Abstract
The authors describe poly(dimethylsiloxane)-coated carbon nanofibers (CNF-PDMS) for solid-phase microextraction of polycyclic aromatic hydrocarbons (PAHs). The fibers were prepared by a sol–gel method and are stable at temperatures up to 350 °C, probably due to the chemical bonds between the coating and the fiber surface. The fibers can be re-used up to 180 times. The CNFs enhance the surface area of the coating compared to that of a plain PDMS fiber and, accordingly, provide higher extraction efficiency. Following thermal desorption, the PAHs were quantified by GC with FID detection. Under optimized experimental conditions, the detection limits of the method range from 5 to 20 pg mL−1, and response is linear in the 0.017 to 100 ng mL−1 range. The repeatability and reproducibility vary between 4.8 % and 8.6 %, and between 4.1 % and 10.2 %, respectively. The method was successfully utilized for the analysis of PAHs in (spiked) water samples, with satisfactory recoveries in the range of 90.1–99.9 %.

We describe a novel adsorbent consisting of poly(dimethylsiloxane) grafted onto carbon nanofibers (CNF-PDMS). It was applied to the solid phase microextraction of polycyclic aromatic hydrocarbons from environmental water samples.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Polycyclic aromatic hydrocarbons (PAHs) are released to the environment as a result of incomplete combustion of organic materials such as coal, oil, wood, meat, gas and tobacco. They possess relatively low water solubility [1] and they exert carcinogenic and mutagenic effects on human health. The Environmental Protection Agency (EPA) has classified 16 PAHs as priority pollutants [2]. Due to their trace level concentration, it is required and vital to develop efficient preconcentration procedures and selective and sensitive separation and analysis techniques. So far various techniques such as solid-phase microextraction (SPME) [3–5] and magnetic solid-phase extraction (MSPE) [6, 7] have been coupled with different analytical instrumentation methods for the extraction, separation and pre-concentration of PAHs in environmental samples.
SPME is a simple, fast, sensitive, and convenient equilibrium-based sample preparation technique that allows the integration of sampling, extraction, concentration and sample introduction steps [8]. The sorbent coating, used to adsorb the analytes from samples, is a key part for the extraction ability of SPME. It is evident that future advances in SPME will greatly depend on new developments in sorbent chemistry and coating technology that will enable preparation of stable coatings from advanced material systems providing the desired selectivity and performance in SPME [9].
The sol–gel technology for creating microextraction sorbents with a uniform film, developed by Malik and co-workers [10], is considered a ground breaking event in the field of sorbent-based sorptive microextraction and has catalyzed the creation of hundreds of novel sol–gel based sorbents with unique selectivity, extraction sensitivity, and numerous applications. Among others, some noteworthy advantages of the sol–gel technology include: use of inexpensive raw materials; mild reaction conditions; unprecedented level of uniformity in the coating; adjustable coating thickness via sol solution design and/or controlling reaction conditions; ability to create the sorbent coating on the surface of substrates of any geometrical shape (both inside and outside); numerous ways to fine tune the selectivity of microextraction sorbents; fast mass transfer rate during extraction, due to the inherently porous and open structure of the sol–gel derived sorbents; formation of covalent bonding between the sol–gel hybrid polymeric network and the host substrate, leading to a remarkably high thermal, chemical, and solvent stability of the microextraction sorbents [11].
It was expected that the large specific surface areas of nanomaterials improves the detection sensitivity and allows for a miniaturization of the device. Therefore, in sample treatment, NPs have been extensively employed to design novel extraction techniques focused on isolation and/or preconcentration of target analytes from different samples [12, 13]. Moreover, nanomaterials can also be functionalized with various chemical groups to increase their affinity toward target compounds, which makes them designable for selective extracting target analytes in complex matrices, e.g. environmental and biological samples [14–17]. Since the extraction adsorbent SPME is the core for accomplishing trace amount analysis, developing adsorbent with high extraction efficiency is still an attractive task.
Carbon nanofibers (CNF) are one of the carbonic materials with unique physicochemical and mechanical properties, high porosity and high specific surface area, which can be used as sorptive materials for the adsorption of organic contaminates. The excellent adsorption capabilities of CNFs can be attributed to the distortion of planar graphene sheets into a helical or cylindrical fashion. As a consequence, carbon nanoparticles readily experience fluctuating and induced dipole moments, which results in excellent van der Waals adhesion to organic species. This effect along with their ability to establish non-covalent π-stacking interactions accounts for improved extraction capacity of either nonpolar or moderately polar organic compounds [18, 19].
The chemical vapour deposition (CVD) was applied in this work to synthesize the CNFs. Then hydroxyl-terminated poly(dimethylsiloxane) (PDMS) grafted onto CNFs by the covalent functionalization (CNF-PDMS). Finally, the functionalized product (CNF-PDMS) was used as extraction phase to prepare the sol–gel SPME fiber in combination with gas chromatography-flame ionization detector (GC–FID) for the determination of PAHs from water samples.
Experimental
Instrumental
GC analyses were performed using a gas chromatograph (Shimadzu-17 A, Tokyo, Japan, www.shimadzu.com) with a split/splitless injection port and a flame ionization detection system (FID). Hydrogen gas (H2) was supplied for FID by using a Shimadzu OPGU-2200 s hydrogen generator (Tokyo, Japan, www.shimadzu.com). Nitrogen (99.999 %) was employed as a carrier gas, and its flow-rate was adjusted to 1 mL min−1. The gas chromatograph was equipped with a Shimadzu Hicap CBP1-M25–025 capillary fused silica column (25 m, length; 0.25 mm I.D.; 0.22 m, film thickness; stationary phase, 5 % phenyl 95 % dimethyl polysiloxane). The column was held at 80 °C for 5 min and increased to 280 °C at a rate of 5 °C min−1 and held for 2 min. The detector temperature was held at 300 °C. The injector temperature was set at 300 °C and fiber desorption was carried out in the splitless mode for 5 min. To mix various solution ingredients, an ultrasonic bath (Parsonic 15S, Pars Nahand Engineering Co. Iran, www.pnec.co.ir), was employed at a frequency of 28 kHz. Scanning electron microscopy (SEM) (Hitachi S-3400 N, www.hitachi-hightech.com) and transmission electron microscopy (Leo, model 912 AB, Germany, http://www.leo-em.co.uk/) were used to characterize the morphology and size of the CNFs. Also, the surface characteristics of the coating fibers were studied by SEM (LEO, model 1450VP, Germany, http://www.leo-em.co.uk/).
Reagents and standards
Hydroxy-terminated poly(dimethylsiloxane) (PDMS) was purchased from Fluka (Buchs, Switzerland), and methyltrimethoxysilane (MTMOS), poly(methylhydrosiloxane) (PMHS), trifluoroacetic acid (TFA) (99 %), hydrochloric acid, nitric acid, and sodium hydroxide were purchased from Merck (Darmstadt, Germany, www.merck.com). Analytical reagents grade tetrahydrofuran (THF), methanol and toluene were also purchased from Merck (Darmstadt, Germany). Toluene and tetrahydrofuran (THF) were dried, deoxygenated, and distilled from Na/benzophenone ketyl before use. Polycyclic aromatic hydrocarbons include naphthalene, fluorene, phenanthrene, anthracene and pyrene were purchased from Merck (Darmstadt, Germany). The stock solutions of the analytes (10 mg mL−1) were prepared in methanol and the working solutions were obtained daily by appropriate dilution with bidistilled water. All the stock solutions were stored at a temperature of 4 °C. All water samples were collected in 250 mL amber glass bottles, previously washed with methanol and ultrapure water and rinsed with the sample. They were filled up completely and analyzed as soon as they arrived at the laboratory.
Synthesis of CNFs
Carbon nanofibers were synthesized by pyrolysis of acetylene on catalytic Fe particles deposited on polyacrylonitrile (PAN)-based carbon fibers (CF) (Toho Tenax America, Inc.). Iron catalysts were deposited by solution dipping. First carbon fibers (CFs) were immersed into an iron nitrate nonahydrate solution (100 mM) and followed by ultrasonic agitation for 2 h. Then they were dried and calcinated at 200 °C under air flow for 2 h to remove the nitrate components and make the desired catalyst coating on the surface of the CF. CVD was applied to grow the CNFs on the CF at atmospheric pressure and the temperature at 600 °C for 30 min. This process was fulfilled by a catalytic reaction of an acetylene flow rate of 50 standard cubic centimeters per minute (sccm) over Fe/CF in the reactor under a flow rate of H2/N2 (100, 100 sccm, respectively). At the end of the run time, the C2H2 flow was stopped, the heater was turned off and then the reactor was cooled under the flow of N2.
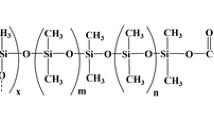
Preparation of carbon nanofibers modified with poly(dimethylsiloxane) (CNF-PDMS)
First, 1 g of CNFs was dispersed into a round-bottomed glass flask containing 50 mL of 60 % nitric acid. The solution was then shaken in an ultrasonic cleaning bath for 30 min. Next the mixture was refluxed at 120 °C and maximum stirrer rate for 24 h. After having been cooled to the room temperature, the mixture was filtered on a 0.22 μm filter (Millipore, Bedford, MA, USA). Finally, the CNFs were washed thoroughly with re-distilled water several times until the pH of the filtrate was neutral. The filtered solid was dried in the vacuum oven at 60 °C for 12 h, obtaining carboxylic acid-functionalized CNFs (CNF-COOH). To convert the surface-bound carboxyl groups into acyl chloride groups, 500 mg of carboxylated CNFs powder was dispersed into 20 mL of SOCl2 at 70 °C for 24 h. The excess thionyl chloride was removed under vacuum and CNF–COCl were washed with dry THF and stored under nitrogen. Then CNF-COCl was mixed with 1 g of PDMS in 20 mL of toluene/THF solvent mixture (v/v 3/1) and 2 mL of triethylamine. The mixture was stirred for 48 h at 80 °C under a nitrogen atmosphere. The solid product, which was CNF-PDMS, was washed repeatedly with dry tetrahydrofuran to remove the untreated PDMS, and then dried under vacuum for 24 h at 100 °C.
Fiber preparation
Prior to sol–gel coating, the protective polyimide layer of a 1 cm segment of a 2 cm fused-silica fiber was removed by the flame of a gas burner. The fused silica fiber was then rinsed with ultrapure water and dipped into 1 M NaOH solution for 1 h to expose the maximum number of silanol groups on the surface of the fiber. Subsequently, the fiber was placed in 0.1 M HCl solution for 30 min to neutralize the remaining NaOH, cleaned again with ultrapure water, and dried at room temperature.
The sol solution was prepared as follows: 25 mg of CNF-PDMS powder and 100 mg of PDMS were added to 100 μL methylene chloride in an Eppendorf tube and dissolved by ultrasonic agitation for 5 min. Then 100 μL MTMOS and 30 μL of PMHS were added to the solution and the mixture was sonicated for another next 5 min. A 60-μL portion of 95 % TFA in water was subsequently added to the resulting solution under ultrasonic agitation. The mixture was then centrifuged at 10,000 rpm for 5 min, and the top clear sol solution was used for fiber coating. A sol–gel coating was formed on the outer surface of the treated fiber end, after the fiber was dipped vertically into the sol–gel solution for 1 h. For each fiber, this coating process was repeated several times until the desired thickness of the coating was obtained. After coating the substrate with the sol solution, the coated substrate was removed from the sol solution and kept in the desiccator overnight for solvent evaporation and aging of the sol–gel coating. Prior to use, the CNF-PDMS fibers were conditioned in the injector of a GC (1 h at 100 °C, 1 h at 200 °C and then 1 h at 300 °C) under 1 mL min−1 of nitrogen. For comparison purposes, a PDMS coating fiber that did not contain any CNFs was prepared in a similar manner.
Headspace SPME procedure
To prevent damage to the fiber coating and elimination of the matrix effect on the fiber headspace-SPME (HS-SPME) was utilized. For HS-SPME mode, 15 mL of sample was placed in a 20 mL sample vial together with a magnetic stirring bar and sealed with PTFE septum. The fiber was carefully introduced directly into the headspace above the aqueous phase and the solution was stirred at a rate of 1000 rpm provided by the stirring hotplate, at 50 °C for 40 min. After extraction, the fiber was immediately inserted into the GC injection port at 270 °C for thermal desorption, and the chromatographic peak area of the analyte was used for quantitation and to examine the extraction efficiency of a SPME fiber. Triplicate analyses were performed for each sample.
Results and discussion
The chemistry of the CNF-PDMS sol–gel coating
To enhance of extraction efficiency, CNF-PDMS was selected as the extraction phase. OH-terminated PDMS in the coating can not only increase the length of network but also help to spread the stationary phase on the silica surface uniformly. PMHS is used as the deactivation reagent. Also, MTMOS, TFA (with 5 % v/v water), and methylene chloride were used as the inorganic precursor, sol–gel catalyst and solvent system, respectively. The creation of the sol–gel CNF-PDMS coating on the fused-silica substrate involved a number of reactions including: (1) controlled catalytic hydrolysis of the sol–gel precursor, MTMOS; (2) polycondensation of hydrolyzed MTMOS, resulting in a growing three-dimensional inorganic silica network; (3) random incorporation of sol–gel active PDMS and CNF-PDMS polymer into the evolving sol–gel network; and (4) chemical anchorage of the growing sol–gel network via condensation to the fused silica substrate.
Characterization of sol–gel CNF-PDMS coated fiber
The morphology of the prepared CNFs was investigated through a scanning electron microscope (SEM) and transmission electron microscope (TEM). Figure 1a indicates that CNFs with very thin diameter were formed uniformly on the carbon fiber. The CNFs exhibit a clean surface with long and smooth fibrous morphology according to the SEM images of Fig. 1a and b. The TEM image of Fig. 2 shows the structure on CNF surfaces and no distinct defect is found.
Figure 3 shows the SEM images of the CNF-PDMS fiber surfaces at different magnifications. The results indicated that the CNF-PDMS SPME surface possesses high porous structure. This increases the available surface area and improves mass transfer during extraction and desorption. CNFs can be seen clearly in the coating. The presence of CNFs in the surface structure of CNF-PDMS fibers can be lead to enhancement of the surface area and consequently adsorption efficiency of the fiber.
Thermal stability and lifetime of the coating
The thermal stability of the CNF-PDMS fiber was studied by HS-SPME of PAHs in aqueous solution. The fiber was conditioned 1 h at different temperatures (250 °C up to 350 °C), and then were used to extract the analytes. The extraction efficiencies of the fiber were not affected by the conditioning process, which demonstrated the excellent thermal stabilities of the fiber. The lifetime of a fiber coating is also important for practical application. The lifetime testing of the fibers was investigated by extracting PAHs from water sample. The results indicate that the extraction efficiency of CNF-PDMS fiber did not decline even after being extracted 180 times, showing a high durability (Fig. 4).
Optimization of extraction and desorption conditions
The following parameters were optimized: (a) desorption conditions; (b) extraction temperature; (c) extraction time; and (d) concentration of NaCl. Respective data and figures are given in the Electronic Supporting Material (ESM). The following experimental conditions were found to give best results: (a) desorption temperature: 250 °C, desorption time: 5 min; (b) extraction temperature: 50 °C (Fig. S1, ESM); (c) extraction time: 40 min (Fig. S2, ESM); and (d) the concentration of NaCl: 30 % w/v (Fig. S3, ESM).
The study of performance of CNF-PDMS fibers
To prove the advantage of usage of CNFs in the extraction of PAHs, two kinds of fibers (PDMS and CNF-PDMS) with the same thickness were used to extract of desired compounds at the optimized conditions. According to Fig. 5, the extraction efficiency of the CNF-PDMS fiber for target compounds was better than the PDMS and higher amounts of analytes were extracted. This enhancement was due to the presence of CNFs in the coating that exhibit considerable porosity and higher specific surface areas for the extraction of desired analytes.
Also, the extraction profile of the CNF-PDMS fiber was compared with two commercially available fibers, PDMS (100 μm) and PDMS/DVB (65 μm) fibers in the analysis of PAHs under the optimized conditions. Figure 5 shows this comparison, where one can conclude that even with a lesser thickness, the extraction ability of the CNF-PDMS fiber (18 μm) is better than PDMS and PDMS/DVB. The reason for this event is the high surface area of CNFs and porous surface structure of sol–gel coated fiber.
Validation of the method
The analytical characteristics of the optimized HS-SPME with CNF-PDMS coating fiber in terms of its linear range, repeatability, reproducibility and limits of detection (LODs) were investigated to estimate the efficiency and the feasibility for application to the analysis of environmental samples. The results are shown in Table 1. The SPME procedure showed linear range of 0.017–100 ng mL−1 for the PAHs under study. The correlation coefficients were between 0.9990 and 0.9997. Limit of detection (LOD), calculating from the lowest concentration of calibration curves with a signal-to-noise ratio of 3, ranged from 0.005 to 0.02 ng mL−1. The repeatability of this method is shown in Table 1, and its determination was done in five consecutive extractions at concentrations of 0.06, 5, and 50 ng mL−1 of the PAHs. Also, Table 1 represents the fiber-to-fiber reproducibility (batch-to-batch) of the sol–gel-coated CNF-PDMS fibers. Three identically sol–gel-coated CNF-PDMS fibers prepared in three batches were tested for the extraction of PAHs at three concentration levels (0.06, 5, and 50 ng mL−1).
The proposed method was compared with a variety of previously reported for determination of PAHs in literature. The distinct features of the methods were summarized in Table 3. This method had wide linear dynamic range and lower or comparable LODs than other reported methods. The effectiveness of the present sorbent can be ascribed; a) the three-dimensional network structure of sol–gel coating which has larger surface area, and (b) using CNFs with high surface area as sorbent modifiers will also be able to provide the enhanced adsorption efficiency for the analyte.
Real sample analysis
The established HS-SPME method using CNF-PDMS sol–gel coating fiber was applied to determine selected PAHs compounds in different water samples. Well water, rain water, river water and wastewater were collected from Sabzevar, Razavi Khorasan Province, Iran. Water samples were stored in amber-glass bottles without headspace and maintained in the dark at 4 °C until their analysis. All samples were analyzed in triplicate by the optimized method. The quantitative results of these real samples are listed in Table 2. Some of the PAHs compounds were detected in the wastewater at trace levels. In order to investigate the performance of the established method, the CNF-PDMS sol–gel coating fiber was applied to extract these samples spiked at three concentration levels (0.06, 5 and 50 ng mL−1) for each compound. The relative recoveries and determination precisions are listed in Table 2. The results showed that the relative recoveries of PAHs compounds (n = 3) ranged from 90.1 to 99.9 % and the RSDs were between 4.3 and 11.3 %. These results clearly demonstrated that the accuracy of the method for the analysis of PAHs in real samples was quite satisfactory.
Conclusions
A new SPME fiber made from PDMS grafted onto CNFs (CNF-PDMS) by sol–gel technique. To the best of our knowledge, this represents the first report on the creation and use of sol–gel CNF-PDMS coating in SPME. Compared with commercial PDMS (100 μm) and PDMS/DVB (65 μm) fibers, the sol–gel-coated CNF-PDMS fiber (18 μm) showed superior extraction efficiency for all the PAHs analyzed. The synthesized fiber had a long lifetime (used over 180 times for headspace SPME) and good thermal stability up to 350 °C without showing significant changes in its properties after being heated for 1 h in the GC injection port. This offers new potentials in ultratrace analysis of contaminants.
References
Wong PK, Wang J (2011) The accumulation of polycyclic aromatic hydrocarbons in lubricating oil over time—a comparison of supercritical fluid and liquid-liquid extraction methods. Environ Pollut 112:407–415
U.S. EPA Method 61040 CFR Part 136, App. A-National Environment Methods, 2004.
Zhang X, Zang ZH, Wang JT, Wang C, Wu QH, Wang Z (2015) Porous carbon derived from aluminum-based metal organic framework as a fiber coating for the solid-phase microextraction of polycyclic aromatic hydrocarbons from water and soil. Microchim Acta 182:2353–2359
Abolghasemi MM, Yousefi V, Rafiee E (2014) Polyoxotungstate nanoclusters supported on silica as an efficient solid-phase microextraction fiber of polycyclic aromatic hydrocarbons. Microchim Acta 181:1807–1814
Abolghasemi MM, Yousefi V, Hazizadeh B (2014) An inorganic-organic hybrid material based on ZnO nanoparticles anchored to a composite made from polythiophene and hexagonally ordered silica for use in solid-phase fiber microextraction of PAHs. Microchim Acta 181:639–645
Amiri A, Baghayeri M, Kashmari M (2016) Magnetic nanoparticles modified with polyfuran for the extraction of polycyclic aromatic hydrocarbons prior to their determination by gas chromatography. Microchim Acta 183:149–156
Bunkoed O, Kanatharana P (2015) Extraction of polycyclic aromatic hydrocarbons with a magnetic sorbent composed of alginate, magnetite nanoparticles and multiwalled carbon nanotubes. Microchim Acta 182:1519–1526
Arthur CL, Pawliszyn J (1990) Solid phase microextraction with thermal desorption using fused silica optical fibers. Anal Chem 62:2145–2148
Aziz-Zanjani MO, Mehdinia A (2014) A review on procedures for the preparation of coatings for solid phase microextraction. Microchim Acta 181:1169–1190
Chong SL, Wang DX, Hayes JD, Wilhite BW, Malik A (1997) Sol − Gel Coating Technology for the preparation of solid-phase microextraction fibers of enhanced thermal stability. Anal Chem 69:3889–3898
Amiri A (2016) Solid-phase microextraction-based sol–gel technique. Trends Anal Chem 75:57–74
Lucena R, Simonet BM, Cárdenas S, Valcárcel M (2011) Potential of nanoparticles in sample preparation. J Chromatogr A 1218:620–637
Ghaemi F, Amiri A, Yunus R (2014) Methods for coating solid-phase microextraction fibers with carbon nanotubes. Trends Anal Chem 59:133–143
Sarafraz-Yazdi A, Amiri A, Rounaghi G, Hosseini HE (2011) A novel solid-phase microextraction using coated fiber based sol–gel technique using poly(ethylene glycol) grafted multi-walled carbon nanotubes for determination of benzene, toluene, ethylbenzene and o-xylene in water samples with gas chromatography-flam ionization detector. J Chromatogr A 1218:5757–5764
Sarafraz-Yazdi A, Amiri A, Rounaghi G, Eshtiagh-Hosseini H (2012) Determination of non-steroidal anti-inflammatory drugs in water samples by solid-phase microextraction based sol–gel technique using poly(ethylene glycol) grafted multi-walled carbon nanotubes coated fiber. Anal Chim Acta 720:134–141
Jiang R, Zhu F, Luan T, Tong Y, Liu H, Ouyang G, Pawliszyn J (2009) Carbon nanotube-coated solid-phase microextraction metal fiber based on sol-gel technique. J Chromatogr A 1216:4641–4647
Zhang W, Sun Y, Wu C, Xing J, Li J (2009) Polymer-functionalized single-walled carbon nanotubes as a Novel Sol − Gel solid-phase micro-extraction coated fiber for determination of poly-brominated diphenyl ethers in Water samples with gas chromatography − electron capture detection. Anal Chem 81:2912–2920
Chigome S, Darko G, Torto N (2011) Electrospun nanofibers as sorbent material for solid phase extraction. Analyst 136:2879–2889
Zhang L, Aboagye A, Kelkar A, Lai C, Fong H (2014) A review: carbon nanofibers from electrospun polyacrylonitrile and their applications. J Mater Sci 49:463–480
Xu H, Ding Z, Lv L, Song D, Feng YQ (2009) A novel dispersive liquid–liquid microextraction based on solidification of floating organic droplet method for determination of polycyclic aromatic hydrocarbons in aqueous samples. Anal Chim Acta 636:28–33
Zanjani MRK, Yamini Y, Shariati S, Jonsson JA (2007) A new liquid-phase microextraction method based on solidification of floating organic drop. Anal Chim Acta 585:286–293
Maghsoudi S, Noroozian E (2012) HP-SPME of volatile polycyclic aromatic hydrocarbons from Water using multiwalled carbon nanotubes coated on a steel fiber through electrophoretic deposition. Chromatographia 75:913–921
Behzadi M, Noroozian E, Mirzaei M (2013) A novel coating based on carbon nanotubes/poly-Ortho-phenylenediamine composite for headspace solid-phase microextraction of polycyclic aromatic hydrocarbons. Talanta 108:66–73
Wei M-C, Jen J (2007) Determination of polycyclic aromatic hydrocarbons in aqueous samples by microwave assisted headspace solid-phase microextraction and gas chromatography/flame ionization detection. Talanta 72:1269–1274
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The author(s) declare that they have no competing interests
Electronic supplementary material
ESM 1
(DOCX 5643 kb)
Rights and permissions
About this article
Cite this article
Amiri, A., Ghaemi, F. Thermally stable carbon nanofibers functionalized with poly(dimethylsiloxane) for solid-phase microextraction of polycyclic aromatic hydrocarbons prior to GC analysis. Microchim Acta 183, 1917–1924 (2016). https://doi.org/10.1007/s00604-016-1832-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00604-016-1832-5