Abstract
A jelly-like form of carbon dots (C-dots) was prepared by microwave-assisted synthesis from citric acid in the presence of tetraoctylammonium bromide. The effect of the concentration of tetraoctylammonium bromide was examined. The synthesized carbon dots were characterized by UV–vis, XRD, FTIR, fluorescence and HR-TEM. Fluorescence extends from 350 to 600 nm, and the corresponding excitation wavelengths range from 300 to 460 nm. Quantum yields are at around 0.11. A cytotoxicity study showed carbon dots to be cell permeable and biocompatible which renders them appropriate for imaging applications. The dots were used to image HeLa cell lines via the blue fluorescence of the dots.

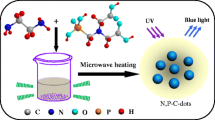
C-dots were synthesized from citric acid by microwave heating in presence of varying concentrations of tetraoctylammonium bromide (TOAB) as a micellar template. The excellent optical properties of the nanoparticles make them well suitable for bio-imaging of HeLa cells.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Since the discovery of carbon dots about a decade ago, carbon dots have received considerable attention due to their distinctive chemical, optical, thermal, mechanical, and electronic properties [1]. Particularly, the desirable properties of chemical stability [2] and biocompatibility [3, 4] are ideally suited for carbon dots to be feasibly applied in the fields of material science, biochemistry, and biomedicine. Due to the strongly fluorescent, color-tunable [5], readily water soluble, and nontoxic properties, immense applications from this material has boosted. Such as the fields in catalysis [6, 7], bio sensing [8], theranostics [9], and bio imaging [10–14].
Unlike quantum dots prepared from other materials such as CdSe, InAs, and so forth, other advantages of carbon dots are their high quantum yields [15] and non-blinking fluorescent features [16]. To solve the blinking issue for these materials, many attempts have been made to control the photoluminescence fluctuations; the most adopted strategy is the shell engineering of the hetero-structured core/shell configuration of the nanodots [17]. Even then the treatment is strenuous and the technical expertise is required. Therefore, the non-blinking feature of carbon dots is important for the applications in chemical analysis and bio-analysis as the blinking may result in signal loses while performing real-time tracking in living cells [10]. In this respect, carbon dots possessed an obvious advantage over other materials.
General synthetic methodologies of carbon dots can be categorized as top-down and bottom-up protocols [16, 18]. The top-down approach consists of laser ablation, arc discharge, acid dehydration, and electrochemical oxidation from carbon source [19]. On the contrary, thermal carbonization, microwave or ultrasonic treatments from precursors belong to bottom-up approach [20]. Some of the methodologies required delicate instrumentation or are tedious in procedures or harmful chemical reagents are unavoidable, making the synthesis technically unfeasible to perform in general laboratories. Among all the available protocols, microwave-assisted methods are efficient, time-saving, cost-effective, environment-friendly, and narrow size-distributed through the homogeneous heating [21, 22]. Therefore, microwave-assisted heating was employed in the synthetic procedure, which was attempted to provide a facile and feasible route of preparing carbon dots without surface passivation or heteroatom doping.
Tetraoctylammonium bromide (TOAB) has been applied as the mediator of biosensors [23], capping reagent of nanomaterials [24–26] and utilized in micro-emulsion synthesis of nanocrystals [27, 28]. We presented the application of TOAB in the synthetic procedure of carbon dots, the formation of carbon dots from precursor citric acid under microwave-assisted heating occurred and encapsulated inside the micelles of TOAB [29]. The synthesized products exhibited narrow size distribution of the particles which has advantages over than other synthetic methods as no post treatments required for the synthesis.
The preparation protocol of carbon dots the authors presented exhibited a jelly form of carbon dots, which has not been presented to date, to the best of our knowledge. Cytotoxicity study of the synthesized carbon dots was conducted and it showed low toxicity and hence biocompatible to cells which is an essential prerequisite as bio-imaging agents or for bio-analysis practices [30]. In-vitro cellular uptake experiments of the carbon dots were performed in human cancer cells. The results suggested that the synthesized carbon dots were prospective for the applications of cell labeling through a straightforward incubation method.
Experimental section
Materials and methods
Citric acid and ethyl alcohol were purchased from J.T. Baker (USA; www.jtbaker.com & www.avantormaterials.com). Tetraoctylammonium bromide (98 %) was purchased from Sigma-Aldrich (USA; www.sigmaaldrich.com). Quinine sulphate was supplied by Alfa Aesar (Great Britain; www.alfa.com). Ultrapure water was provided from a Milli-Q Plus water purification system (18.2 MΩ cm, Millipore, and Bedford, MA, USA) and was used for all experiments.
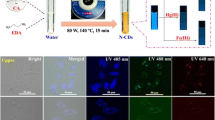
HeLa cell line [BCRC 60005] was purchased from Bioresource Collection and Research Center [BCRC] (Taiwan; www.bcrc.firdi.org.tw). The fluorescent microscope images were obtained by using an inverted Olympus IX73 fluorescent microscope with a barrier filter 420 nm for an excitation wavelength between 330 and 385 nm.
Synthesis of carbon dots
Five grams of citric acid was accurately measured as the precursor molecule of carbon dots. 0.1 M TOAB aqueous solution was prepared and 2, 4, 6, and 8 mL of TOAB solution was transferred into the aforementioned citric acid individually (which is denoted as C-dots I, II, III, and IV, respectively). The solutions were heated with a domestic microwave oven (at maximum 500 watt). The solution was heated and the volume decrease was visually observed along with the color turning from transparent to yellowish-red. The optimization of reaction time in the microwave-assisted heating was performed. The results suggested that carbon dots can be synthesized for the duration of 2–3 min. After 3 min yellowish color was clearly observed in all concentrations of carbon dots. To confirm the formation of carbon dots the product was UV-light illuminated at 365 nm and the characteristic blue color fluorescence of carbon dots was demonstrated. A jelly type of carbon dots was obtained which is a distinctive variety from the C-dots from previous published literatures. Liquid form of carbon dots was used for the characterization of carbon dots in UV and fluorescence measurements; it was dissolved in ethyl alcohol and diluted with DI (deionized) water. The gel form of C-dots which we prepared is freely soluble in minimum quantity of ethyl alcohol and the volume was made up by diluted with DI water. The yellowish mixture of C-dots was isolated by centrifugation at 12,000 rpm (17,226 g) for 10 min. It was further purified by dialysis against Milli-Q water for 20 h under constant stirring condition. The product was collected and the solution was concentrated in a rotary vacuum evaporator (Büchi, Germany; www.buchi.com) and frozen dried further for the quantification of C-dots.
Characterization of carbon dots
The morphology and uniformity of C-dots were measured by a high resolution transmission electron microscopy (HR-TEM) (JEOL, Japan). Spectral properties of C-dots were studied by a UV–vis spectroscopy (Thermo, Evolution 201, and USA). Fluorescence spectroscopy (Hitachi-2700, Japan) was used to examine the fluorescence property of C-dots at different excitation wavelengths; a standard quartz cuvette having a path length of 1.0 cm was used. Functional groups on the surface of C-dots were studied using a Fourier transform infrared spectrometer (FT-IR) (Perkin Elmer, USA). The crystallinity of C-dots was studied using an X-ray diffraction (XRD) (Philips, the Netherlands). A green argon laser with excitation wavelength 514 nm (Photon energy 2.41 eV) was applied to conduct the photoluminescence (PL) study of C-dots prepared from various contents ranging from 2 to 8 mL of TOAB.
Quantum yield measurement
The quantum yield (Q) of the C-dots has been calculated using the following equation, Eq. 1. Quinine sulphate (Q is 0.54) was chosen as a reference to determine Q. Where Q denotes for quantum yield, I represents measured integrated emission intensity, n denotes the refractive index, and E is the optical density. R represents the fluorophore of known quantum yield for reference [31].
Cell labeling and cytotoxicity studies
HeLa cells were cultured in Dulbecco’s Modified Eagle’s Medium (Sigma, St. Louis, Missouri, USA) with 10 % fetal bovine serum (Gibco, Grand Island, New York, USA), 1 % non-essential amino acids (Biosource, Camarillo, California, USA), 1 % penicillin/streptomycin (Sigma, St. Louis, Missouri, USA), 1 % sodium pyruvate (Sigma, St. Louis, Missouri, USA), and 1.5 g L−1 sodium bicarbonate (Sigma, St. Louis, Missouri, USA) in 5 % CO2 at 37 °C.
The cells were trypsinized and seeded in tissue culture plates at an initial cell density of ~106 cells/well. The cells were treated with C-dots at a final concentration of 0.1 mg mL−1. After incubated at 37 °C for 24 h, the cells were washed three times with fresh media and imaged with an inverted Olympus IX73 fluorescent microscope with excitation wavelength of 330–385 nm for blue region images collection.
Cytotoxicity study was performed by the Trypan blue staining assay. HeLa cells were staining by Trypan blue dye [32]. HeLa cells were trypsinized and re-suspended in culture medium. The cells were seeded to a tissue culture plate with 0.5 mL full of medium and kept for overnight at 37 °C and 5 % CO2. The C-dots solution of different amounts was loaded to each well. After incubation for 24 h, 0.4 % Trypan blue was added to each well and after 5 min, stained cells is counted to determine the cell viability. Unstained cells are counted as living cells and blue stained cells were counted as dead cells.
Results and discussion
A simple, straightforward microwave-assisted synthesis of carbon dots from the carbon source citric acid and micellar template of TOAB was employed, as shown from the schematic illustration in Fig. 1. A jelly-like form of carbon dots were obtained from this approach, which has a distinctive appearance than other published synthetic methods. The synthesized carbon dots were characterized by FT-IR, fluorescence, PL, UV–vis, and XRD measurements as well as HR-TEM micrographs.
In Fig. 2a, UV–vis spectra of carbon dots exhibited strong absorbance below 400 nm, which attributes to the overlap of n-π* transition of C = O and π-π* transition of C = C functional groups, can be validated from the analysis of FT-IR spectra in later discussion. For the cases of C-dots III and IV, the absorbance was significant while for C-dots II the weak absorbance indicated the preparation condition in C-dots II was unfavorable and negligible absorbance was observed in the case of C-dots I.
The XRD pattern of carbon dots in Fig. 2b demonstrated a broad single peak centered at ca. 20° (2θ) which is similar to those fabricated from other approaches, having highly disordered carbon with a predominantly amorphous structure corresponding to an interplanar spacing of 3.55 Å which is larger than the spacing between (002) planes in bulk graphite (3.34 Å). From the results of C-dots II, III, and IV, this peak becomes more observable while for C-dots I the peak is not characteristic of carbon dots.
The functional groups on the surface of carbon dots can be interpreted from the analysis of FT-IR spectra in Fig. 2c. The intense broad peak around 3300 cm−1 can be assigned to the O-H stretching and the broadness of the peak suggested a large number of OH group. The prominent peak near 1750 cm−1 was attributed to the C = O vibration, and the small shoulder around 1650 cm−1 can be indexed to the C = C or CONH2 vibration. Moreover, the peak near 1200 cm−1 can be assigned to the alkoxy C-O-C stretching. The synthesized carbon dots were highly hydrophilic and good dispersion in water due to the polar functional groups on the surface.
Strong PL emission of carbon dots with the peak maximum at 2.12 eV (585 nm) was observed with UV–vis irradiation, as shown in Fig. 2d, the C-dots IV showed the most intense PL at 2.12 eV compared to C-dots I, II, and III. Taking this manifestation together with the optical photographs from Fig. 5 it is obviously indicative of the characteristic of carbon dots. The PL mechanism of carbon dots has been presented based on the density functional theory (DFT), which indicates that PL energy of carbon dots depends on the extent of graphitic or amorphous feature of the core while the quantum yield depends on the surface defects and functional groups [33]. However, the reason and mechanism behind the multicolor luminescence from a single compound is still unknown to date and more research and exploration need to be done.
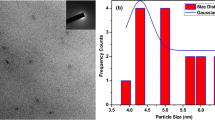
For the investigation of morphology and average particle size of carbon dots, HR-TEM micrographs of carbon dots were shown in Fig. 3. Except the particles converged into significant clusters for carbon dots I shown in Fig. 3a, the synthesized carbon dots II, III, and IV (in Fig. 3b, c, and d, respectively) revealed a crystalline structure and were well dispersed in water without aggregation and were practically spherical in shape with an average diameter of 3 nm, without any size selection or separation procedure. The results exhibited the average size of carbon dots is in narrow range.
As revealed in Fig. 4c, the fluorescence spectra of carbon dots displayed fluorescent emission at around 350–600 nm with the corresponding excitation wavelength of 300–460 nm with 20 nm increment, which exhibited the wavelength-dependent nature of carbon dots for excitation and emission spectra. The maximum intensity of emission observed at 375 nm was excited at 300 nm. As the excitation wavelength increased from 300 to 460 nm, the intensity of fluorescence decreased accordingly and shifted to longer wavelength (red shift). Some suggested the functional groups on the surface of carbon dots are attributed to this phenomenon while some suggested it is because of the defects on the surface. And it is worth to note that the fluorescence intensity decreases in reverse order to the excitation wavelength. Regarding the quantum yield of carbon dots, the quantum yield of C-dots I, II, III, and IV was calculated with reference to quinine sulphate. The quantum yield of C-dots IV was calculated as 11 % which is the highest yield among carbon dots I-IV which indicates it has the most intense fluorescence property compare to others.
The carbon dots aqueous solution showed colorless transparent under visible light as shown in Fig. 5a, b, whereas strong blue fluorescence emitted under UV light irradiation (λex = 365 nm) was observed, as demonstrated in Fig. 5b, d. This bright fluorescence is the characteristic of carbon dots, combining with the features of tunable emission, high biocompatibility and low cytotoxicity making carbon dots as one of the potential candidates for applications in bio-imaging. Due to their intense fluorescence, non-toxic and high water solubility C-dots can be used as biological imaging agents efficiently. However, for cellular targeting and subcellular level the functionalization on the surface of C-dots is necessary. Interestingly, we found that small nano-sized C-dots entered into cells without any further functionalization. In order to capture the cell images, C-dots were mixed with cells culture media along with cells, incubated for 24 h, and the washed cells were then imaged under bright field and fluorescence mode, respectively. In Fig. 6a, b the images of cells without C-dots were shown. For comparison, fluorescence images of cells with C-dots in Fig. 6c, d were shown respectively. Figure 6a, c in left panel indicated cells capturing in bright field mode and Fig. 6b, d exhibited cells image capturing in fluorescence mode. The low visibility displayed in Fig. 6b is due to the absence of C-dots but, by contrast, very intense blue fluorescence color of cells with C-dots was demonstrated in Fig. 6d. For being able to be applied for bio-imaging, toxicity of carbon dots is a definite concern. The most adopted is the Trypan blue assay of human cancer cells [34]. Shown in Fig. 7 is the cytotoxicity study of various concentrations of carbon dots on HeLa cells, the living cells which were not colored by Trypan blue remained a majority while the blue stained dead cells were in a comparative minority. The particle size distributions of these C-dots were revealed in Fig. 8 in the form of histogram. The study shows the cell viability was more than 95 % after treatment with even 1–5 mg mL−1 carbon dots, which is quite high concentration applied for imaging studies. The toxicity evaluation of carbon dots in vivo was considered nontoxic as well from other report [35]. Furthermore, the stability test of C-dots was conducted with fluorescence at 375 nm and excited at 300 nm, as shown in Fig. 9. It demonstrated that the C-dots are highly stable after long term excitation. Therefore, carbon dots appear to be low toxicity to cells according to various research reports.
Conclusions
We presented a facile synthesis of carbon dots via microwave-assisted heating from the carbon source citric acid and the micellar template of TOAB. The product displayed a jelly-like form of carbon dots, which has not yet been presented from the literatures. The synthesized carbon dots without surface passivation demonstrated water soluble, narrow size distribution with excellent optical properties, great chemical stability, and biocompatibility through the characterization. From HeLa cell imaging, strong fluorescence was observed after 24 h incubation for intracellular uptake, it presents great opportunities from experimental research to practical applications and develops them a promising candidate as fluorescent imaging agents for the applications in bio-imaging and bio-sensing.
References
Blau W, Ji W (2012) Feature issue introduction: nanocarbon for photonics and optoelectronics. Opt Mater Express 2(6):891–892
Zhou J, Sheng Z, Han H, Zou M, Li C (2012) Facile synthesis of fluorescent carbon dots using watermelon peel as a carbon source. Mater Lett 66(1):222–224
Liu R, Wu D, Liu S, Koynov K, Knoll W, Li Q (2009) An aqueous route to multicolor photoluminescent carbon dots using silica spheres as carriers. Angew Chem 121(25):4668–4671
Yang S-T, Cao L, Luo PG, Lu F, Wang X, Wang H, Meziani MJ, Liu Y, Qi G, Sun Y-P (2009) Carbon dots for optical imaging in vivo. J Am Chem Soc 131(32):11308–11309
Kozák OE, Datta KKR, Greplová M, Vc R, Kašlík J, Zbořil R (2013) Surfactant-derived amphiphilic carbon dots with tunable photoluminescence. J Phys Chem C 117(47):24991–24996
Li H, He X, Kang Z, Huang H, Liu Y, Liu J, Lian S, Tsang CHA, Yang X, Lee ST (2010) Water‐soluble fluorescent carbon quantum dots and photocatalyst design. Angew Chem Int Ed 49(26):4430–4434
Zhu C, Zhai J, Dong S (2012) Bifunctional fluorescent carbon nanodots: green synthesis via soy milk and application as metal-free electrocatalysts for oxygen reduction. Chem Commun 48(75):9367–9369
Zhu A, Qu Q, Shao X, Kong B, Tian Y (2012) Carbon‐dot‐based dual‐emission nanohybrid produces a ratiometric fluorescent sensor for in vivo imaging of cellular copper ions. Angew Chem 124(29):7297–7301
Zheng M, Liu S, Li J, Qu D, Zhao H, Guan X, Hu X, Xie Z, Jing X, Sun Z (2014) Integrating oxaliplatin with highly luminescent carbon dots: an unprecedented theranostic agent for personalized medicine. Adv Mater 26(21):3554–3560
Chen Y, Liang H (2014) Applications of quantum dots with upconverting luminescence in bioimaging. J Photochem Photobiol B Biol 135:23–32
Ding H, Zhang P, Wang T-Y, Kong J-L, Xiong H-M (2014) Nitrogen-doped carbon dots derived from polyvinyl pyrrolidone and their multicolor cell imaging. Nanotechnology 25(20):205604
Jiang C, Wu H, Song X, Ma X, Wang J, Tan M (2014) Presence of photoluminescent carbon dots in Nescafe® original instant coffee: Applications to bioimaging. Talanta 127:68–74
Mehta VN, Jha S, Kailasa SK (2014) One-pot green synthesis of carbon dots by using Saccharum officinarum juice for fluorescent imaging of bacteria (Escherichia coli) and yeast (Saccharomyces cerevisiae) cells. Mater Sci Eng C 38:20–27
Wang Z, Qu Y, Gao X, Mu C, Bai J, Pu Q (2014) Facile preparation of oligo (ethylene glycol)-capped fluorescent carbon dots from glutamic acid for plant cell imaging. Mater Lett 129:122–125
Liang Q, Ma W, Shi Y, Li Z, Yang X (2013) Easy synthesis of highly fluorescent carbon quantum dots from gelatin and their luminescent properties and applications. Carbon 60:421–428
da Silva JCE, Gonçalves HM (2011) Analytical and bioanalytical applications of carbon dots. TrAC Trends Anal Chem 30(8):1327–1336
Pisanello F, Leménager G, Martiradonna L, Carbone L, Vezzoli S, Desfonds P, Cozzoli PD, Hermier JP, Giacobino E, Cingolani R (2013) Non‐blinking single‐photon generation with anisotropic colloidal nanocrystals: towards room‐temperature, efficient, colloidal quantum sources. Adv Mater 25(14):1974–1980
Algarra M, Pérez-Martín M, Cifuentes-Rueda M, Jiménez-Jiménez J, da Silva JE, Bandosz T, Rodríguez-Castellón E, Navarrete JL, Casado J (2014) Carbon dots obtained using hydrothermal treatment of formaldehyde. Cell imaging in vitro. Nanoscale 6(15):9071–9077
Wang F, Pang S, Wang L, Li Q, Kreiter M, Liu C-Y (2010) One-step synthesis of highly luminescent carbon dots in noncoordinating solvents. Chem Mater 22(16):4528–4530
Yang X, Zhuo Y, Zhu S, Luo Y, Feng Y, Dou Y (2014) Novel and green synthesis of high-fluorescent carbon dots originated from honey for sensing and imaging. Biosens Bioelectron 60:292–298
Mazzier D, Favaro M, Agnoli S, Silvestrini S, Granozzi G, Maggini M, Moretto A (2014) Synthesis of luminescent 3D microstructures formed by carbon quantum dots and their self-assembly properties. Chem Commun 50(50):6592–6595
Wang J, Cheng C, Huang Y, Zheng B, Yuan H, Bo L, Zheng M-W, Yang S-Y, Guo Y, Xiao D (2014) A facile large-scale microwave synthesis of highly fluorescent carbon dots from benzenediol isomers. J Mater Chem C 2(25):5028–5035
Ye C, Zhong X, Yuan R, Chai Y (2014) A novel ECL biosensor based on C 60 embedded in tetraoctylammonium bromide for the determination of glucose. Sensors Actuators B Chem 199:101–107
Teh GB, Nagalingam S, Tilley RD, Ramesh S, Lim YS (2009) Colloidal synthesis of silicon nanocrystals Via inverse micelles microemulsion. Zeitschrift für Physikalische Chemie Int J Res Phys Chem Chem Phys 223(12):1417–1426
Nair SS, John SA, Sagara T (2009) Simultaneous determination of paracetamol and ascorbic acid using tetraoctylammonium bromide capped gold nanoparticles immobilized on 1, 6-hexanedithiol modified Au electrode. Electrochim Acta 54(27):6837–6843
Stowell CA, Korgel BA (2005) Iridium nanocrystal synthesis and surface coating-dependent catalytic activity. Nano Lett 5(7):1203–1207
Miranda OR, Dollahon NR, Ahmadi TS (2006) Critical concentrations and role of ascorbic acid (vitamin C) in the crystallization of gold nanorods within hexadecyltrimethyl ammonium bromide (CTAB)/tetraoctyl ammonium bromide (TOAB) micelles. Cryst Growth Des 6(12):2747–2753
Tilley RD, Warner JH, Yamamoto K, Matsui I, Fujimori H (2005) Micro-emulsion synthesis of monodisperse surface stabilized silicon nanocrystals. Chem Commun 14:1833–1835
Miranda OR, Ahmadi TS (2005) Effects of intensity and energy of CW UV light on the growth of gold nanorods. J Phys Chem B 109(33):15724–15734
Baker SN, Baker GA (2010) Luminescent carbon nanodots: emergent nanolights. Angew Chem Int Ed 49(38):6726–6744
Hu S-L, Niu K-Y, Sun J, Yang J, Zhao N-Q, Du X-W (2009) One-step synthesis of fluorescent carbon nanoparticles by laser irradiation. J Mater Chem 19(4):484–488
Lin H, Lin T, Juang J (2007) Abl deregulates Cdk5 kinase activity and subcellular localization in Drosophila neurodegeneration. Cell Death Differ 14(3):607–615
Zhu B, Sun S, Wang Y, Deng S, Qian G, Wang M, Hu A (2013) Preparation of carbon nanodots from single chain polymeric nanoparticles and theoretical investigation of the photoluminescence mechanism. J Mater Chem C 1(3):580–586
Monteiro-Riviere NA, Inman AO, Zhang L (2009) Limitations and relative utility of screening assays to assess engineered nanoparticle toxicity in a human cell line. Toxicol Appl Pharmacol 234(2):222–235
Yang S-T, Wang X, Wang H, Lu F, Luo PG, Cao L, Meziani MJ, Liu J-H, Liu Y, Chen M (2009) Carbon dots as nontoxic and high-performance fluorescence imaging agents. J Phys Chem C 113(42):18110–18114
Acknowledgments
The authors acknowledge the financial support from the Ministry of Science and Technology of Taiwan and the assistance from National Sun Yat-Sen University. The authors appreciate Mr. Bosgum Wu for his help in the acquisition of chemicals and materials.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Bhaisare, M.L., Talib, A., Khan, M.S. et al. Synthesis of fluorescent carbon dots via microwave carbonization of citric acid in presence of tetraoctylammonium ion, and their application to cellular bioimaging. Microchim Acta 182, 2173–2181 (2015). https://doi.org/10.1007/s00604-015-1541-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00604-015-1541-5