897–4902
Mu Q, Xu H, Li Y, Ma S, Zhong X (2014) Adenosine capped QDs based fluorescent sensor for detection of dopamine with high selectivity and sensitivity. Analyst 139:93–98
Acknowledgments
This work was supported by PhD start-up grants SWU113112, SWU113111 from Southwest University, National Natural Science Foundation of China (21505108) and Chongqing Key Laboratory for Advanced Materials and Technologies of Clean Energies (Grant cstc2011pt).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
The author(s) declare that they have no competing interests
Electronic Supplementary Material
ESM 1
(DOC 430 kb)
Rights and permissions
About this article
Cite this article
Wang, B., Chen, Y., Wu, Y. et al. Synthesis of nitrogen- and iron-containing carbon dots, and their application to colorimetric and fluorometric determination of dopamine. Microchim Acta 183, 2491–2500 (2016). https://doi.org/10.1007/s00604-016-1885-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00604-016-1885-5