Abstract
We describe the synthesis of a hybrid nanocomposite consisting of MoS2 and reduced graphene oxide (MoS2/rGO) by a one-pot hydrothermal process, and the use of the material in an amperometric sensor. Scanning electron microscopy and nitrogen adsorption-desorption tests revealed that the nanocomposite possesses a porous and flower-like structure and a large specific surface. Cyclic voltammetry and differential pulse voltammetry demonstrated the nanocomposite to perform excellently in the electrochemical oxidation of ascorbic acid (AA), dopamine (DA), and uric acid (UA), respectively. The oxidation potentials are well separated (oxidation peaks at −64, 168 and 320 mV vs. Ag/AgCl) for AA, DA, and UA, and the respective detection sensitivities are strongly enhanced (0.12, 4.11 and 1.59 μA μM−1 cm−2). The sensor was applied to the detection of the three species in spiked human serum samples where it gave recoveries ranging from 98.9 to 104.1 % (for n = 3).

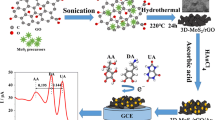
MoS2/reduced graphene oxide (MoS2/rGO) hybrid nanocomposite was synthesized by a one-pot hydrothermal process and used to modify a glassy carbon electrode for the simultaneous detection of ascorbic acid (AA), dopamine (DA), and uric acid (UA).
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Ascorbic acid (AA), dopamine (DA) and uric acid (UA) are considered as crucial small biomolecules in the physiological processes of human metabolism. AA, i.e. vitamin C, is one of the essential nutrients for most mammal, but also associated with various disorders in human body, such as scurvy, mental illness, hepatic disease, infertility, common cold, cancer and AIDS [1, 2]. As one of the most important catecholamine neurotransmitters, DA plays a vital role in the function of central nervous, renal, hormonal and cardiovascular systems, abnormal levels of which may lead to serious neurological disorders, like Parkinson’s disease and schizophrenia [3, 4]. UA is the primary end product of purine metabolism, abnormal levels of which in urine and serum was a symptom of several diseases such as gout, hyperuricemia, pneumonia and Lesch-Nyhan syndrome [5, 6]. On the other hand, AA, DA, and UA usually co-exist in human physiological fluids. The simultaneous determination of these three small biomolecules is a challenging issue for the prediction and prevention of multiple diseases. Electrochemical methods are an effective toolbox for the detection of AA, DA, or UA due to their high sensitivity, simplicity, low costs and fast response for analysis [7–12]. However, these three species usually foul the detection electrodes and can be oxidized at almost the same potential at the traditional electrodes [13, 14]. Therefore, the technique difficulties for electrochemically simultaneous determination lie in how to achieve distinguishable oxidation potentials and sensitive current response at modified electrode.
Graphene oxide (rGO) has been considered as a versatile scaffold for nanomaterials to form hybrid nanocomposite with improved properties [15–23]. Compared with pure graphene (GN), rGO was hydrophilic and easily dispersed into many solvents for various modification, due to its defects and numerous oxygen functions (-OH, C=O, -COOH). Up to now, rGO has been used as an effective support matrix to disperse or stabilize noble metal such as Ag, Au, Pt, Pd, and alloys [7–11]. In addition, those hybrid nanocomposites combining rGO sheets with noble metal nanomaterials have been applied to electrochemically simultaneous detection of AA, DA, and UA. However, those methods to composite rGO sheets with precious metal nanomaterials suffered from some disadvantages such as high cost, multiple-step and time-consuming processes [7–11]. Furthermore, the obtained analytical performance based on those nanocomposites, was highly demanded to be improved. For instance, the detection sensitivity was still not high enough and the separation of the oxidation potential was of necessity to be further extended. Therefore, it is urgently necessary to develop alternatives to noble metals to obtain a low-cost and high-performance rGO-based nanocomposite for electrochemically simultaneous detection of small biomolecules in practical applications.
MoS2-based materials exhibit superior performance in the energy conversion and storage field including solar cells, electrocatalysis, supercapacitors and lithium ions batteries, due to their unique physical and chemical properties such as graphene-like 2D structure, tunable band gap, edge/defective sites and so on [24, 25]. Although there have been increasing reports on the MoS2-based materials for bioactive macromolecules detection like DNA, micro-RNA and protein [26–28], it is still desirable to develop new MoS2-based nanocomposite for the detection of small biomolecules such as AA, DA, and UA. Herein, MoS2/rGO nanocomposite with porous nanostructure, large specific surface area and good electric conductivity, was prepared simply by a one-pot hydrothermal treatment. The MoS2/rGO nanocomposite combining rGO and MoS2 was used as electrode material to successfully construct a selective, stable and reproducible electrochemical biosensor for simultaneous detection of AA, DA, and UA.
Experimental
Reagents and apparatus
Graphene oxide (GO) powders (JCGO-99-1-150n; the contents of O and N tested by X-ray photoelectron spectroscopy (XPS) were 20.31 and 1.71 %, respectively) were purchased from JCNANO (Nanjing, China, www.jcnano.com). All the other chemicals were of analytical grade from commercial sources and used without further purification unless otherwise specified. High-resolution transmission electron microscopy (HRTEM) images were taken from a JEOL Model JEM-2100F TEM at 200 kV (www.jeol.de/electronoptics-en). Scanning electron microscopy (SEM) images were determined with a Hitachi S-4800 microscope (www.hitachi.com). XPS was conducted using an Escalab 250 X-ray Photoelectron Spectroscope (ThermoFisher, American, www.thermoscientific.com). Fourier transform infrared (FTIR) spectra were recorded on FTIR spectroscope (Tensor 37, Bruker, Germany, www.bruker.com). X-ray diffraction (XRD) analysis was performed on a Rigaku D/max-IIB X-ray diffractometer using Cu Kα radiation (λ = 1.5418 Å) (www.rigaku.com). An ultrasonic bath (KQ218, 40 kHz, 100 W, China, www.ks-csyq.com) was used as the ultrasonic source. In all the procedures, the double-distilled water (resistivity =18.2 MΩ cm−1) was obtained from an Olst ultrapure K8 apparatus (Olst, Ltd., www.olst.cn). All the electrochemical measurements were carried out at room temperature on CHI-842C electrochemical workstation (Chen-hua Instruments Inc., Shanghai, China, www.chinstruments.com). A three-electrode system was used in the experiment with a glassy carbon electrode (GCE) (ca. 0.0707 cm2) as the working electrode, an Ag/AgCl electrode (saturated KCl) as reference electrode and a Pt wire electrode as counter electrode, respectively. Phosphate buffer (PB) comprising NaH2PO4, Na2HPO4, and KCl was used as the supporting electrolyte throughout the experiment without otherwise specification.
Synthesis of MoS2/rGO nanocomposite
For a typical synthesis [29, 30], 50 mg of GO powders were dispersed into 100 mL of H2O and ultrasonicated until the mixture turned yellow-brown and clear, indicating GO aqueous suspensions were obtained. Afterward, 0.6 g of Na2MoO4 2H2O and 0.75 g of NH2CSNH2 were dissolved into the above GO suspensions (30 mL) and stirred until the solution became homogeneous. Then the mixture solution was transferred into a 50 mL Teflon-lined autoclave and heated in an oven at 220 °C for 24 h. After cooling to room temperature naturally, the black precipitates obtained were centrifuged and washed with double-distilled water and anhydrous ethanol in sequence for three times and then dried under vacuum at 50 °C overnight. For comparison, pure MoS2 was prepared with the same procedure in the absence of GO.
Electrode preparation
To prepare the working electrode, the dried and ground MoS2/rGO nanocomposite was dispersed in a mixed solution (the volume ratio of DMF vs water was 3:1) and ultrasonicated until the mixed solution was homogeneous. The concentration of composite suspension was 10 mg mL−1. Then, 10 μL of suspensions were cast onto a mirror-polished and clean GCE and dried under an infrared lamp. The obtained modified electrode was denoted as MoS2/rGO/GCE and stored at room temperature when not in use. For comparison, the pure MoS2 modified GCE (MoS2/GCE) was prepared in the same way.
Results and discussion
Characterization of the nanocomposite
As shown in Fig. 1a, the pure MoS2 with sheet-like nanostructure tended to tightly stack together, which implied the aggregated nature of the synthesized pure MoS2. In contrast, the MoS2/rGO nanocomposite (Fig. 1b) displayed a loose porous and flower-like nanostructure consisting of 2D petal-like nanoflakes. A representative lamellar structure of the pure MoS2 and MoS2 component in MoS2/rGO nanocomposite can be seen in the inset of Fig. 1a and b, consistent with the (002) plane of hexagonal atomic lattices in bulk MoS2. The interlayer distance of 0.63 nm for MoS2 component in MoS2/rGO nanocomposite was slightly larger than that of 0.61 nm in the pure MoS2, which corresponded to the previous literatures [31, 32].
Crystalline structures of MoS2 and MoS2/rGO nanocomposite were investigated by XRD. As shown in Fig. 2a, both samples displayed the similar typical hexagonal structure of crystalline MoS2 (JCPDS 37-1492). The primary peak of both appeared around 14.06°, corresponding to the diffraction from (002) plane of MoS2 with the layer-to-layer spacing of 0.62 nm [29, 30]. This was also consistent well with the HRTEM results, indicating successful preparation of the nanocomposite. The nitrogen adsorption-desorption isotherms of MoS2 and the MoS2/rGO nanocomposite exhibited type IV with type H3 hysteresis loop and possessed mesoporous structure (Fig. 2b). The specific surface area of MoS2/rGO nanocomposite was measured to be 96.5 m2 g−1, which was much larger than that of MoS2 (23.6 m2 g−1). The MoS2/rGO nanocomposite exhibited an average pore size of 14.13 nm which was slightly smaller than 17.69 nm of MoS2 (BJH analysis, the inset of Fig. 2b). Those results implied that increased specific surface area and unique porous nanostructure of the nanocomposite may provide more active sites for the adsorption of target molecules, which contributed to enhancing electrocatalytic performance of the modified electrode [33].
The compositions of the pure MoS2 and MoS2/rGO nanocomposite and chemical states of C, S, and Mo element were then characterized by XPS. The XPS results showed that both samples consisted of Mo, S, C, and O element, and the atomic ratio of Mo to S for both was approximately 1:2 (Fig. S1). As shown in Fig. 3a, four different peaks of C(O)-O, C=O, C-O-C and sp2-hydridized C-C proved a considerable degree of oxidation in GO while the C 1 s peak-fitting results of MoS2/rGO nanocomposite (Fig. 3b) just exhibited C-C peak and smaller peaks of C=O and C-O-C compared with that of GO. The disappearance of the C(O)-O peak obviously suggested the effective reduction of GO during the one-pot hydrothermal treatment, which was in accordance with FTIR analysis (Fig. S2). As shown in Fig. S3A, the main doublet located at binding energies of 162.03 and 163.18 eV consisted well with the S 2p3/2 and S 2p1/2 of MoS2, respectively. The S 2 s and Mo 3 days peaks of pure MoS2 are shown in Fig. S3B, and three resolved peaks can be observed. The two main peaks of Mo 3d5/2 (229.13 eV) and Mo 3d3/2 (232.33 eV) were characteristic of MoS2 and the peak around 226.15 eV was in accordance with S 2 s of MoS2. As for the MoS2/rGO nanocomposite, the above binding energies were all slightly larger than that of pure MoS2 [30]. This result indicated a possible electron transfer between MoS2 layer and rGO sheets in the nanocomposite, which may effectively increase the conductivity and improve the catalytic activity [31].
Electrochemical behaviors of AA, DA, UA at the modified electrode
As displayed in Fig. S4A-C, the individual electrooxidation of AA, DA, and UA occurred at the bare GCE with a large overpotential at different levels and extremely low current response, indicating a sluggish electron transfer process at the bare GCE due to the electrode fouling resulted from the oxidation product of small biomolecules [13, 14]. Compared with the bare GCE, an obvious electrooxidation peak with distinct potential shift and high current response appeared at the MoS2/rGO/GCE because of the enhanced conductivity and catalytic activity. As shown in Fig. S4D, a broad, overlapped and quite small anodic peak appeared at bare GCE while three well-resolved and large oxidation peaks stood out at the MoS2/rGO/GCE. This result obtained demonstrated that the MoS2/rGO/GCE had a rather better capacity to effectively distinguish the oxidation potentials of the three biomolecules than the bare GCE. From Fig. S4A to 4D, the MoS2/GCE only presented a large background current response and no remarkable separating ability. To further verify the separating ability and current sensitivity of the MoS2/rGO/GCE, differential pulse voltammetry (DPV) curves for the oxidation of AA, DA, and UA in their mixture at MoS2/rGO/GCE were shown in Fig. S5. It can be seen that the DPV peaks of these three species were well-resolved with the electrooxidation potentials at about −64 mV (AA), 168 mV (DA), and 320 mV (UA), respectively. Thus, the separation of peak potentials at MoS2/rGO/GCE was 232 and 152 mV for AA-DA and DA-UA, respectively, which was large enough to determine AA, DA, and UA simultaneously. Effects of solution pH and scan rate on the peak potential and current of electrochemical oxidation of the three species were also investigated by DPV (Fig. S6 and S7).
Simultaneous determination of AA, DA, and UA
As shown in Figs. 4, 5 and 6, the DPV responses of MoS2/rGO/GCE toward AA, DA, or UA were displayed when the concentration of one species changed with the other two keeping constant. The peak current of AA was linear to the AA concentrations in the range from 12 to 5402 μM with a regression equation of I p,AA (μA) = 0.0108C AA (μM) + 0.738 (R 2 = 0.9994). The detection sensitivity was calculated to be 0.12 μA μM−1 cm−2 based on the slope of the regression equation divided by electroactive surface area (ECSA) of the MoS2/rGO/GCE (Fig. S8). The corresponding limit of detection (LOD) was 0.72 μM (S/N = 3). As for DA and UA, the peak currents were proportional in the concentration range from 5 to 545 and from 25 to 2745 μM, respectively. The linear functions were I p,DA (μA) = 0.364C DA (μM) + 2.758 (R 2 = 0.9988) and I p,UA (μA) = 0.141C UA (μM) + 0.339 (R 2 = 0.9986), respectively. The corresponding detection sensitivity of DA and UA was 4.11 and 1.59 μA μM−1 cm−2 while the LOD of DA and UA were 0.05 and 0.46 μM, respectively. The various analytical performances of the electrochemical sensor were compared with those reported in the literature (Table S1). Notably, compared with those high-cost noble metal-based biosensors, MoS2/rGO/GCE exhibited a higher sensitivity, lower detection limit and wider linear range. The MoS2/rGO nanocomposite was thus one of the promising candidates for selective and sensitive determination of AA, DA, and/or UA.
Selectivity, stability and reproducibility
To investigate the anti-interference ability of MoS2/rGO/GCE, the peak current ratio was compared as shown in Fig. S9. I 0, I 1, I 2 meant the DPV current response for 1 mM AA, 100 μM DA, 200 μM UA and I for 1 mM of various interferential compounds and ions. Peak current ratio changed less than ±6 % (Fig. S9), indicating the biosensor exhibited good selectivity for the detection of AA, DA and/or UA. The stability was quite essential for nanocomposite modified electrode to construct the biosensor [34]. The stability of MoS2/rGO/GCE was investigated by CV with 50 circles of continuous scan in 5 mM [Fe(CN)6]3-/4- aqueous solution containing 0.1 M KCl. The results showed that there was a negligible decrease of the voltammetric current during the whole procedures, suggesting that an excellent stability of the electrode (Fig. S10). The reproducibility was studied by five modified electrode prepared independently using the same batch of composite material. By comparing the peak currents in 0.1 M PB (pH 7.0) containing 1 mM AA, 100 μM DA and 200 μM UA, the relative standard deviation (R.S.D) for AA, DA, and UA were 3.52, 2.78, 3.23 %, respectively, indicating excellent reproducibility.
Real sample analysis
To verify the feasibility of the MoS2/rGO/GCE in practical analysis, it was applied to simultaneously detect the concentration of AA, DA, and UA in human serum samples using standard addition method. All the samples was filtrated and diluted with PB (pH 7.0) before the measurements. The analysis data were summarized in Table S2, showing the potential practical application of our biosensor for real samples.
Conclusions
In summary, a facile strategy for preparing MoS2/rGO hybrid nanocomposite has been developed by a one-pot hydrothermal synthesis. Electrochemical measurements demonstrated that the nanocomposite can improve electrocatalytic performance toward oxidation of AA, DA, and UA by enlarging peak separation and increasing peak current due to its unique porous nanostructure, large specific surface area and good electric conductivity. Simultaneous detection of AA, DA, and UA was achieved in a wide concentration range with high sensitivity and good selectivity. The biosensor herein may be applied to selectively detect other bioactive biomolecules such as epinephrine, norepinephrine, etc. Furthermore, other disulphide-based nanocomposites with rGO may also possess a wide range of applications in biosensors.
References
Zheng YJ, Huang ZJ, Zhao CF, Weng SH, Zheng W, Lin XH (2013) A gold electrode with a flower-like gold nanostructure for simultaneous determination of dopamine and ascorbic acid. Microchim Acta 180:537–544
Zhao DY, Fan DW, Wang JP, Xu CX (2015) Hierarchical nanoporous platinum-copper alloy for simultaneous electrochemical determination of ascorbic acid, dopamine, and uric acid. Microchim Acta 182:1345–1352
Wang Y, Li YM, Tang LH, Lu J, Li JH (2009) Application of graphene-modified electrode for selective detection of dopamine. Electrochem Commun 11:889–892
Wang HW, Ren FF, Yue RR, Wang CQ, Zhai CY, Du YK (2014) Macroporous flower-like graphene-nanosheet clusters used for electrochemical determination of dopamine. Colloids Surf A 448:181–185
Du J, Yue RR, Yao ZQ, Jiang FX, Du YK, Yang P, Wang CY (2013) Nonenzymatic uric acid electrochemical sensor based on graphene-modified carbon fiber electrode. Colloids Surf A 419:94–99
Wang Y, Xiao Y (2012) Glassy carbon electrode modified with poly(dibromofluorescein) for the selective determination of dopamine and uric acid in the presence of ascorbic acid. Microchim Acta 178:123–130
Yan J, Liu S, Zhang ZQ, He GW, Zhou P, Liang HY, Tian LL, Zhou XM, Jiang HJ (2013) Simultaneous electrochemical detection of ascorbic acid, dopamine and uric acid based on graphene anchored with Pd-Pt nanoparticles. Colloids Surf B 11:392–397
Wu GH, Wu YF, Liu XW, Rong MC, Chen XM, Chen X (2012) An electrochemical ascorbic acid sensor based on palladium nanoparticles supported on graphene oxide. Anal Chim Acta 745:33–37
Zhou CL, Li S, Zhu W, Pang HJ, Ma HY (2013) A sensor of a polyoxometalate and Au-Pd alloy for simultaneously detection of dopamine and ascorbic acid. Electrochim Acta 113:454–463
Wang X, Wu M, Tang WR, Zhu Y, Wang LW, Wang QJ, He PG, Fang YZ (2013) Simultaneous electrochemical determination of ascorbic acid, dopamine and uric acid using a palladium nanoparticle/graphene/chitosan modified electrode. J Electroanal Chem 695:10–16
Wang CQ, Du J, Wang HW, Zou CE, Jiang FX, Yang P, Du YK (2014) A facile electrochemical sensor based on reduced graphene oxide and Au nanoplates modified glassy carbon electrode for simultaneous detection of ascorbic acid, dopamine and uric acid. Sens Actuators B 204:302–309
Huang YP, Miao YE, Ji SS, Tjiu WW, Liu TX (2014) Electrospun carbon nanofibers decorated with Ag-Pt bimetallic nanoparticles for selective detection of dopamine. ACS Appl Mater Interface 6:12449–12456
Huang JS, Liu Y, Hou HQ, You TY (2008) Simultaneous electrochemical determination of dopamine, uric acid and ascorbic acid using palladium nanoparticle-loaded carbon nanofibers modified electrode. Biosens Bioelectron 24:632–637
Sheng ZH, Zheng XQ, Xu JY, Bao WJ, Wang FB, Xia XH (2012) Electrochemical sensor based on nitrogen doped graphene: simultaneous determination of ascorbic acid, dopamine and uric acid. Biosens Bioelectron 34:125–131
Chen D, Tang LH, Li JH (2010) Graphene-based materials in electrochemistry. Chem Soc Rev 39:3157–3180
Chen D, Feng HB, Li JH (2012) Graphene oxide: preparation, functionalization, and electrochemical applications. Chem Rev 112:6027–6053
Liu N, Liu ZM, Han HL, Ma ZF (2014) Graphene oxide reduced directly by redox probes for multiplexed detection of tumor markers. J Mater Chem B 2:3292–3298
Hao MJ, Liu N, Ma ZF (2013) A new luminol chemiluminescence sensor for glucose based on pH-dependent graphene oxide. Analyst 138:4393–4397
Wang LY, Liu N, Ma ZF (2015) Novel gold-decorated polyaniline derivatives as redox-active species for simultaneous detection of three biomarkers of lung cancer. J Mater Chem B 3:2867–2872
Guo SJ, Dong SJ (2011) Graphene nanosheet: synthesis, molecular engineering, thin film, hybrids, and energy and analytical applications. Chem Soc Rev 40:2644–2672
Wang Y, Shao YY, Matson DW, Li JH, Lin YH (2010) Nitrogen-doped graphene and its application in electrochemical biosensing. ACS Nano 4:1790–1798
Lei W, Si WM, Xu YJ, Gu ZY, Hao QL (2014) Conducting polymer composites with graphene for use in chemical sensors and biosensors. Microchim Acta 181:707–722
Sanghavi BJ, Wolfbeis OS, Hirsch T, Swami NS (2015) Nanomaterial-based electrochemical sensing of neurological drugs and neurotransmitters. Microchim Acta 182:1–41
Wang H, Feng HB, Li JH (2014) Graphene and graphene-like layered transition metal dichalcogenides in energy conversion and storage. Small 10:2165–2181
Huang X, Zeng ZY, Zhang H (2013) Metal dichalcogenide nanosheets: preparation, properties and applications. Chem Soc Rev 42:1934–1946
Su S, Chao J, Pan D, Wang LH, Fan CH (2015) Electrochemical sensors using two-dimensional layered nanomaterials. Electroanalysis 27:1062–1072
Ge J, Ou EC, Yu RQ, Chu X (2014) A novel aptameric nanobiosensor based on self-assembled DNA-MoS2 nanosheet architecture for biomolecule detection. J Mater Chem B 2:625–628
Huang JH, Ye L, Gao X, Li H, Xu JB, Li ZG (2015) Molybdenum disulfide-based amplified fluorescence DNA detection using hybridization chain reactions. J Mater Chem B 3:2395–2401
Min SX, Lu GX (2012) Sites for high efficient photocatalytic hydrogen evolution on a limited-layered MoS2 cocatalyst confined on graphene sheets―the role of graphene. J Phys Chem C 116:25415–25424
Li HL, Yu K, Fu H, Guo BJ, Lei X, Zhu ZQ (2015) MoS2/graphene hybrid nanoflowers with enhanced electrochemical performances as Anode for lithium-ion batteries. J Phys Chem C 119:7959–7968
Li YG, Wang HL, Xie LM, Liang YY, Hong GS, Dai HJ (2011) MoS2 nanoparticles grown on graphene: an advanced catalyst for the hydrogen evolution reaction. J Am Chem Soc 133:7296–7299
Xie JF, Zhang H, Li S, Wang RX, Sun X, Zhou M, Zhou JF, Lou XW, Xie Y (2013) Defect-rich MoS2 ultrathin nanosheets with additional active edge sites for enhanced electrocatalytic hydrogen evolution. Adv Mater 25:5807–5813
Punckt C, Pope MA, Aksay IA (2014) High selectivity of porous graphene electrodes solely due to transport and pore depletion effects. J Phys Chem C 118:22635–22642
Xu T, Liu N, Yuan J, Ma ZF (2015) Triple tumor markers assay based on carbon-gold nanocomposite. Biosens Bioelectron 70:161–166
Acknowledgments
This research was financed by grants from Beijing Natural Science Foundation (2132008) and the Project of the Construction of Scientific Research Base by the Beijing Municipal Education Commission.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
ESM 1
(DOCX 1014 kb)
Rights and permissions
About this article
Cite this article
Xing, L., Ma, Z. A glassy carbon electrode modified with a nanocomposite consisting of MoS2 and reduced graphene oxide for electrochemical simultaneous determination of ascorbic acid, dopamine, and uric acid. Microchim Acta 183, 257–263 (2016). https://doi.org/10.1007/s00604-015-1648-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00604-015-1648-8