Abstract
We describe an indirect method for the voltammetric determination of Gd(III) ion. It is based on competitive extraction of Gd(III) into an ion-imprinted polymer (IIP) on the surface of a carbon paste electrode (CPE). The nano-sized IIP was synthesized via suspension polymerization in silicone oil and deposited on the CPE. The IIP-modified CPE was then incubated with solutions containing Pb(II) and a solution containing both Pb(II) and Gd(III) ions. The oxidative stripping differential pulse voltammetric signal for Pb(II) was utilized to determine the competitively extracted quantity of Pb(II). The presence of Gd(III) reduces the quantity of electroactive lead ions in the IIP located on the CPE. No such effect was observed for the case of a nonimprinted CPE. The effect of various factors on response were optimized. The drop in the signal for Pb(II) as a result of addition of Gd(III) is proportional to the concentration of Gd(III). The voltammetric response is linearly related to the concentration of Gd(III) in the 6.0 nM to 48 μM range, and the detection limit is 4.5 nM at an SNR of 3. The relative standard deviation is 3.7 % (for n = 5). The electrode is selective for Gd(III) even in the presence of other lanthanide ions. The method was applied to the determination of G(III) in synthetic and in spiked real samples.

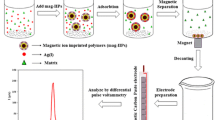
Schematic representation of the decrease in DPV signal of probe ion as a result of its replacement with Gd3+ ions in the selective sites of the IIP particles, existing on the carbon paste electrode surface.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Gadolinium has been classified as one of the rare earth elements. The rare earth elements are divided into the lanthanide and actinide series. Gadolinium and other lanthanide oxides are widely used in the preparation of glass fibers, gasoline-cracking catalysts, optical glasses and polishing compounds. Gadolinium yttrium garnets, fabricated utilizing gadolinium element, are used as phosphors for color TV tubes. This element is also used for the manufacturing of compact discs and computer memories [1, 2]. In addition, gadolinium based compounds are widely employed as a contrast agent for magnetic resonance imaging [3].
Because of the increasing applications of gadolinium in modern industries, its measurement has become important in recent years. Various methods such as: inductively coupled plasma-mass spectroscopy [4, 5], inductively coupled plasma atomic emission spectroscopy [6], laser-based multi step resonance ionization [7], phosphorescence [8] and spectrofluorimetry [9] have been reported for Gd monitoring. However, these methods are expensive and time-consuming. As a result, design of an inexpensive and simple technique which can provide satisfactory selectivity to determine Gd in the presence of other lanthanides is of great value.
Among different available analytical methods the electrochemical techniques can be referred as a really efficient approach, because of their high sensitivity, low cost, high selectivity and easy automation. However, direct electroanalysis of Gd3+ ions, using sensitive electrochemical methods like differential pulse voltammetry (DPV), is not possible; because, this ion is electrically inactive in aqueous solutions and using the traditional electrode materials. The electrochemical methods, used for Gd3+ analysis, have been limited to some PVC-based membrane ion selective electrodes [10, 11].
Molecularly imprinted polymer (MIP) can selectively recognize a molecule among closely related structural analogues [12–15]. Similar to MIPs, ion imprinted polymer (IIPs) can recognize a target ion among other metallic ions. Numerous IIP materials have been reported for various kinds of metal ions [16–20].
Previously, we developed a new methodology for Eu3+ determination based on Eu3+-imprinted polymer modified carbon paste (CP) electrode [21]. In that work Eu3+ concentration in a solution was estimated by monitoring of the decrease in the DPV signal of an electroactive probe ion as the result of competitive extraction of Eu3+ in the IIP sites, occupied by the probe ions. Following the mentioned methodology and in order to extend the developed technique, in this study, a DPV method was used for Gd3+ determination. Herein, the Gd3+-imprinted polymer was used as the recognition element of carbon paste electrode. In order to overcome the problem of electrochemically inactivity of Gd3+, an electroactive probe ion was utilized to trace the Gd3+ adsorption in the IIP, situated in the electrode surface. This is the first indirect voltammetric determination of Gd3+ via the IIP-based carbon paste electrode. Compared to our previous work, in this approach the detection limit of the method has been lowered significantly by changing the kind of the electroactive probe ion (from copper ion to lead ion).
Experimental
Instrument and reagents
Electrochemical data was obtained using a potentiostat/galvanostat model PGSTAT302, Metrohm. Carbon paste electrodes, modified with IIP or non imprinted polymer (NIP), were used as a working electrode. An Ag/AgCl electrode and a platinum wire were used as the reference and counter electrodes, respectively. Methacrylic acid (Merck, Germany http://www.merckgroup.com/), Vinyl pyridine (VP) and divinyl benzene (DVB) (Sigma-Aldrich, USA http://www.sigmaaldrich.com/united-states.html) were purified by distillation under reduced pressure. 2, 2′-(2- methyl propionitrile) was obtained from Acros Organic, Geel, Belgium (http://www.exportersindia.com/acrosorganics/) and used as an initiator. Gd(NO3)3 and other lanthanides were from Merck, Germany. Other chemicals were of analytical grade and purchased from Merck, Germany.
Preparation of MIP nanoparticles by suspension polymerization in silicon oil
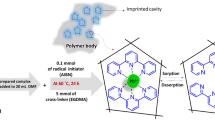
In order to prepare, nano-sized IIP, 0.3 mmol of Gd(NO3)3 (template), 1.8 mmol of vinyl pyridine (functional monomer and complexing ligand), 0.6 mmol of MAA (functional monomer and complexing ligand), 12 mmol of DVB (cross-linker) were dissolved in 5 mL of acetonitrile (porogen). After 1 h, 0.05 g of AIBN (initiator) was added to the mixture. The obtained mixture was transferred to silicon oil (100 mL), purged previously with a stream of nitrogen gas for 15 min. The mixture was then mixed vigorously by a mechanical mixer (1000 rpm for 10 min). This was followed by ultrasonication for 22 min. Before polymerization, a stream of nitrogen gas was blown throughout the solution for 15 min. The polymerization reaction was carried out in a water bath, fixed at 70 °C, for 24 h. The obtained polymeric powder was first washed with petroleum ether and then toluene. After every washing step, high speed centrifugation was applied for the separation of the polymeric particles from the solvents. To extract the non-reacted monomers from the polymer, the particles were further washed with methanol and acetonitrile. Then, Gd3+ ions were removed from the polymer by suspending of the particles of the synthesized polymer in a solution of sodium acetate and EDTA for 2 h. Finally, the polymer was successively rinsed with distillated water and dried in vacuum at 50 °C, overnight. In order to prepare the non-imprinted polymer (NIP), exactly, the above-described method was utilized in the absence of Gd3+.
Preparation of the modified electrodes
In order to construct the IIP- and NIP-modified carbon paste electrodes, (IIP-CP and NIP-CP), 0.05 g of graphite was blended with 0.03 g of nano-sized Gd3+-IIP or the related NIP for 10 min. Then, the graphite/IIP (or graphite/NIP) blend was added to the melted n-eicosane (heated at 45–50 °C) and mixed with a stainless steel spatula. Finally, the paste obtained was used to fill a hole (2.00 mm in diameter, 3 mm in depth) at the end of an electrode body, previously heated at 45 ° C. The surface of the electrode was smoothed with the aid of a smooth paper sheet.
Rebinding experiments
The prepared IIP-modified electrodes were inserted into the solution containing both Gd3+ (and other aimed ions) and Pb2+ (pH = 7), being at stirring state (500 rpm). Then, the electrode was placed in the electrochemical cell, filled with 10 mL of HCl solution (0.10 M). In order to record the electrochemical signal, pre-potential of −1.0 V was applied to the electrode for 20 s to reduce the adsorbed probe ions (Pb2+) and then the differential pulse stripping voltammetry was performed in the potential range of −0.8 to −0.3 V. The obtained signals were compared with the signal of the electrode, immersed in the pure Pb2+solution. The concentration of Pb2+ in both described solutions was the same in all experiments.
Results and discussion
Synthesis of Gd(III)-imprinted polymer and its characterization
In order to synthesize Gd3+-imprinted polymer, MAA and VP were contacted with Gd3+ (mole ratio 2:6:1). MAA and VP acted either as functional monomers or complexing ligands, canceling the need to additional complexing ligand. This strategy not only resulted in a simple IIP synthesis procedure but also cancelled the risk of removal of the ligands during the washing stage; because, the functional monomers, providing the coordination bonding sites in the IIP, were attached to the polymer network as a result of the polymerization reaction. The so-called suspension polymerization in silicon oil method was utilized as the polymerization method in order to obtain nano-sized imprinted polymers. Scanning electron microscopy images of the synthesized polymeric particles are shown in Fig. 1. It is apparent that the nano-sized polymeric particles are obtained by employing the described synthesis method. Size distribution chart, represented in the inset of the image, indicates that the mean size of the IIP particles is about 50 nm. The FT-IR spectra were also used to investigate further the structure of the obtained polymers. The FT-IR spectra of the unleached IIP, leached IIP and NIP are shown and described in details in Electronic Supplementary Material (Fig. S1, ESM).
Selection of probe ion
Gd3+ is a non-electroactive ion among lanthanides and thus direct electroanalysis methods like voltammetry can not be used for its determination. Therefore, an indirect method was chosen for the investigation of the recognition property of the newly developed Gd3+-IIP. For this purpose, a CP electrode was modified with Gd3+-IIP and immersed in a proper electroactive probe ion solution. By this means, the electroactive ion was adsorbed in the selective sites of the IIP, situated in the carbon paste electrode. The differential pulse voltammetry signal of the described electrode was proportional to the amount of the electroactive probe ions, adsorbed in the electrode surface. However, in the presence of Gd3+ ions the electroactive probe ions had to compete with Gd3+ to occupy the selective sites of the IIP. This led to a considerable fall in the population of the electroactive probe ion in the selective sites of the IIP. Such a described effect decreased the voltammetric response of the electrode. The extent of the signal reduction was proportional to both Gd3+ affinity to the sites of the IIP and the number of Gd3+ ions contributed in the competition. Figure 2 illustrates schematically the explained methodology.
The selection of probe ion, however, is very important. The probe ion not only must have a good electroactivity but also have to compete strongly with the target ion for the occupying of the selective site of the IIP. That is to say, the probe ion kind can affect both sensitivity and selectivity of the determination method. Figure 3 represents the results of indirect analysis of Gd3+ ion by using two different electroactive probe ions. As can be seen, in the case of Pb2+ the decrease in electrode signal, as a result of competitive adsorption of Gd3+, is considerably bigger than that observed when using Cu2+ as the probe ion. This can be attributed to the inherent bigger differential pulse voltammetry signal of Pb2+ ions on CP electrode, compared to that of Cu2+. Therefore, removing of Pb2+ ions by replacing of target ions can lead to higher drop in initial signal, compared to exchanging of the same number of Cu2+ with Gd3+ ions. However, the difference in affinities of theses probe ions to the ion-selective sites of the IIP may be a key point in this case.
Comparison of the recognition characteristics of the ion imprinted polymer with non-imprinted polymer
After selection of a proper probe ion for indirect analysis of Gd3+, the effectiveness of the ion-selective sites of the IIP for Gd3+ recognition was further evaluated. In order to reach this aim, the Gd3+ recognition capability of the IIP was compared with that of the NIP particles. In Fig. 4, the voltammograms of (a) and (b) represent the DPV signal obtained for the IIP-CP electrode after 10 min incubation in Pb2+ and Pb2+ plus Gd3+ solutions, respectively. On the other hand, the voltammograms of (c) and (d) are related to the NIP-CP electrode for Pb2+ and Pb2+ plus Gd3+solutions, respectively. It is clear that the IIP-CP electrode creates bigger Pb2+ signal, compared to the NIP-CP electrode, suggesting that the adsorption of Pb2+ in the IIP is noticeably higher than that in the NIP material. This may be assigned to the selective sites of the IIP which do not exist in the NIP material. Furthermore, it can be seen that Pb2+ signal in the case of IIP-CP electrode is decreased considerably in the presence of Gd3+ as the target ions; however, the NIP-CP signal is not affected significantly in the presence of Gd3+. These results indicate that the competition between Gd3+ and Pb2+ is just for occupying the selective sites of the IIP. In other words, there is no noticeable competition between Gd3+ and Pb2+ in order to take the non-selective sites of the IIP; because, the influence of Gd3+ on the Pb2+ signal of the NIP-CP electrode is very smaller than that on the IIP-CP electrode signal. This experiment suggests that the competitive extraction of Gd3+ ion in the IIP-CP, in the presence of Pb2+ ion, can be effectively used for the selective determination of Gd3+ in the aimed solutions.
differential pulse voltammetry responses of the IIP-CP (“a” and “b”) and NIP-CP (“c” and “d”) electrodes immersed in the solution of pure Pb2+ (“a” and “c”) and that containing both Pb2+ and Gd3+(b and d); [Pb2+] = 5 × 10−7 mol. L−1, lanthanides ion concentration = 3 × 10−7 mol. L−1, Extraction condition: extraction time = 15 min, agitation speed:400 rpm; electrochemical analysis: 15 ml HCl solution(0.1 mol. L−1)
The effect of different conditions on the efficiency of the method
Fig. S2a (ESM) represents the dependence of the response of the IIP-CP electrode to the IIP amount in the carbon paste. It is apparent that the electrode response to Pb2+ increases with enhancement in the IIP till a limited amount and then it keeps constant. However, the electrode response to the mixture of Pb2+ and Gd3+ increases continuously as a result of enhancement in the IIP amount. The availability of higher amount of the IIP on the electrode decreases the need to compete between the target and probe ions; since, in such a case, Gd3+ ions can enter in the sites, not occupied by Pb2+ ions, leading to no suitable information from the target ion recognition by the IIP sites. According to this figure, at a certain amount of the IIP (3 mg) a maximum competition is observed between Gd3+ and Pb2+ to occupy the positions of the selective sites. Therefore, in order to obtain meaningful signal as a result of target ion recognition, it is necessary to use the optimum amount of the IIP in the modified electrode composition.
The effect of extraction solution pH on the competitive recognition of Gd3+ by the IIP-CP electrode is shown in Fig. S2b (ESM). It is obvious that adjusting the solution pH at the range of 6–8 leads to the best condition for the recognition of Gd3+ by the IIP; because, at this pH range there is a maximum signal difference between the electrode immersed in the Pb2+ solution and that incubated in the Pb2+ plus Gd3+ solution. Increasing of the acidity of the environment nullify the capability of the functional groups of the IIP sites to link to both Pb2+ and Eu2+ via coordination bonding. This is because of protonation of the nitrogen groups of vinyl pyridine in the selective sites of the IIP as a result of increasing in H+ concentration. On the other hand, the swelling of the IIP particles as well as the interference effect of hydroxide ions on Pb2+ and Eu2+ in alkaline condition may decrease the interaction of both ions with the IIP sites. Therefore, a neutral pH was chosen as an appropriate pH condition for the competitive recognition of Gd3+.
The effect of competitive extraction time on the electrode response was also checked (Fig. S2c, ESM). Herein, the adsorption of the probe ion is initially increased and after a definite time, the adsorption increment rate is decreased strongly. The first adsorption regime is ascribed to the up-taking of Pb2+ ion by the high affinity selective sites and the second is related to the adsorption of probe ions to the non-specific binding sites of the IIP. The adsorption behavior of the IIP in the presence of both target and probe ions, however, differ noticeably from that of the previously described behavior. This indicates that there is a real competition between Gd3+ and Pb2+ for accessing the binding sites of the IIP. It can be seen, that the difference of two curves meets a maximum amount at the extraction time of 10 min. Thus, this extraction time was chosen as the best option for the evaluation of the recognition characteristic of the Gd3+-IIP.
Analytical characterization of the electrode modified with the Gd(III)-imprinted polymer
The interference effects of various species on the target ion analysis by the IIP-CP electrode were examined. The maximum concentrations of foreign species that caused a relative error of 5 % in the analytical signal were accepted as tolerance limit. The obtained results are given in Table 1. According to results obtained, the developed sensor response was not influenced by presence of 100-fold excess of Ca2+, Mg2+ and Zn2+ as well as 50-fold excess of Hg2+, Co2+, Mn2+, Ni2+, Fe3+ and Ag+. Presence of 20-fold excess of Cd2+, Cu2+ and Pt2+ influenced significantly the electrode signal. Among the tested lanthanide ions, the interfering effect of Eu3+ and Sm3+ was the highest and observed at about 7-fold concentration excess. Dy3+ and Er3+ showed interfering effect when their concentrations were 10 times higher than Gd3+ concentration. La3+ and Ce3+ showed no significant effect on Gd3+ response up to 15-foldconcentration excess.
Figure 5(I) shows the successive decreases in the initial voltammetric signal of the probe ion as a result of Gd3+ concentration increment in the solution. The depicted results were used for the plotting of an applicable calibration curve. For this aim, the voltammetric responses of the IIP-CP electrodes, obtained for the solutions of different concentrations of Gd3+ and fixed concentration of Pb2+ (3 × 10−7 mol L−1) were subtracted from the voltametric signal of the IIP-CP electrode, incubated in the pure Pb2+ solution (3 × 10−7 mol L−1). Figure 5(II) represents the obtained calibration curve in which the decrease in voltammetric signal is plotted as a function of Gd3+ concentration in the solution. As can be seen, this electrode exhibits concentration linear range of 6 × 10−9 –4.8 × 10−7 mol L−1. The detection limit was calculated to be equal to 4.5 × 10−9 mol L−1 (S/N). Relative standard error percent of 5 separate determinations by the sensor was found to be 3.71 %.
decreasing of the IIP-CP signal for Pb2+ as function of addition of different concentration of Gd3+ (a= 0.0 b=6.0 × 10−9, c=3.0 × 10−8, d=1.0 × 10−7, e=1.8 × 10−7, f g=2.8 × 10−7, h=3.2 × 10−7,i=3.8 × 10−7, j=4.5 × 10−7) (I) and the plot of the decrease amounts as a function of Gd3+ concentration used as the calibration curve of the method (II); I0: the electrode signal in the pure Pb2+ solution (3 × 10−7 mol. L−1) I: the electrode signal in the solution containing Pb2+ (3 × 10−7 mol. L−1) and different concentration of Gd3+
In Table 1, some analytical characteristics of this method are compared with some previously reported methods. It can be seen that compared to Gd(III) membrane electrodes, spectrofluorimetric technique and optical sensor our developed technique can led to very better detection limit. Among the depicted methods, the inductively coupled plasma-mass spectroscopy method, coupled with solid phase extraction (SPE-ICP-MS) shows relatively better detection limit, in comparison to this method. However, our developed method is cheaper, easier and much rapid, compared to the SPE-ICP-MS method.
The developed method was used to determine Gd3+ in several synthetic and real samples. Since, no Gd(III) was found in the tested real samples, the samples were spiked with the known amounts of Gd(III) solutions and then their Gd3+ contents were determined by both developed technique and reference method. A comparison with an inductively coupled plasma emission spectrometry (ICP–OES), used as reference method, suggested that the developed voltammetric determination method gave reliable results in a wide range of samples (Table 2).
Conclusion
Nano-sized Gd3+-imprinted polymer was prepared and used as a selective recognition element of a carbon paste electrode. An indirect voltammetric method based on competitive extraction of Gd3+ in the IIP-CP electrode, in the presence Pb2+ was introduced for highly selective and sensitive determination of Gd (III) in water samples. The competition of Gd3+ ions against probe ions to occupy the Gd3+-selective sites of the IIP, existing in CP electrode, lead to an effective reduction in the DPV signal of the electrode to the probe ion. Both Cu2+ and Pb2+ were tested as probe ions and the later was found to be the better option; since, it led to better sensitivity in target ion analysis. The IIP amount, used for the CP modification, was a crucial factor for achievement the best condition. This developed electrode showed high selectivity to Gd3+ determination, even in the presence of other lanthanide ions. The sensor was capable of determination of Gd3+ in spiked water samples.
References
Kirk OR, Othmer FD (1982) Encyclopedia of chemical technology. Wiley, New York, pp 840–851
Xinmin Z, Hao W, Heping Z, Qiang S (2007) Luminescent properties of SrGa″2S″4: Eu2+ and its application in green-LEDs. J Rare Earths 25:701–705
Collidge TA, Thompson PC, Mark PB, Traynor JP, Jardine AG, Morris S, Simpson TWM (2007) Gadolinium-enhanced MR imaging and nephrogenic systemic fibrosis: retrospective study of a renal replacement therapy cohort. Radiology 245:168–175
Salonia JA, Gasquez JA, Martinez LD, Cerutti S, Kaplan M, Olsina RA (2006) Inductively coupled plasma optical emission spectrometric determination of gadolinium in urine using flow injection on-line sorption preconcentration in a knotted reactor. Instrum Sci Technol 34:305–316
Isnard H, Brennetot R, Caussignac C, Caussignac N, Chartier F (2005) Investigations for determination of Gd and Sm isotopic compositions in spent nuclear fuels samples by MC ICP-MS. Int J Mass Spectrom 246:66–73
Normann PT, Joff P, Martinsen I, Thomsen HS (2000) Quantification of gadodiamide as Gd in serum, peritoneal dialysate and faeces by inductively coupled plasma atomic emission spectroscopy and comparative analysis by high-performance liquid chromatography. J Pharm Biomed Anal 22:939–947
Blaum K, Geppert C, Schreiber W, Hengstler J, Muller P, Nortershauser W, Wendt K, Bushaw B (2002) Trace determination of gadolinium in biomedical samples by diode-laser-basedmulti-step resonance ionization mass spectrometry. Anal Bioanal Chem 372:759–765
Gong Z, Zhang Z (1997) Room temperature phosphorescence optosensing for gadolinium. Mikrochim Acta 126:117–121
Berg B, Mainka E, Ache HJ (1989) Determination of gadolinium in aquous solutions by time-resolved fluorimetry. Anal Bioanal Chem 333:766–767
Ganjali MR, Rezapour M, Norouzi P, Salavati-Niasari M (2005) A new pentadentate S-N schiffs base as a novel ionophore construction of a novel Gd(III) membrane sensor. Electroanalysis 17:2032–2036
Ganjali MR, Norouzi P, Alizadeh T, Tajarodi A, Hanifehpour Y (2007) Fabrication of a highly selective and sensitive Gd(III)-PVC membrane sensor based on N-(2-pyridyl)-N-(4- nitrophenyl)thiourea. Sens Actuators B 120:487–493
Pardieu E, Cheap H, Vedrine C, Lazerges M, Lattach Y, Garnier F, Remita S, Pernelle C (2009) Molecularly imprinted conducting polymer based electrochemical sensor for detection of atrazine. Anal Chim Acta 649:236–245
Alizadeh T (2010) Preparation of molecularly imprinted polymer containing selective cavities for urea molecule and its application for urea extraction. Anal Chim Acta 669:94–101
Yoshimatsu K, Reimhult K, Krozer A, Mosbach K, Sode K, Ye L (2007) Uniform molecularly imprinted microspheres and nanoparticles prepared by precipitation polymerization: the control of particle size suitable for different analytical applications. Anal Chim Acta 584:112–121
Zhang T, Liu F, Chen W, Wang J, Li K (2001) Influence of intramolecular hydrogen bond of templates on molecular recognition of molecularly imprinted polymers. Anal Chim Acta 450:53–61
Alizadeh T, Ganjali MR, Zare M (2011) Application of an Hg2+ selective imprinted polymer as a new modifying agent for the preparation of a novel highly selective and sensitive electrochemical sensor for the determination of ultratrace mercury ions. Anal Chim Acta 689:52–59
Kala R, Biju VM, Rao TP (2005) Synthesis, characterization, and analytical applications of erbium(III) ion imprinted polymer particles prepared via γ-irradiation with different functional and crosslinking monomers. Anal Chim Acta 549:51–58
Zhao J, Han B, Zhang Y, Wang D (2007) Synthesis of Zn(II) ion-imprinted solid-phase extraction material and its analytical application. Anal Chim Acta 603:87–92
Liu Y, Chang X, Yang D, Guo Y, Meng S (2005) Highly selective determination of inorganic mercury(II) after preconcentration with Hg(II)-imprinted diazoaminobenzene–vinylpyridine copolymers. Anal Chim Acta 538:85–91
Metilda P, Prasad K, Kala R, Gladis JM, Prasada RT, Naidu GRK (2007) Ion imprinted polymer based sensor for monitoring toxic uranium in environmental samples. Anal Chim Acta 582:147–153P
Alizadeh T, Amjadi S (2013) Synthesis of nano-sized Eu3+ imprinted polymer and its application for indirect voltammetric determination of europium. Talanta 106:431–439
Hennebruder K, Wennrich R, Mattusch J, Stark HJ, Engewald W (2004) Determination of gadolinium in river water by SPE preconcentration and ICP-MS. Talanta 63:309–316
Bautista RD, Jimenez AI, Jimenez F, Arias JJ (1996) Simultaneous spectrofluorimetric determination of europium, dysprosium, gadolinium and terbium using chemometric methods. Talanta 43:421–429
Zamani HA, Rajabzadeh G, Ganjali MR, Norouzi P (2007) Determination of gadolinium(III) ions in soil and sediment samples by a novel gadolinium membrane sensor based on 6-methyl-4-{[1-(2-thienyl)methylidene]amino}3-thioxo-3,4-dihydro-1,2,4-triazin-5-(2H)-one. Anal Chim Acta 598:51–57
Gupta VK, Singha AK, Kumawat LK (2013) A novel gadolinium ion-selective membrane electrode based on 2-(4-phenyl-1, 3-thiazol-2-yliminomethyl) phenol. Electrochim Acta 95:132–138
Zare-Dorabei R, Norouzi P, Ganjali MR (2009) Design of a novel optical sensor for determination of trace gadolinium. J Hazard Mater 171:601–605
Acknowledgments
The Authors wish to acknowledge the vice-presidency of research, University of Mohaghegh Ardabili, for financial support of this work.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
ESM 1
(PDF 329 kb)
Rights and permissions
About this article
Cite this article
Alizadeh, T., Ganjali, M.R. & Alizadeh, H. Competitive extraction of Gd(III) into a carbon paste electrode impregnated with a nano-sized Gd(III)-imprinted polymer as a new method for its indirect voltammetric determination. Microchim Acta 182, 1205–1212 (2015). https://doi.org/10.1007/s00604-014-1419-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00604-014-1419-y