Abstract
We describe the synthesis of molecularly imprinted core-shell microparticles via a metal chelating strategy that assists in the creation of selective recognition sites for albumin. Porcine serum albumin (PSA) was immobilized on silica beads via copper(II) chelation interaction. A solution containing 2-hydroxyethyl methacrylate and methacrylic acid as the monomers was mixed with the above particles, and free radical polymerization was performed at 25 °C. Copper ion and template were then removed to obtain PSA-imprinted core-shell particles (MIPs) with a typical diameter of 5 μm. The binding capacity of such MIP was 8.9 mg protein per gram of MIPs, and the adsorption equilibrium was established within <20 min. The imprinting factor for PSA reached 2.6 when the binding capacity was 7.7 mg protein per gram of MIPs. The use of such MIPs enabled PSA to be selectively recognized even in presence of the competitive proteins ribonuclease B, cytochrome c, and myoglobin. The results indicate that this imprinting strategy for protein may become a promising method to prepare MIPs for protein recognition.

Preparation and rebinding of surface imprinted particles for target protein recognition
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The current opinion about molecular imprinting is an attractive mimetic technology to create specific binding sites geometrically and chemically complementary to the template molecules with non-biological strategy [1, 2], which is highly similar to the conception of “antigen-antibody”. In recent, this general approach have been used successfully and widely for small weight template recognition (<1000Da) [3–7]. During the past decade, the imprinted materials toward macromolecules, especially proteins, were synthesized [8]. Although there are many inherent problems to address, such as the huge molecular size, conformational flexibility and complexity, solubility, and sensitivity to environment of the proteins, protein imprinted materials have been used in various fields, such as biosensors [9–11], separated medium [12–15], protein [16, 17] and man-made enzyme inhibitors [18].
At present, the formats of protein imprinted materials can be typified into 3D and 2D imprinting types [2]. Universally, the binding sites of 3D imprinting polymers are formed throughout bulk material, which is originated from small molecule imprinting, but suffer from the heterogeneity of recognition sites and poor mass transfer [19]. As contrast, the binding sites of 2D imprinted polymers are formed at the material surface and this strategy gains the popularity in the research of protein imprinted materials due to several advantages, such as easy imprinted sites accessibility, fast target molecule recognition, easy template removal and minor influence of protein natural structure [20, 21].
Grafted imprinting is a novel kind of surface imprinting method to immobilize template with covalent or non-covalent interaction on the surface of film or particle, followed by the polymerization of monomers and cross-linkers to form the surface imprinting recognition sites [12, 15, 22–24]. As a result, effective template utilization and more imprinting sites are easily achieved by this method. The metal chelating, as a way to graft the template, became popular in the field of molecular imprinting. 14 Deductively, the metal chelating interaction is much stronger than non-covalent interaction, which would favor the template immobilization on the surface of the matrix. Additionally, the metal ion and protein are removed by EDTA or other chelant with mild condition, which would lead to the easy template removal and formation of recognition sites.
In our previous work of surface protein imprinted materials with metal coordination [15], cooper ion was immobilized on the surface of silica nanoparticles through imidoacetic acid groups, then the template protein was immobilized by metal chelating interaction. By sol–gel polymerization, a thin silica-skeleton shell with average thickness about 20 nm was formed, which was able to capture and recognize template protein. However, non-specific absorption was obvious mainly due to the property of silica skeleton. For further biological application, the imprinted shell should be hydrophilic and had low non-specific absorption towards the distribution [25]. To decrease non-specific absorption on the materials, the employment of the hydrophilic monomer, such as acrylamide, has been proved as an available strategy instead of the silicon agents. Herein, as shown in Scheme 1, a type of surface imprinted core-shell particles with copper ion chelating and hydrophilic polymer shell for special recognition of porcine serum albumin (PSA) was prepared. After the copper ion and template protein removal, the imprinted core-shell hydrophilic particles attempted to recognize PSA with high specification, fast recognition speed and low non-specific absorption.
Materials and methods
Materials
Macroporous silica particles (5 μm, 1000 Å) were obtained from Fuji Chemical Co. Ltd. (Kusugai, Japan). Methacrylic acid, N, N, N′, N′-tetramethylethylenediamine, ammonium persulfate, vinyltrimethoxysilane, 3-aminopropyltriethoxysilane, 3-Glycidoxypropyltrimethoxysilane (GLYMO) and imidoacetic acid (IDA) were obtained from Acros Organics (NJ, USA, www.acros.com). Formic acid was obtained from Fluka Chemical Co. (Buchs, Switzerland). Porcine serum albumin (PSA Mw 66.0 kDa, pI 5.1), ribonuclease B (RNB, Mw 11.7 kDa, pI 8.8), cytochrome C (Cyt-c, Mw 12.3 kDa, pI 10.6), myoglobin (Mb, Mw 14.3 kDa, pI 5.2), and 2-Hydroxyethyl methacrylate were obtained from Sigma-Aldrich Chemical Co. (St. Louis, MO, USA, www.sigmaaldrich.com). HPLC-grade acetonitrile was purchased from Merck (Darmstadt, Germany, www.merck-china.com). Ethylene diamine tetraacetic acid (EDTA) was purchased from Tianhe Chemical Co. (Tianjin, China). Sodium thiosulphate, potassium bromide, sodium dihydrogen phosphate, disodium hydrogen phosphate dodecahydrate and copper sulfate pentahydrate were purchased from Kermel Chemical Co. Ltd. (Tianjin, China). Potassium iodide was purchased from SCR Chemical Co. (Shanghai, China). Potassium bromate was purchased from Guangfu Chemical Co. Ltd. (Tianjin, China). Water was purified by a Milli-Q system (Millipore, Molsheim, France, www.millipore.com). All inorganic reagents were of analytical reagent grade, and all the other solvents were of HPLC grade and used without further purification.
Preparation of functional silica particles
The functionalization of the silica particles was performed with three steps as followings:
-
(a)
Silica particles (100 mg) were homogenized in 2.0 mL of methanol, and then 50.0 μL of vinyltrimethoxysilane was added. This reaction was allowed to proceed at 25 °C for 36 h with stirring. The mixture was centrifuged and washed twice by methanol to remove residual vinyltrimethoxysilane after this reaction.
-
(b)
Vinyl-silica particles prepared by step (a) were mixed with 1.0 mL of the compound of IDA-GLYMO solution and 52.5 μL of ammonia. This step of reaction was performed at 60 °C for 36 h with stirring. And then the mixture was centrifuged and washed as step (a) when the reaction completed.
-
(c)
At last, the functional silica was homogenized in 2.0 mL of water, and then 10.0 μL of 3-aminopropyltriethoxysilane was added into the reaction system. This reaction was allowed to proceed at 60 °C for 24 h with stirring. After centrifugation and rinse as the same step above mentioned, the vinyl-IDA-aminopropyl functional silica particles were dried at 70 °C for 24 h.
Immobilization of Cu2+and protein on the functional silica particles
To immobilize Cu2+ on the surface of silica, 0.4 mL of 100 mM CuSO4 solution and 0.60 mL of phosphate buffer (pH = 7.4) was mixed, then the mixture was added into 20 mg of functional silica particles. And then 1 mL of 0.20 mg · mL−1 of PSA solution was added into the Cu2+ immobilized silica in order to form Cu2+-PSA immobilized silica particles in 10 mM of phosphate buffer (pH = 7.4) at 4 °C for several hours. After incubation, the mixture was centrifuged and washed twice by 10 mM of phosphate buffer (pH = 7.4) to remove residual protein in the solution. The non-imprinted polymer (NIP) was prepared by the same procedure without the PSA addition.
Preparation of protein imprinted particles
This imprinted material was directly prepared by in situ free radical-initiated polymerization on the surface of Cu2+-PSA silica particles. The pre-polymerization mixture, which contained methacrylic acid (145 μL) and 2-Hydroxyethyl methacrylate (455 μL), were dissolved in 9.40 mL of phosphate buffer (pH 7.4, 10 mM) and purged with N2 for 20 min. In the next step, 20 mg of Cu2+-PSA silica particles were homogenized in 2.0 mL of pre-polymerization mixture, and then 5.0 μL of N, N, N′, N′-tetramethylethylenediamine and 7.5 μL of 10 % ammonium persulfate (w/v) solution were added into the mixture separately. The reaction was performed at 25 °C for 18 h. After polymerization, the mixture was centrifuged at about 1882 g (~3000 rpm) and washed twice by water. And then, 1.0 mL of 100 mM EDTA aqueous solution was added to remove the copper ion from the particles. The elution solution, the mixture with 20 % formic acid (v/v), 40 % acetonitrile (v/v) and 40 % water, was added to remove the template protein from the surface of the imprinted particles. After the removal of EDTA and acid by water, imprinted particles were dried at room temperature by vacuum.
Morphologic observation
For Transmission electron microscopy (TEM) imaging, imprinted particles (2 mg) were washed with ethanol, and then were dried at 70 °C for 2 h. A Tecnai G2 Spirit microscope (Tecnai G2, FEI, Eindhoven, Netherlands) was operated at 120 kV for the TEM measurement.
Chromatographic analysis
HPLC analysis was performed by using a Shimadzu HPLC system constituted by two LC-20 AD Solvent Delivery Units, an SUS-20A gradient controller and an SPD-20A Detector (Shimadzu, Kyoto, Japan). The analytical column was obtained from Sipore Co. (C8, 5 μm, 150 mm × 4.6 mm I.D., Dalian, China). A Chromatocorder 12 from SIC (Tokyo, Japan) was employed for data analysis.
For the chromatographic analysis with gradient elution, mobile phase A was acetonitrile/water (5:95, v/v, containing 0.1 % trifluoroacetic acid), and mobile phase B was acetonitrile/water (90:10, v/v, containing 0.1 % trifluoroacetic acid). The gradient was set as follows: 0–40 min, 20–90 % B (v/v). The flow rate was 1.0 mL · min−1. The injection volume was 20 μL for all samples, and the wavelength of UV detector was set at 214 nm.
Adsorption kinetics
The kinetic adsorption measurements was carried out by incubating 20.0 mg MIP or NIP particles with 1.00 mL 0.40 mg · mL−1 of PSA in phosphate buffer (pH = 7.4) solution at 4 °C. Then, the concentrations of PSA at different time intervals, 1 min, 5 min, 20 min, 60 min and 120 min were monitored by triplicate HPLC experiments.
Binding capacity
The binding capacity was measured by incubating 20.0 mg of MIP or NIP particles with 1.00 mL of phosphate buffer (pH = 7.4) with a range of PSA concentrations of 0.05 ~ 0.50 mg · mL−1 at 4 °C for 120 min. Then the supernatant was separated and analyzed by HPLC. The amount of PSA bound on the imprinted particles was calculated from the concentration differences before and after incubation. The concentrations of PSA in solution were determined by two parallel HPLC experiments.
Selectivity
To demonstrate the recognition specificity of the prepared MIPs to PSA, 20 mg MIP and NIP particles were respectively incubated in 10 mM phosphate buffer at pH 7.4 containing PSA, RNB, Cyt-c and Mb at 4 °C for 24 h. The concentration of each protein was 0.30 mg · mL−1. After incubation, the supernatant was separated, and the imprinted particles were washed twice by 5 mL phosphate buffer, followed by 5 min elution with 1.00 mL of buffer composed of 20 % formic acid (v/v), 40 % acetonitrile (v/v) and 40 % water (v/v). The eluted supernatant was separated. Then, both the incubated and the eluted supernatant were analyzed by HPLC. The concentration of each protein in solution was determined by the duplicate HPLC experiments.
Results and discussion
Design of the protein imprinted particles
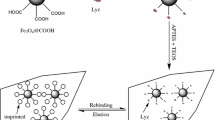
The preparation of albumin from procine serum (PSA) surface imprinted particles by metal chelated strategy was shown in Fig. 1. The silica particles with average diameter of 5 μm were chosen as the matrix to prepare the imprinted core-shell particles for the potential packing of chromatographic column. Three functional groups, including vinyl, IDA and aminopropyl, were modified on the surface of silica one by one and the subsequence of the three modifications was critical. Vinyl group was decorated on the silica particles firstly in order to make sure that the polymerization was performed around the particles. The amount of the double bonds at the surface of silica was 26.0 ± 1.41 μmol · g−1, according to the Br2 addition reaction method (seen in Supporting Information) [26]. And then IDA group was immobilized on the matrix with truss arm for the metal ions capture and further the huge protein (66 kDa) chelation. IDA-GLYMO group is much larger than the vinyl group, therefore, IDA was modified after the modification of vinyl group to minimize the steric hindrance. Finally, to introduce the non-covalent interaction between the monomer and the matrix, a small amount of aminopropyl group was modified on the surface by ammonium persulfate hydrolysis. Although the steric hindrance of IDA-GLYMO group remained, aminopropyl groups are modified successfully on the silica surface mainly because of the higher reactivity of 3-aminopropyltriethoxysilane than vinyltrimethoxysilane.
With the IDA group and copper ion, the proteins were immobilized on the surface of wide-pore modified silica particles (5 μm, 1000 Å, with blue colour due to the copper ion chelated) by metal chelated interaction in pH 7.4 phosphate buffer. After protein immobilization, the particles were rinsed by 1.0 mL of 10 mM phosphate buffer in order to sweep away the protein which was not immobilized successfully on the surface of silica particles. It should be mentioned that 5 μm silica particles were applied as a model silica particle here due to their potential application as the chromatographic or electrochromatographic stationary phase.
To decrease the non-specific adsorption and increase the selective affinity towards the target protein, the formed imprinted particles should be hydrophilic. 2-Hydroxyethyl methacrylate, with neutral and excellent hydrophilicity because of the presence of the hydroxyl group, was chosen as the main monomer. Simultaneously it provided hydrogen bonding interactions with the template protein, which was rich in carboxyl, hydroxyl and amino group. In the field of molecular imprinting, slight electrostatic interaction was usually applied to form recognition sites especially in the protein imprinting [27–29]. Therefore, small amount of methacrylic acid was added as the co-monomer. The mole ratio of the two monomer above (2-hydroxyethyl methacrylate: methacrylic acid) was optimized as 3:1. During the polymerization, the template was interacted with the functional monomers to form hydrogen bonding and ionic interactions. Hydrogen bonding was the main interaction between the protein and the polymer. And it existed between carboxyl, hydroxyl and amino groups of the monomer and the side chain groups of the amino acid. Additionally, ionic interaction between template protein and monomer was retained due to the methacrylic acid carboxyl group after the polymerization.
After the polymerization, water was used to wash up the unreacted monomer, and EDTA was added to dislodge the copper ion from the particles for the further extraction of the protein. The mixture containing 20 % formic acid, 40 % acetonitrile (v/v) and 40 % water was used to remove PSA from the imprinted particles, which change the pH and surface charges of the polymer shell to favor the protein extraction from the particles and avoid the ruining of the silica skeleton in the core.
Morphologic observation
TEM images were measured for the size, shape and thin imprinted shell of these surface imprinted core-shell particles. As shown in the left image of Fig. 1, the particles were regular spherical, with monodispersed particle size of about 5 μm. Neither aggregation nor solidified polymer was observed between the core-shell particles. This phenomenon indicated that the polymerization was mainly occurred on the surface of particles. As shown in the right image of Fig. 1, the thickness of the imprinted polymer layer was about 20 nm, which was quite similar to the total length of the protein radius and the truss arm. This favored more protein imprinted sites available for the protein recognition.
Binding capacity and kinetic study
To investigate the binding ability of the MIP and NIP core-shell particles, the adsorption kinetics of MIP and NIP was performed. As shown in Fig. 2, for MIP, within 1 min, the adsorption of template protein reached more than 90 % of the maximum, and the saturated adsorption achieved in less than 20 min. For the NIP, the saturated adsorbed capacity was much less than that of MIP. Under this condition, imprinting factor (MIP/NIP) was about 2.2. The short equilibrium time benefited from the surface imprinting, which would favor the accessibility of the recognition sites. Additionally, this fast recognition ability would favor the potential chromatographic application of molecularly imprinted particles.
The saturation adsorption experiments were also investigated. As shown in Fig. 3, the binding capacity increased with the increase of PSA concentrations from 0.10 mg · mL−1 to 0.60 mg · mL−1. The binding capacity curve of NIP reached the saturated adsorption at lower protein concentration, while that of MIP reached the maximum at higher protein concentration. In the saturation absorption, binding capacity of MIP was about 8.9 mg protein per gram of materials, which was much higher than that of NIP, 4.0 mg protein per gram of materials,, while the PSA concentration was 0.40 mg · mL−1 Therefore, the imprinting factor of PSA in saturation adsorption condition was about 2.2. Additionally, the highest IF was about 2.6 at 0.20 mg · mL−1 and 0.30 mg · mL−1of the PSA concentration and higher than that of the saturation condition. Additionally, the IF with the PSA concentration 0.10, 0.50, 0.60 mg · mL−1 was 2.2, 2.1 and 2.0, respectively. As a result, the recognized concentration 0.30 mg · mL−1 was the optimized concentration in the recognized concentration range due to the higher binding capacity (about 7.7 mg protein per gram of materials) and imprinted factor. These facts mentioned above demonstrated that the imprinted particles showed the specific recognition ability to PSA.
Selectivity of the imprinted particles
To observe the selectivity of the imprinted core-shell particles, competitive recognition was undertaken under the optimized conditions (phosphate buffer, 10 mM, pH7.4) with PSA and three competitive proteins, Cyt-c, RNB and Mb, which had different molecularly weight, hydrophilicity and pI. After incubation for 12 h, the imprinted particles were firstly rinsed by the phosphate buffer. Then, the proteins were eluted by the elution (20 % FA + 40 % ACN + 40 % water) from the particles. The eluted protein amount was calculated from the chromatographic peak area of HPLC. As shown in Fig.4, the amounts of PSA eluted from the MIP and NIP was much more than the other competitive proteins. The amount of PSA eluted from the MIP reached to about 7.30 mg protein per gram of materials, while it was 3.85 mg protein per gram of materials for the NIP. Imprinting factor for PSA still reached to 1.90 under the competitive condition, which was lower than that in the above-mentioned saturation experiment. Meanwhile, the imprinting factor of other competitive protein, such as Cyt-c or Mb, was less than 1.00. These results indicated that the imprinted particles recognized PSA in the protein mixture. Deductively, the phenomenon may due to the morphological complementation between the protein and imprinted cavity. The size of template PSA was much larger than other competitive protein and as a result the binding capacity of PSA in competitive recognition was much higher than that of the small competitor, RNB and Cyt-c, which had similar hydrophilicity to PSA.
Selectivity of MIP and NIP particles for four proteins with different Mw, hydrophilic and pI. 20 mg MIP or NIP particles were respectively incubated in 10 mM phosphate buffer at pH 7.4 containing PSA, RNB, Cyt-c and Mb with each concentration 0.30 mg · mL−1 at 4 °C for 24 h. The result was obtained by the duplicate experiments
Additionally, the charge of the hydrophilic polymer surface was slightly negative at this recognition condition (pH = 7.4) mainly due to the presence of the carboxyl group in the co-polymer network skeleton. Although the charge of PSA (pI 5.1) was also negative at this neutral pH and electrostatic exclusion existed between the protein and polymer, PSA still achieved well absorption and recognition on this imprinted material. Additionally, the charge of RNB (pI 8.8) or Cyt-c (pI 10.6) was positive at pH = 7.4 and electrostatic attraction was supposed to exist between the protein and polymer. However, low adsorption towards RNB or Cyt-c appeared in competitive recognition. This proved further that charge interaction did not play an important role in protein recognition.
Conclusion
The protein imprinted hydrophilic core-shell particles with porcine serum albumin as the template, 2-hydroxyethyl methacrylate and methacrylic acid as the hyodrophilic functional monomers, were successfully synthesized with copper ion chelating strategy. Due to the recognition sites located on the hydrophilic polymer shell, the novel porcine serum albumin imprinted core-shell particles recognized the target protein with good selectivity, fast mass transfer speed, high binding capacity and low non-specific recognition. This indicated that the protein imprinted materials would be potential to be used as chromatographic stationary phase in trap column to achieve PSA separation.
References
Mahon CS, Fulton DA (2014) Mimicking nature with synthetic macromolecules capable of recognition. Nat Chem 6(8):665–672. doi:10.1038/nchem.1994
Whitcombe MJ, Chianella I, Larcombe L, Piletsky SA, Noble J, Porter R, Horgan A (2011) The rational development of molecularly imprinted polymer-based sensors for protein detection. Chem Soc Rev 40(3):1547–1571. doi:10.1039/c0cs00049c
Pan G, Zhang Y, Ma Y, Li C, Zhang H (2011) Efficient one-pot synthesis of water-compatible molecularly imprinted polymer microspheres by facile RAFT precipitation polymerization. Angew Chem Int Edit 50(49):11731–11734. doi:10.1002/anie.201104751
Yang KG, Liu ZB, Mao M, Zhang XH, Zhao CS, Nishi N (2005) Molecularly imprinted polyethersulfone microspheres for the binding and recognition of bisphenol A. Anal Chim Acta 546(1):30–36. doi:10.1016/j.aca.2005.05.008
Yang KG, Berg MM, Zhao CS, Ye L (2009) One-pot synthesis of hydrophilic molecularly imprinted nanoparticles. Macromolecules 42(22):8739–8746. doi:10.1021/Ma901761z
Shen XT, Ye L (2011) Molecular imprinting in Pickering emulsions: a new insight into molecular recognition in water. Chem Commun 47(37):10359–10361. doi:10.1039/c1cc13899e
Hao Y, Gao R, Shi L, Liu D, Tang Y, Guo Z (2015) Water-compatible magnetic imprinted nanoparticles served as solid-phase extraction sorbents for selective determination of trace 17beta-estradiol in environmental water samples by liquid chromatography. J Chromatogr A 1396:7–16. doi:10.1016/j.chroma.2015.03.083
Yang KG, Zhang LH, Liang Z, Zhang YK (2012) Protein-imprinted materials: rational design, application and challenges. Anal Bioanal Chem 403(8):2173–2183. doi:10.1007/s00216-012-5840-y
Fukazawa K, Ishihara K (2009) Fabrication of a cell-adhesive protein imprinting surface with an artificial cell membrane structure for cell capturing. Biosens Bioelectron 25(3):609–614. doi:10.1016/j.bios.2009.02.034
Zhou J, Gan N, Li T, Hu F, Li X, Wang L, Zheng L (2014) A cost-effective sandwich electrochemiluminescence immunosensor for ultrasensitive detection of HIV-1 antibody using magnetic molecularly imprinted polymers as capture probes. Biosens Bioelectron 54:199–206. doi:10.1016/j.bios.2013.10.044
Zhang W, Liu W, Li P, Xiao H, Wang H, Tang B (2014) A fluorescence nanosensor for glycoproteins with activity based on the molecularly imprinted spatial structure of the target and boronate affinity. Angew Chem Int Ed 53(46):12489–12493. doi:10.1002/anie.201405634
Li Q, Yang K, Liang Y, Jiang B, Liu J, Zhang L, Liang Z, Zhang Y (2014) Surface protein imprinted core–shell particles for high selective lysozyme recognition prepared by reversible addition–fragmentation chain transfer strategy. ACS Appl Mater Interfaces 6(24):21954–21960. doi:10.1021/am5072783
Yang K, Liu J, Li S, Li Q, Wu Q, Zhou Y, Zhao Q, Deng N, Liang Z, Zhang L, Zhang Y (2014) Epitope imprinted polyethersulfone beads by self-assembly for target protein capture from the plasma proteome. Chem Commun 50(67):9521–9524. doi:10.1039/c4cc03428g
Li QR, Yang KG, Liu JX, Zhang LH, Liang Z, Zhang YK (2013) Transferrin recognition based on a protein imprinted material prepared by hierarchical imprinting technique. Microchim Acta 180(15–16):1379–1386. doi:10.1007/s00604-013-0994-7
Liu J, Yang K, Deng Q, Li Q, Zhang L, Liang Z, Zhang Y (2011) Preparation of a new type of affinity materials combining metal coordination with molecular imprinting. Chem Commun 47(13):3969–3971. doi:10.1039/c0cc05317a
Saridakis E, Khurshid S, Govada L, Phan Q, Hawkins D, Crichlow GV, Lolis E, Reddy SM, Chayen NE (2011) Protein crystallization facilitated by molecularly imprinted polymers. Proc Natl Acad Sci U S A 108(27):11081–11086. doi:10.1073/pnas.1016539108
Saridakis E, Chayen NE (2013) Imprinted polymers assisting protein crystallization. Trends Biotechnol 31(9):515–520. doi:10.1016/j.tibtech.2013.05.003
Cutivet A, Schembri C, Kovensky J, Haupt K (2009) Molecularly imprinted microgels as enzyme inhibitors. J Am Chem Soc 131(41):14699–14702. doi:10.1021/ja901600e
Liu J, Deng Q, Yang K, Zhang L, Liang Z, Zhang Y (2010) Macroporous molecularly imprinted monolithic polymer columns for protein recognition by liquid chromatography. J Sep Sci 33(17–18):2757–2761. doi:10.1002/jssc.201000350
Liu J, Deng Q, Tao D, Yang K, Zhang L, Liang Z, Zhang Y (2014) Preparation of protein imprinted materials by hierarchical imprinting techniques and application in selective depletion of albumin from human serum. Sci Rep 4:5487. doi:10.1038/srep05487
Chen LX, Xu SF, Li JH (2011) Recent advances in molecular imprinting technology: current status, challenges and highlighted applications. Chem Soc Rev 40(5):2922–2942. doi:10.1039/c0cs00084a
Shen XT, Zhou TC, Ye L (2012) Molecular imprinting of protein in Pickering emulsion. Chem Commun 48(66):8198–8200. doi:10.1039/c2cc33572g
Gao RX, Kong X, Wang X, He XW, Chen LX, Zhang YK (2011) Preparation and characterization of uniformly sized molecularly imprinted polymers functionalized with core-shell magnetic nanoparticles for the recognition and enrichment of protein. J Mater Chem 21(44):17863–17871. doi:10.1039/c1jm12414e
Gao R, Mu X, Hao Y, Zhang L, Zhang J, Tang Y (2014) Combination of surface imprinting and immobilized template techniques for preparation of core-shell molecularly imprinted polymers based on directly amino-modified Fe3O4 nanoparticles for specific recognition of bovine hemoglobin. J Mater Chem B 2(12):1733–1741. doi:10.1039/c3tb21684e
Liu J, Yang K, Qu Y, Li S, Wu Q, Liang Z, Zhang L, Zhang Y (2015) An efficient approach to prepare boronate core-shell polymer nanoparticles for glycoprotein recognition via combined distillation precipitation polymerization and RAFT media precipitation polymerization. Chem Commun (Camb) 51(18):3896–3898. doi:10.1039/c4cc10004b
Lu Q, Chen X, Nie L, Luo J, Jiang H, Chen L, Hu Q, Du S, Zhang Z (2010) Tuning of the vinyl groups’ spacing at surface of modified silica in preparation of high density imprinted layer-coated silica nanoparticles: a dispersive solid-phase extraction materials for chlorpyrifos. Talanta 81(3):959–966. doi:10.1016/j.talanta.2010.01.044
Hayden O, Lieberzeit PA, Blaas D, Dickert FL (2006) Artificial antibodies for bioanalyte detection-sensing viruses and proteins. Adv Funct Mater 16(10):1269–1278. doi:10.1002/adfm.200500626
Dechtrirat D, Jetzschmann KJ, Stocklein WFM, Scheller FW, Gajovic-Eichelmann N (2012) Protein rebinding to a surface-confined imprint. Adv Funct Mater 22(24):5231–5237. doi:10.1002/adfm.201201328
Hoshino Y, Urakami T, Kodama T, Koide H, Oku N, Okahata Y, Shea KJ (2009) Design of synthetic polymer nanoparticles that capture and neutralize a toxic peptide. Small 5(13):1562–1568. doi:10.1002/smll.200900186
Acknowledgments
This work was supported by the National Basic Research Program of China (2012CB910601 and 2013CB911202), National Nature Science Foundation (21375128 and 21190043), the Creative Research Group Project of the NSFC (21321064), and the National High Technology Research and Development Program of China (2012AA020202).
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
ESM 1
(DOC 2154 kb)
Rights and permissions
About this article
Cite this article
Li, Q., Yang, K., Li, S. et al. Preparation of surface imprinted core-shell particles via a metal chelating strategy: specific recognition of porcine serum albumin. Microchim Acta 183, 345–352 (2016). https://doi.org/10.1007/s00604-015-1640-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00604-015-1640-3