Abstract
Magnetic oleate-coated Fe3O4 nanoparticles were applied to the extraction of PCBs from fruit juices that were quantified by gas chromatography coupled to triple quadrupole mass spectrometry. Two methods were evaluated: The first method involves a two-step procedure that combines dispersive liquid-liquid microextraction with dispersive micro-solid phase extraction, and the second one involves magnetic solid-phase extraction (mSPE) carried out in a single step. The mSPE procedure is shown to be more sensitive, and therefore, it was optimized and applied to the analysis of PCBs in juices. The detection limits for all target PCBs are below 6 ng∙L−1 for apple juice, and 3 ng∙L−1 for grape juice. The enrichment factor is 125. Analysis of spiked fruit juice samples gave relative recoveries higher than 70 % for all PCBs except for PCB28 and PCB52.

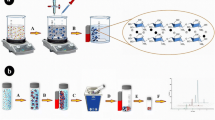
Diagram of the extractive methods using magnetic nanoparticles (MNPs): A) two-step method combining dispersive liquid-liquid microextraction (DLLME) with dispersive micro solid-phase extraction (D-μ-SPE) and B) one-step magnetic solid-phase extraction (mSPE) procedure
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Polychlorinated biphenyls (PCBs) are a group of synthetic organic compounds included in the Stockholm Convention of 2001 as persistent organic pollutants. Although there are 209 possible congeners, research has been mainly focused on seven congeners (PCB28, PCB52, PCB101, PCB118, PCB138, PCB153 and PCB180) which contribute largely to the total amount found in the environment. The presence of PCBs in juices has been scarcely evaluated mainly due to their complexity.
Liquid-liquid extraction or solid phase extraction (SPE) are the methods commonly used for the extraction of PCBs from liquid samples, but solid-phase microextraction, dispersive liquid-liquid microextraction (DLLME), stir-bar sorptive extraction, or QuEChERS extraction also have been applied in order to use low volumes of organic solvents [1–3]. Schellin and Popp [4] described the use of a membrane-assisted solvent extraction method for the determination of PCBs in apple juice, and a DLLME method based on solidification of floating organic droplet for the extraction of PCBs in peach juice was reported by Matsadiq et al. [5]. Recently, magnetic solid-phase extraction (mSPE) methods using nanoparticles (NPs) of magnetite (Fe3O4) have been described as an interesting technique for the analysis of pollutants in liquid samples [6]. In mSPE, the magnetic sorbent is dispersed into the solution and afterwards the analytes can be easily isolated from the sample solution by means of a magnetic force. Extractive methods involving two-step microextraction techniques, DLLME combined with micro solid-phase extraction (D-μ-SPE) using magnetic nanoparticles (MNPs), were described as an alternative to the conventional DLLME, in which the retrieval of the extraction solvent is based on the adsorption by MNPs [7–11].
Several extractive methods using MNPs have been described in the available literature for the determination of PCBs in water samples using different MNPs coatings in each study [12–14]. Recently, our group has reported the application of Fe3O4 MNPs coated with oleate (Ol-) for the determination of PCBs in water and soil leachates [15] and these coated MNPs are promising sorbents in the mSPE of PCBs from complex liquid samples. The application of MNPs in the analysis of pollutants in fruit juices has been mainly focused on pesticide analyses [9, 11, 16–18].
We report on a sensitive, easy and fast extractive method using Ol-coated Fe3O4 MNPs as magnetic sorbent for the extraction and preconcentration of PCBs from juices. Two extractive methods were evaluated: 1) a two step method combining DLLME with D-μ-SPE and 2) a one-step mSPE procedure. Various experimental parameters affecting the extraction efficiencies were optimized and, finally, the selected method was applied to the analysis of PCBs in commercial apple and grape juices. As indicated above, few studies have reported the extraction of PCBs in fruit juice and, to the best of our knowledge MNPs have not been previously used for this purpose.
Experimental
Reagents and standards
Ethyl acetate (EtAc), acetonitrile (ACN), n-hexane and methanol (MeOH), residue analysis grade, were purchased from Scharlab (Spain, www.scharlab.com). Fe (II, III) oxide MNPs (diameter 50-100 nm) and sodium chloride (NaCl) were acquired from Sigma-Aldrich (Germany; www.sigmaaldrich.com). Sodium oleate (65–90 %) was supplied by ACROS Organics (Belgium, www.acros.com). Octanol and sodium hydroxide were supplied by Merck (Germany; www.merck.com) and Panreac (Spain; www.panreac.es), respectively. Ultrahigh-quality water was obtained from a Milli-Q water purification system (Millipore, Spain; http://www.merckmillipore.com).
A mixture of seven PCBs (Code BP-D7) at a concentration of 10 μg mL−1 each, in nonane:toluene (96.5:3.5 v/v, purity > 98 %) and a 5 μg mL−1 nonane:toluene (93:7, v/v) solution of mass-labeled PCB congeners (Code MBP-D7) were purchased from Wellington Laboratories (Canada; www.well-labs.com). Working mixture solution of PCBs (at 2000 ng mL−1) and the internal standard solution, containing 13C12-PCBs (at 600 ng mL−1) were prepared in MeOH. All standard solutions were stored at -20 °C prior to use.
Samples and magnetic nanoparticles
Different commercial brands of apple and grape juices were purchased from supermarkets in Madrid. A total of six different juices, three apple and three grape, were analyzed. The uncoated Fe3O4 MNPs were a commercial product supplied by Sigma-Aldrich (Germany, www.sigmaaldrich.com).
Surface modification of the Fe3O4 MNPs with sodium oleate was carried out as described in previous work [15]. Briefly, 200 mg of Fe3O4 NPs and 30 mL of MilliQ water were maintained at 80 °C in an oven. Then, three portions of 26 mg sodium oleate were added with a time interval of 10 min between each addition and the reaction mixture was kept at 80 °C for another 35 min. Then, the tubes were cooled to room temperature and the Ol-coated MNPs were separated using a neodymium (Nd-Fe-B) block magnet and, finally, they were washed five times with 4 mL of MeOH to remove the excess of oleate from the solution.
Characterization of the Ol-coated MNPs was performed at the “Instituto de Ciencia de Materiales de Madrid” (ICMM/CSIC) and INIA (Spain). The morphology and particle size were examined by transmission electron microscopy (TEM) with a JEOL JEM 2100 operating at 200 kV (Jeol Ltd., Japan; www.jeol.co.jp/en). Simultaneous differential thermal and thermogravimetric analyses (DTA and TGA, respectively) were performed using a SEIKO model EXSTAR 6300 (SEIKO Instrument Inc.; www.sii.co.jp) using a temperature range from room temperature to 800 °C, at 10 °C min-1. The IR spectroscopic study was conducted on a Bruker IFS 66VS spectrometer (www.bruker.com) and on a Jasco FTIR-460 Plus spectrophotometer (www.jascoinc.com).
Apparatus
A Vibromatic Rocking Mixer (Selecta, Spain; www.grupo-selecta.com/es) and an ultrasonic water bath (Raypa, Barcelona, Spain; www.raypa.com) at room temperature, with a generator with an output of 150 W and a frequency of 35 kHz, were used to carried out the dispersion of octanol in the juice samples.
GC–MS/MS analysis was carried out on an Agilent 7890A (Waldbronn, Germany; www.agilent.com) gas chromatograph equipped with a multimode inlet and coupled to a triple quadrupole mass spectrometer, Model 7000 (Agilent, Waldbronn, Germany; www.agilent.com). Analyses were performed based in our previous paper for the determination of PCBs [15]. The quantification of the studied compounds was based on their relative response factor to five calibration standards in the range from 7.5 to 90 ng mL−1. Each calibration level was spiked with 90 ng mL−1 of internal standards, taking into account a preconcentration factor of 125.
Extraction procedures
Dispersive liquid-liquid microextraction and magnetic retrieval
A mixture of 50 μL of 1-octanol and 5 mL of methanol was quickly injected into a 100 mL conical flask containing 25 mL of sample, which was closed with a glass cap and stirred vigorously using a Vibromatic Rocking Mixer at a vibration frequency of 782 oscillations min−1 for 2 min. Subsequently, 30 mg of Ol-coated MNPs were quickly added to the flask that was stirred another 2 min. The MNPs were isolated by placing the magnet on the wall of the flask and the supernatant was discarded. Finally, the MNPs were extracted twice with 2 mL of ACN, spiked with the internal standard solution, concentrated to dryness using an evaporator Genevac EZ-2 (NET Interlab, S.A.L., Spain; www.net-interlab.es), and redissolved in 200 μL of hexane. Then, the extract was analyzed in the GC-MS/MS system.
Magnetic solid-phase extraction procedure
25 mL of juice were diluted with water to a final volume of 50 mL adjusting to pH 7 with 0.4 M NaOH. Then, 5 mL of MeOH and 100 mg of Ol-coated MNPs were added and the mixture was stirred in the vibromatic mixer at 370 oscillations min−1 for 10 min. Afterwards, the MNPs were isolated using the magnet, the supernatant was decanted and a nitrogen stream was passed for 1 min. The adsorbed PCBs were eluted with EtAc (2 × 2 mL). The residues were redissolved with 200 μL of hexane for its chromatographic analysis. For recovery studies, samples were previously spiked with the appropriate working solutions of PCBs in MeOH and internal standard solution was added before to extract concentration. Nevertheless, in the analysis of real samples the internal standards were added before the mSPE procedure.
Statistical Analysis
Data analyses were performed using the statistical package Statgraphics Plus, release 5.0 (Manugistics, Maryland, USA).
Results and discussion
Characterization of the MNPs
Surface modification of Fe3O4 MNPs with sodium oleate was done following a procedure previously used by our group, whose characterisation was reported in a previous paper [15]. The size and shape of the Ol-coated MNPs were examined by TEM and it was observed that the MNPs have cubic shape and variable sizes, sometimes higher than those reported by the commercial supplier (100 nm) (Fig. S1a, Electronic Supplementary Material, ESM). In general, there were no obvious morphological differences between uncoated and coated MNPs suggesting that the grafted oleate layer was thin [15]. FT-IR with two different spectrometers were done to evaluate the surface groups of the coated MNPs. IR spectra of the coated NPs did not show the characteristic bands of the oleate, probably because the amount of oleate adsorbed to the surface was low because itdecreased with the increasing size of the MNPs [15, 19].
The thermal stability of the MNPs was measured using DTA and TGA. The presence of oleate on the surface of the MNPs could be determined taking into account the difference in the temperature of the exothermic transformation corresponding to the oxidative phase that were higher when the MNPs were coated, due to their higher thermal stability. The curves obtained are shown in Fig. S1b (ESM).
Optimization of the two-step procedure based on dispersive liquid-liquid microextraction and magnetic retrieval
The combination of DLLME and magnetic retrieval without the assistance of a disperser solvent had been successfully applied to the extraction of organic pollutants with low water solubility, such as PAHs [8, 10] or carbaryl pesticide [9] from water and triazole fungicides from fruit samples [11]. It has been described that the application of ultrasound or vortex assistance, instead of using a disperser solvent, can facilitate emulsification and the transfer of the target analytes to the immiscible extractive solvent reducing solvent consumption [20]. The combination of DLLME with D-μ-SPE using MNPs overcomes the need of a centrifugation or cooling step used in the conventional DLLME. 1-Octanol was selected as the extractant for several reasons. It has been described as one of the most widely used organic solvents in liquid phase microextraction; 1-octanol is much less toxic than conventional halogenated hydrocarbons; it ensures extraction enrichment because it is immiscible with water and dissolves target analytes well; due to its low vapor pressure and viscosity it is free from loss during agitation and prone to emulsification without a disperser [11]. Finally, although 1-octanol is not generally applied in classical DLLME, it has been described as a good solvent to be extracted and retrieved from an aqueous sample solution by D-μ-SPE rather than the analytes directly [8–11].
Based on these studies, a preliminary assay was done to evaluate the dispersion of 1-octanol on the juice samples. Thus, the formation of small solvent drops in the samples assisted by stirring or ultrasound was evaluated. It was observed that a quick injection of 1-octanol, followed by vigorously stirring in the mechanical shaker or sonication in the ultrasound system, only after the stirring process formation of small drops was observed. Therefore, the mechanical shaking was selected to carry out the DLLME step in further assays.
In a first assay, 50 μL of 1-octanol were quickly injected to 25 mL of a fortified apple juice sample and vigorously stirred for 2 min; then, 30 mg of the Ol-coated MNPs were added and stirred at high speed for 1 min. The recoveries obtained were > 52 % for PCB 28 and PCB 52 and between 33 and 40 % for the other congeners.
The effect of different volumes of 1-octanol and masses of MNPs, the pH effect and the use of MeOH as disperser solvent were evaluated in the DLLME step; and the extraction time was the parameter evaluated in the D-μ-SPE step. The respective data and Figures are given in the ESM (Figures S2 and Figure S3). It was observed a decrease in the recovery of all the analytes at neutral pH (Fig. 1). The following experimental conditions were found to give best results: (a) pH of 3.5 (b) simultaneous injection of a mixture of 50 μL of 1-octanol and 5 mL of methanol into the sample. (c) 2 min extraction time with the MNPs. After the optimization of the main parameters, the recoveries obtained for the PCBs varied from 35 to 77 %, and the best results were obtained for the PCBs with the lower molecular weight (for PCB28, PCB52 and PCB 101 the recoveries were 77, 68 and 60 %, respectively).
The MNPs can retrieve 1-octanol from aqueous solutions via hydrophobic interactions [8], but PCBs are also hydrophobic and they are effectively absorbed by the MNPs directly by mSPE [15]. Shi and Lee [8] compared the use of MNPs in mSPE and in a two-step method for the extraction of PAHs from water samples and suggested that the DLLME step was a contributing factor in the enhancement of the extraction. Similar results have been reported by Mukdasai et al. [9] for the extraction of carbaryl pesticide using other MNPs.
Optimization of the direct magnetic solid-phase extraction procedure
Taking in account that the recoveries obtained by the two step procedure reported above were lower than those expected, the direct application of these MNPs as mSPE sorbent was evaluated. A preliminary assay was carried out based on our previous studies [15]. Thus, 50 mL of sample containing a 10 % of MeOH and a known concentration of PCBs mixture, were extracted by mSPE using 50 mg of Ol-coated MNPs during 10 min and desorption with EtAc. In the preliminary assays the quantification was done by comparing peak areas obtained in samples with those found for standard mixtures. The results showed recoveries from 67 % to 108 %, indicating a potential use of the Ol-coated MNPs as sorbent for mSPE, but some parameters had to be optimized.
First of all, the effect of the sample dilution in the recoveries was evaluated. Recoveries above 100 % were obtained for PCB28 and PCB52, which could be due to a potential matrix effect as a result of the complexity of the sample. Sample dilution is the simplest and the most commonly used method for decreasing the effect of matrix components on the extraction efficiency of DLLME [21] and when complex matrices, such as milk or urine, were extracted by mSPE [22–24]. Thus, the effect of the sample dilution in the recoveries was evaluated at 0, 25 % and 50 % of dilution with water, and it was observed that a 50 % of dilution resulted in high recoveries in most cases. This condition was selected in the following assays. Nevertheless, recoveries higher of 100 % obtained for PCBs 28 and 52 suggested that the potential matrix effects were not sufficiently attenuated with the sample dilution. For this reason, isotopically labeled PCBs were used as internal standards to overcome this effect (see validation section).
The effects of sample pH on the mSPE procedure were evaluated in the range of 3.5 to 9. The pH of the sample was 3.5 and to achieve higher pH values 0.4 M NaOH was added to the diluted sample. The chromatographic responses were significantly higher at pH 7 (Fig. 2); therefore, this pH value was the most adequate for further analyses.
The effect of ionic strength was investigated by adding NaCl in the range of 0 to 20 %. Results showed that recoveries did not increase with a 5 % of NaCl and decreased with the increase of NaCl concentration (Figure S4). Therefore, salt was not added in the following assays. Finally, the influence of the amount of MNPs on the recovery was evaluated using 75, 100 and 150 mg and the statistical analysis confirmed that 100 mg of MNPs gave the best recoveries for all the compounds (Figure S5). Based on the experimental results shown above, the optimal conditions for the mSPE procedure were as follows: 100 mg of MNPs were added to 50 mL of diluted samples (1:1, v/v), at pH 7, containing a 10 % of MeOH, followed by a 10 min extraction. These results pointed out that the procedure applied to analyze PCBs in soil leachates [15] required modifications such as, sample dilution, an increase in the amount of MNPs used, and pH 7, to be used in more complex liquid samples, such as fruit juices.
The mSPE procedure provided better recoveries of the target analytes than the two-step procedure. Therefore, the mSPE method was validated and applied to the analysis of PCBs in apple and grape juices.
Magnetic solid-phase extraction method validation
The identification and quantification of PCBs were done by GC-MS/MS. Two sets of standard solutions, one set was solvent-based and the other was prepared spiking blank apple juice extracts, were injected to determine if the chromatographic responses of target analytes were affected by matrix components. The slopes obtained by plotting concentration, at five levels, against peak area for both solutions, were compared. It was observed that the slope ratio obtained with external standard quantification, for the two sets of calibration solutions, was > 1 for all the PCBs (from 1.2 to 1.6) showing a matrix effect. Hence, isotopically labeled PCBs were used as internal standards, because it was observed that isotope labeled standards counteracted matrix effects. The calibration curves allowed the quantification PCBs in juice in the range of 0.05 to 0.7 ng mL−1 with a good linearity (correlation coefficients ≥0.995).
Recovery through the method was tested by adding known amount of analytes (to reach final concentrations of 0.7 and 0.2 ng mL−1) to three replicate apple and grape juice samples that were analyzed following the mSPE method described above. Unspiked blank samples were previously analyzed to determine the possible presence of these compounds. Recoveries obtained by mSPE at these fortification levels ranged from 70 to 85 % for all PCBs except for PCB28 and PCB52, with standard deviations < 6.5, see Table 1. Although the recovery rates for these two compounds were low, the method is consistent because these standard deviations fulfilled the requirements of IUPAC [25].
Table 2 shows a comparative summary of the mSPE method for juice using Ol-coated MNPs with other methods reported for the determination of PCBs in liquid samples. The efficiency obtained was in the range reported in other studies [12, 15]; although, in general, the values were lower than those reported because juice samples are more complex than the samples analyzed in those studies.
The repeatability of the chromatographic determination was determined by injecting nine times fortified blank apple and grape extracts, obtained with Ol-coated MNPs within a given day and the RSD calculated for the studied compounds ranged from 2.1 to 9.4. Within-laboratory reproducibility of the chromatographic determination was evaluated during different days and was found to be lower than 9 % for all the compounds, expressed as RSD. The repeatability and reproducibility of the whole procedure was determined for grape juice spiked at 0.2 ng mL−1. Thus, the repeatability was evaluate by analysing six replicates within a given day and reproducibility by determining the recoveries of three replicates within 3 days on different weeks with RSD lower than 4 % and 7 %, respectively.
The limits of detection (LODs) and limits of quantification (LOQs) of the method were determined by analysis of ten replicates of apple and grave juice extracts spiked at 0.06 ng mL−1 (taking in account a preconcentration factor of 125). LODs were calculated with the following equation: LOD = t99*S, where t99 is the Student’s t value for a 99 % confidence level and n-1 degrees of freedom and S is the standard deviation of the replicate analyses [30]. LOQs were calculated as 10 times the standard deviation of the results [30]. LODs ranged from 1.6 to 5.4 ng L−1. These limits were similar to those obtained for PCBs in soil leachates using the same coated MNPs and lower to those reported by other authors for the determination of PCBs in water samples, using other nanocomposites or extractive methods (Table 2). These values are in the same range of those reported by Matsadiq et al. [5] using DLLME based on solidification of floating organic droplet and gas chromatography-electron capture detection for PCBs in peach juices, with values from 3.0 to 6.7 ng L−1.
Application to real samples
The validated mSPE method was applied to the determination of PCBs in commercial apple and grape juices. The results revealed that none of the seven target PCBs were found in these juice samples. A representative MRM chromatogram of the quantifier transitions of PCBs in a standard solution containing 15 ng mL−1 of each compound and in a grape juice extract are depicted in Fig. 3a and b, respectively.
Analytical methods for the analysis of PCBs are widely reported [31] and, while there is sufficient data available on PCBs levels in foodstuffs, there is less information on their evaluation in fruits and vegetables [32, 33] and it should be remarked the scarcity of data regarding these contaminants in fruit juices. Llobet et al. [33] reported the levels of PCBs in 108 foodstuffs samples (12 fruits), and found a total concentration of PCBs in fruit of 4.5 ng kg-1. Schellin and Popp [4] described a membrane-assisted solvent extraction method for the extraction of PCBs from river water, white wine and apple juice and no PCBs were found in the samples. Matsadiq et al. [5] described a DLLME method for the determination of PCBs, organochlorine and pyrethroid pesticides in peach juices, pulps and peels, and the target analytes were not found in the samples, except for PCB 101, which was detected.. Recently, Dasgupta et al. [34] described a DLLME method for the extraction of pesticides, dioxin-like PCBs and PAHs in water and juices; although, this method was only applied to the screening of pesticides in the juice samples. The low LOQ obtained with the mSPE method using Ol-coated MNPs allow the detection and quantification of these PCBs at the levels detected in peach juice by Matsadiq et al. [5].
Conclusions
In this work, two extractive methods using Ol-coated MNPs were evaluated for the extraction of PCBs from fruit juices. In the two-step extraction method lower amounts of MNPs were needed but the recoveries were lower than with the mSPE procedure. The mSPE procedure provided LOQs lower to those reported by other authors for the determination of PCBs in water samples. It was observed that the sample matrix could affect the behavior of the PCBs and isotope labeled internal standards were used to compensate matrix effects. The method provided a pre-concentration factor of, at least, 125. No PCBs residues were found in the real samples analyzed. The present work demonstrates that Ol-coated Fe3O4 MNPs, as mSPE adsorbent, has good applicability for the extraction of these persistent pollutants that could be present in juice samples.
References
Westbom R, Thorneby L, Zorita S, Mathiasson L, Bjorklund E (2004) Development of a solid-phase extraction method for the determination of polychlorinated biphenyls in water. J Chromatogr A 1033:1–8
Shi J-W, Zhao Y-G, Fu Z-J, Li J-G, Wang Y-F, Yang T-C (2012) Development of a screening method for the determination of PCBs in water using QuEChERS extraction and gas chromatography-triple quadrupole mass spectrometry. Anal Sci 28:167–173
Rezaei F, Bidari A, Birjandi AP, Hosseini MRM, Assadi Y (2008) Development of a dispersive liquid-liquid microextraction method for the determination of polychlorinated biphenyls in water. J Hazard Mater 158:621–627
Schellin M, Popp P (2003) Membrane-assisted solvent extraction of polychlorinated biphenyls in river water and other matrices combined with large volume injection-gas chromatography-mass spectrometric detection. J Chromatogr A 1020:153–160
Matsadiq G, Hu H-L, Ren H-B, Zhou Y-W, Liu L, Cheng J (2011) Quantification of multi-residue levels in peach juices, pulps and peels using dispersive liquid-liquid microextraction based on floating organic droplet coupled with gas chromatography-electron capture detection. J Chromatogr B 879:2113–2118
Xie L, Jiang R, Zhu F, Liu H, Ouyang G (2014) Application of functionalized magnetic nanoparticles in sample preparation. Anal Bioanal Chem 406:377–399
Tay KS, Abd Rahman N, Bin Abas MR (2013) Magnetic nanoparticle assisted dispersive liquid-liquid microextraction for the determination of 4-n-nonylphenol in water. Anal Methods 5:2933–2938
Shi ZG, Lee HK (2010) Dispersive liquid-liquid microextraction coupled with dispersive mu-solid-phase extraction for the fast determination of polycyclic aromatic hydrocarbons in environmental water samples. Anal Chem 82:1540–1545
Mukdasai S, Thomas C, Srijaranai S (2013) Enhancement of sensitivity for the spectrophotometric determination of carbaryl using dispersive liquid microextraction combined with dispersive m-solid phase extraction. Anal Methods 5:789–796
Wang N, Shen R, Yan Z, Feng H, Cai Q, Yao S (2013) Magnetic retrieval of an extractant: fast ultrasound-assisted emulsification liquid-liquid microextraction for the determination of polycyclic aromatic hydrocarbons in environmental water samples. Anal Methods 5:3999–4004
Li Y, Yang X, Zhang J, Li M, Zhao X, Yuan K, Li X, Lu R, Zhou W, Gao H (2014) Ultrasound-assisted emulsification magnetic microextraction: a fast and green method for the determination of triazole fungicides in fruit juice. Anal Methods 6:8328–8336
Cao X, Chen J, Ye X, Zhang F, Shen L, Mo W (2013) Ultrasound-assisted magnetic SPE based on Fe3O4-grafted graphene for the determination of polychlorinated biphenyls in water samples. J Sep Sci 36:3579–3585
Zeng S, Cao Y, Sang W, Li T, Gan N, Zheng L (2012) Enrichment of polychlorinated biphenyls from aqueous solutions using Fe3O4 grafted multiwalled carbon nanotubes with poly dimethyl diallyl ammonium chloride. Int J Mol Sci 13:6382–6398
Zeng S, Gan N, Weideman-Mera R, Cao Y, Li T, Sang W (2013) Enrichment of polychlorinated biphenyl 28 from aqueous solutions using Fe3O4 grafted graphene oxide. Chem Eng J 218:108–115
Pérez RA, Albero B, Tadeo JL, Molero E, Sanchez-Brunete C (2015) Application of magnetic iron oxide nanoparticles for the analysis of PCBs in water and soil leachates by gas chromatography-tandem mass spectrometry. Anal Bioanal Chem 407:1913–1924
Deng X, Chen X, Lin K, Ding G, Yao P (2013) Rapid and selective determination of trace benzimidazole fungicides in fruit juices by Magnetic solid-phase extraction coupled with high-performance liquid chromatography-fluorescence detection. Food Anal Methods 6:1576–1582
Zhang J, Li M, Li Y, Li Z, Wang F, Li Q, Zhou W, Lu R, Gao H (2013) Application of ionic-liquid-supported magnetic dispersive solid-phase microextraction for the determination of acaricides in fruit juice samples. J Sep Sci 36:3249–3255
Li Z, Hou M, Bai S, Wang C, Wang Z (2013) Extraction of imide fungicides in water and juice samples using magnetic graphene nanoparticles as adsorbent followed by their determination with gas chromatography and electron capture detection. Anal Sci 29:325–331
Zhang L, He R, Gu H-C (2006) Oleic acid coating on the monodisperse magnetite nanoparticles. Appl Surf Sci 253:2611–2617
Leong M-I, Fuh M-R, Huang S-D (2014) Beyond dispersive liquid-liquid microextraction. J Chromatogr A 1335:2–14
Saraji M, Boroujeni MK (2014) Recent developments in dispersive liquid-liquid microextraction. Anal Bioanal Chem 406:2027–2066
Ye L, Wang Q, Xu J, Shi Z-G, Xu L (2012) Restricted-access nanoparticles for magnetic solid-phase extraction of steroid hormones from environmental and biological samples. J Chromatogr A 1244:46–54
Gao Q, Luo D, Bai M, Chen Z-W, Feng Y-Q (2011) Rapid determination of estrogens in milk samples based on magnetite nanoparticles/polypyrrole magnetic solid-phase extraction coupled with liquid chromatography-tandem mass spectrometry. J Agric Food Chem 59:8543–8549
Zhang S, Niu H, Zhang Y, Liu J, Shi Y, Zhang X, Cai Y (2012) Biocompatible phosphatidylcholine bilayer coated on magnetic nanoparticles and their application in the extraction of several polycyclic aromatic hydrocarbons from environmental water and milk samples. J Chromatogr A 1238:38–45
Thompson M, Ellison SR, Wood R (2002) Harmonized guidelines for single laboratory validation of methods of analysis (IUPAC Technical Report). Pure Appl Chem 74:835–855
Ozcan S (2011) Analyses of polychlorinated biphenyls in waters and wastewaters using vortex-assisted liquid-liquid microextraction and gas chromatography-mass spectrometry. J Sep Sci 34:574–584
Ozcan S, Tor A, Aydin ME (2009) Determination of selected polychlorinated biphenyls in water samples by ultrasound-assisted emulsification-microextraction and gas chromatography-mass-selective detection. Anal Chim Acta 647:182–188
Chen X, Ding N, Zang H, Yeung H, Zhao R-S, Cheng C, Liu J, Chan TWD (2013) Fe3O4@MOF core-shell magnetic microspheres for magnetic solid-phase extraction of polychlorinated biphenyls from environmental water samples. J Chromatogr A 1304:241–245
Karamani AA, Douvalis AP, Stalikas CD (2013) Zero-valent iron/iron oxide-oxyhydroxide/graphene as a magnetic sorbent for the enrichment of polychlorinated biphenyls, polyaromatic hydrocarbons and phthalates prior to gas chromatography-mass spectrometry. J Chromatogr A 1271:1–9
Ripp J (1996) Analytical detection limit guidance” Wisconsin department of natural resources, 1st edition, Madison, United States of Wisconsin, 07–33
Muir D, Sverko E (2006) Analytical methods for PCBs and organochlorine pesticides in environmental monitoring and surveillance: a critical appraisal. Anal Bioanal Chem 386:769–789
Grassi P, Fattore E, Generoso C, Fanelli R, Arvati M, Zuccato E (2010) Polychlorobiphenyls (PCBs), polychlorinated dibenzo-p-dioxins (PCDDs) and dibenzofurans (PCDFs) in fruit and vegetables from an industrial area in northern Italy. Chemosphere 79:292–298
Llobet JM, Bocio A, Domingo JL, Teixido A, Casas C, Muller L (2003) Levels of polychlorinated biphenyls in foods from Catalonia, Spain: estimated dietary intake. J Food Prot 66:479–484
Dasgupta S, Banerjee K, Utture S, Kusari P, Wagh S, Dhumal K, Kolekar S, Adsule PG (2011) Extraction of pesticides, dioxin-like PCBs and PAHs in water based commodities using liquid-liquid microextraction and analysis by gas chromatography-mass spectrometry. J Chromatogr A 1218:6780–6791
Acknowledgments
This study was financed by the Ministry of Science and Innovation-National Institute for Agricultural and Food Research and Technology, INIA, Project number “RTA 2011-00047-00-00”. Authors wish to express their gratefulness to Dr. M.P. Morales, researcher of the Department of Biomaterials and Bioinspired Materials, and to the Scientific Technical Services of Infrared Spectroscopy (ICMM; CSIC) for her help and advice in the characterization of the MNPs and for the IR analysis, respectively. David Ibarra (INIA-CIFOR) is greatly acknowledged for FTIR analysis.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
ESM 1
(DOCX 1363 kb)
Rights and permissions
About this article
Cite this article
Pérez, R.A., Albero, B., Tadeo, J.L. et al. Oleate functionalized magnetic nanoparticles as sorbent for the analysis of polychlorinated biphenyls in juices. Microchim Acta 183, 157–165 (2016). https://doi.org/10.1007/s00604-015-1617-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00604-015-1617-2