Abstract
Background
In addition to the direct power of anticancer drugs, the effectiveness of anticancer therapy depends on the host immune function. The present study investigated whether or not the reduction rate and histological response of preoperative chemotherapy were related to the immune microenvironment surrounding a primary tumor of the rectum.
Methods
Sixty-five patients received preoperative chemotherapy followed by resection from 2012 to 2014; all of these patients were retrospectively analyzed. CD3, CD8, and FoxP3 were immunohistochemically examined as markers for T lymphocytes, cytotoxic T lymphocytes, and regulatory T lymphocytes (Treg), respectively. The correlation between the tumor-infiltrating lymphocyte composition and the tumor reduction rate and histological response to neoadjuvant chemotherapy was investigated.
Results
The average tumor reduction rate was 41.5% ± 18.8%. According to RECIST, 47 patients (72.3%) achieved a partial response (PR), and 1 patient (1.5%) achieved a complete response (CR). Eight patients (12.3%) showed a grade 2 histological response, and 2 (3.1%) showed a grade 3 response. A multivariate analysis demonstrated that a low Treg infiltration in stromal cell areas was significantly associated with the achievement of a PR or CR [odds ratio (OR) 7.69; 95% confidence interval (CI) 1.96–33.33; p < 0.01] and a histological grade 2 or 3 response (OR 11.11; 95% CI 1.37–98.04; p = 0.02).
Conclusion
A low Treg infiltration in the stromal cell areas may be a marker of a good response to neoadjuvant chemotherapy in patients with locally advanced rectal cancer.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Background
In the treatment of locally advanced rectal cancer (LARC), neoadjuvant chemoradiotherapy (NACRT) has been used to shrink tumors, increase the frequency of radical resection, and reduce local recurrence [1,2,3]. However, radiotherapy (RT) has a harmful effect on the anorectal function and the patient’s quality of life after surgery. Several randomized trials have demonstrated that the prevalence of bowel dysfunction in patients who underwent low anterior resection with RT was almost twice as high as that in patients after surgery alone [4,5,6,7,8]. Furthermore, RT may cause urinary and sexual dysfunction. Thus, neoadjuvant chemotherapy (NACT) was introduced as a new treatment to avoid this functional deterioration.
Tumor-infiltrating lymphocytes (TILs) are considered to represent the primary host immune response to tumors and are associated with long-term outcomes in several cancers [9,10,11,12]. Regarding colorectal cancer (CRC), a meta-analysis by Mei et al. revealed that high generalized tumor inflammatory infiltration was associated with a good overall survival [13]. They also concluded that a high degree of cytotoxic T lymphocyte (CD8+ T lymphocyte; CTL) but not T lymphocyte (CD3+ T lymphocyte) or regulatory T lymphocyte (Foxp3+ T lymphocyte; Treg) infiltration indicated an improved overall survival. Therefore, TILs and the infiltration of some T lymphocyte subsets are important factors associated with the prognosis of CRC.
Tregs express the transcription factor Foxp3 and suppress the activation of T cells. High Treg infiltration is reported to be associated with a poor prognosis in several cancers [14, 15]. In contrast, in CRC, high Treg infiltration was reported to be associated with a good prognosis in a previous large-scale cohort study [16]. One possibility for this paradox is that Tregs may limit the tumorigenic effects of inflammation mediated by Th17 cells. Alternatively, Tregs in the tumor area may merely be indirect indicators of the antitumor immune response, with tumor-specific CTLs being activated and expanding, thereby resulting in an increase in Tregs.
Several mechanisms are now well known to enhance antitumor immunity with both chemotherapy and RT [17,18,19,20,21,22,23]. Similar to other chemotherapy agents, treatment with 5-fluorouracil (5-FU) causes tumor cells to release HMGB1, a major hallmark of immunogenic cell death [24], and 5-FU may preferentially lead to the depletion of Tregs over other T lymphocyte subsets [25]. In breast cancer, a complete pathological response is associated with the disappearance of Tregs after NACRT [26]. In rectal cancer, a low density of Foxp3+ Tregs in post-treatment resected specimens has been reported to be associated with a good treatment response after CRT, whereas pretreatment Tregs have not been reported to be associated with the treatment response [27]. Although there are several reports concerning the involvement of Tregs in the local tumor microenvironment response to CRT for LARC, the association between Tregs and the local tumor microenvironment response during chemotherapy remains unclear.
The present study investigated whether or not the tumor reduction rate in patients who underwent NACT for LARC was associated with the condition of the immune microenvironment surrounding the primary tumor, especially post-treatment Tregs.
Methods
Patients
All 65 patients (males, n = 47; females, n = 18) who underwent preoperative chemotherapy followed by resection of a primary lesion at Yokohama City University Hospital and Yokohama City University Medical Center between January 2012 and December 2014 were enrolled in the present study (Table 1). The median age was 63 (range 27–78) years old. The indication of neoadjuvant chemotherapy was age < 80 years old, rectal cancer, cT3/T4, and lymph node involvement.
This study was conducted with the approval of the institutional review board at the Yokohama City University Graduate School of Medicine.
Preoperative chemotherapy
The preoperative chemotherapy regimen consisted of 6 courses of modified FOLFOX6 [oxaliplatin (85 mg/m2), levofolinate calcium (200 mg/2), 5-fluorouracil (400 mg/m2) on day 1, followed by 2,400 mg/m2 continuous infusion over 46 h every 2 weeks] with or without panitumumab. In cases with wild-type RAS, panitumumab [Pmab (6 mg/kg on day 1 every 2 weeks)] was administered. The median dose of oxaliplatin was 100% (range 50–100%) among the 65 patients. The median interval between preoperative chemotherapy and surgery was 27.4 days.
To evaluate the effect of preoperative chemotherapy, the reduction rate calculated using the maximum dimension of the primary lesion measured on computed tomography (CT) or magnetic resonance imaging (MRI) before and after chemotherapy and the histological response of the surgical specimen were used. The reduction rate was defined, according to response evaluation criteria in solid tumors (RECIST 1.1) as a complete response (CR), partial response (PR), stable disease (SD), or progressive disease (PD) [28]. The histological response was defined according to the Japanese classification of colorectal carcinoma: Grade 0 (no effect), no evidence of effect; Grade 1a (very slight effect), viable tumor cells occupying > 2/3 of the tumor area; Grade 1b (slight effect), viable tumor cells remain in more than 1/3 but less than 2/3 of the tumor area; Grade 2 (considerable effect) viable tumor cells remain in less than 1/3 of the tumor area; and Grade 3 (CR) no viable tumor cells remain. The pathological stage was defined according to the TNM classification, as follows: Stage 0, 2 (3%); Stage I, 13 (20%); Stage II, 20 (31%); Stage III, 22 (34%); Stage IV, 8 (12%). Downstaging was achieved in 41 patients (63.1%). Further details on these patients are provided in Table 1.
Immunohistochemistry
Surgical specimens were immunohistochemically analyzed to detect CD3, CD8, Foxp3, CD86, and CD206. The antibodies and cellular expression are listed in Table 2. We performed immunostaining as previously reported [29, 30]. We prepared 4-μm-thick tissue sections from each specimen. These sections were deparaffinized in xylene and rehydrated through a graded series of alcohol washes. We blocked the endogenous peroxidase activity of the specimens by immersing the slides in absolute methanol solution containing 0.3% hydrogen peroxide for 30 min at room temperature. We carried out antigen retrieval using autoclave pretreatment in citrate buffer solution. After washing in phosphate-buffered saline, specimens were saturated and then incubated with the primary antibody. We visualized immunohistochemical reactions with a DAB kit (Dako, Carpinteria, CA, USA). Finally, the sections were counterstained with hematoxylin and examined microscopically. For a negative control, we omitted the primary antibody but followed all other steps.
Immunohistochemical evaluations
Cytoplasmic staining for each marker was regarded as positive immunoreactivity; however, non-nucleated small fragments were not counted. The numbers of TILs in the cancer cell (CC) areas and stromal cell (ST) areas and macrophages in peritumoral tissues were counted manually. After selecting three different fields, we determined the average counts for each patient. CC areas were counted in the densest field using a × 40 objective lens. The fields within 1000 μm of CC nests at the advancing edge of a tumor in surgically resected specimens with positively stained ST areas were counted using a × 40 objective lens. A representative area (× 40 objective lens, 0.22 mm2) from tumoral tissue or adjacent normal glandular tissue was selected for the quantitative analysis of macrophage infiltration.
Two investigators (SK and KI), who were blinded to the patients’ clinical information, counted the numbers of each type of immune cell under the instructions of the pathologist. In cases of discrepancy, a consensus was achieved using a multi-head microscope.
Statistical analyses
The χ2 test or Fisher’s exact probability test was used to compare the proportions of categorical variables. The Mann–Whitney U test was used to compare continuous variables. Optimal cut-off points for cell numbers were selected using the median cell numbers. Univariate and multivariate Cox proportional hazards models were used to explore the association of the TIL expression and the pathological response. Following the univariate analysis, variables with p values of < 0.05 were selected for the multivariate analysis by logistic regression. We generated survival curves using the Kaplan–Meier method and determined statistically significant differences between two groups by employing the log-rank test. The relapse-free survival (RFS) was defined as the time from curative surgery to recurrence or to the date of death or loss to follow-up. p values of < 0.05 were considered to indicate statistical significance.
We performed statistical analyses using the Dr. SPSS II software package (SPSS Inc., Chicago, IL, USA).
Results
Tumor reduction rate
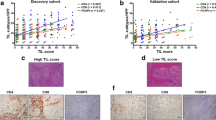
The average tumor reduction rate was 41.5 ± 18.8%. According to RECIST, a PR was achieved in 47 patients (72.3%) and a CR was achieved in 1 patient (1.5%). The relationships between the tumor reduction rate and TIL are shown in Fig. 1. Infiltration of CC areas by CD3, CD8, and FoxP3+ lymphocytes was not correlated with tumor reduction. Furthermore, the infiltration of M1 (CD86) and M2 (CD206) macrophages was not associated with tumor reduction. In contrast, the higher infiltration of CD8+ lymphocytes (r = 0.272, p = 0.028) and lower infiltration of FoxP3+ lymphocytes in ST areas (r = − 0.265, p = 0.033) was correlated with the tumor reduction rate. RAS mutations were identified in 49.2% (n = 32) of patients. We observed no significant association between the RAS mutational status and the number of TILs, including Tregs.
Correlation between lymphocyte or macrophage subsets and tumor reduction rate. The high infiltration of CD8 + lymphocyte in ST areas was correlated with the tumor reduction rate (r = 0.272, p = 0.03) (d). The low infiltration of FoxP3 + lymphocyte in ST areas was correlated with the tumor reduction rate (r = − 0.265, p = 0.03) (f). CD3 as a marker for T cells; CD8 as a marker for cytotoxic T cells; Foxp3 as a marker for regulatory T cells; CD86 as a marker for M1 macrophages; CD206 as a marker for M2 macrophages; CC, cancer cell; ST, stromal cell
The results of a univariate analysis of the correlation of lymphocyte and macrophage numbers with the response to chemotherapy are shown in Table 3. Patients were classified into high and low groups according to the median values. The tumor reduction rate was analyzed using the χ2 test to determine the ratio of patients with a PR or CR. The prevalence of a low number of Foxp3 + lymphocytes in ST areas was significantly increased in the patients with a PR or CR compared with that of a high number of Foxp3 + lymphocytes. The histological response was analyzed using the χ2 test to determine the ratio of patients with grade 2 and 3 histology. The group with a low number of Foxp 3 + lymphocytes in ST areas showed a significantly high rate of patients with grade 2 and 3 histology.
Univariate and multivariate analyses of factors associated with a PR or CR
The results of the univariate and multivariate analyses of factors related to a PR or CR (according to RECIST) are shown in Table 4. Excluding treatment-related factors, eight non-immunologic factors and eight immunologic factors were included in the univariate analysis. Three factors were significantly associated with the achievement of a PR or CR: pretreatment CEA ≥ 5.0 μg/ml, a low Foxp3 + lymphocyte infiltration in ST areas, and a low CD206 + macrophage infiltration. Factors with a p value of < 0.05 in the univariate analysis were included in a multivariate analysis using stepwise selection. Low Foxp3 + lymphocyte infiltration in ST areas proved to be significantly associated with the achievement of a PR or CR [odds ratio (OR) 7.69; 95% confidence interval (CI) 1.96–33.33; p < 0.01].
Univariate and multivariate analyses of factors associated with grade 2 or 3 histology
The results of univariate and multivariate analyses of factors associated with grade 2 or 3 histology are shown in Table 4. Two factors were significantly associated with grade 2 or 3 histology: a low Foxp3 + lymphocyte infiltration in CC and in ST areas. The factors with a p value of < 0.05 in the univariate analysis were included in a multivariate analysis using stepwise selection. A low Foxp3 + lymphocyte infiltration in ST areas was significantly associated with grade 2 or 3 histology (OR 11.11; 95% CI 1.37–98.04; p = 0.02).
Correlation between the normalized CEA after chemotherapy and a good response to chemotherapy
Of the 65 patients, 33 (50.8%) had elevated CEA levels before chemotherapy. The CEA level normalized in 20 patients after chemotherapy. Patients with normalized CEA levels after treatment were more likely to achieve a PR or CR and grade 2 or 3 histology than patients with non-normalized CEA levels. However, there were no significant differences in the Foxp3 + lymphocyte infiltration in ST areas between the normalized and non-normalized CEA groups.
Correlation between Foxp3 + lymphocytes in ST areas and recurrence
The median follow-up time for the RFS was 51.8 months. During the follow-up period, 18 patients developed recurrent disease. The Kaplan–Meier method was used to estimate the RFS. The RFS curve for Foxp3 + lymphocytes in ST areas is shown in Fig. 2. The analysis of Foxp3 + staining in ST areas demonstrated no significant difference in the RFS between patients with low and high Foxp3 + lymphocyte counts (p = 0.93). The 3-year RFS rates among patients with high and low Foxp3 + lymphocyte counts were 75.0% and 74.2%, respectively.
Discussion
We showed that the tumor microenvironment, especially Foxp3 + lymphocytes in ST areas, was associated with the tumor reduction rate and the histological response of rectal cancer to preoperative chemotherapy. Our data suggest that Tregs in ST areas may inhibit the response to NACT and represent a therapeutic target in rectal cancer.
Whether or not the infiltration of Foxp3 + TILs is associated with the response to NACRT remains unclear, with conflicting findings reported. Indeed, a low Foxp3 + TIL infiltration after NACRT has been reported to be associated with a complete response in some reports [31], whereas other studies have reported that the density of Foxp3 + TILs before and after preoperative treatment was not associated with the response to NACRT [27, 32]. Whether or not the infiltration of Foxp3 + TILs is associated with the response to NACT also remains unclear. The density of Foxp3 + TILs after NACT was reported to be positively associated with downstaging of the primary lesion, although the density before and after preoperative treatment was not associated with the pathological response to NACT [33]. In one pilot study, Foxp3 + cells were increased in two patients who showed a poor response to NACT, whereas Foxp3 + cells were greatly decreased in patients who had a complete response [34]. Consistent with previous results, Tregs in ST areas had a significant relationship with the tumor reduction rate in patients who underwent NACT for LARC. This result suggests that Tregs have a negative effect on the response of rectal cancer to chemotherapy.
Tregs undergo rapid turnover relative to other T lymphocyte subsets and are selectively depleted by several chemotherapy drugs, including 5-FU [25, 35, 36]. During neoadjuvant therapy, Tregs may be selectively depleted in patients who respond to treatment, which provides an opportunity to mount an effective antitumor immune response. Alternatively, the presence of fewer pretreatment Tregs in the tumor environment may enable more effective antitumor immunity-induced neoadjuvant therapy. Although we did not investigate pretreatment Tregs in this study, McCoy et al. reported that there was no strong relationship between pretreatment Tregs and the treatment response, indicating the specific importance of the presence of Tregs after treatment [27]. Further studies will be needed to investigate the relationship between pretreatment Tregs and the response to chemotherapy.
In the present study, a high density of CTLs in ST areas was correlated with the tumor reduction rate in the univariate analysis but not with a pathological response. In previous studies, a low infiltration of CTLs in both biopsy samples obtained before treatment and resected specimens obtained after treatment was significantly associated with a poor pathological response in patients who underwent NACT for LARC [33]. Necrotic tumor cell death caused by both chemotherapy and radiotherapy has been reported to induce the release of tumor antigens and promote the recruitment of immune cells to the tumor by mediators, such as interferon-γ, which is a proinflammatory cytokine [37]. Thus, both chemotherapy and RT reportedly promote antitumor immunity. In addition, CTL infiltration reportedly does not increase after NACT but remarkably increases after NACRT. Thus, RT is considered to activate the antitumor immunity more strongly than chemotherapy. Because NACT is a relatively new strategy compared with NACRT, further studies may be needed.
We observed no significant association between the RAS mutational status and the distribution of TILs, including Tregs. Although several studies and meta-analyses concerning the prognostic significance of KRAS mutations have been reported, the results remain controversial, with only some of them indicating an impact of mutant KRAS on the clinical outcome [38, 39]. Furthermore, the association between the KRAS mutation status and the densities of TILs, including Tregs, has not yet been evaluated. Although whether or not KRAS is involved in the induction of regulatory T cells has not been determined, Tregs are reportedly required for KRAS-mediated lung tumorigenesis [40]. Another study reported that tumor cells expressing mutated KRAS generated suppressive Tregs and that silencing KRAS significantly reduced this ability [41]. However, we observed no significant association between the RAS mutational status and the distribution of Tregs in the present study.
In our study, although low Foxp3 + lymphocyte infiltration in ST areas was associated with the tumor reduction rate and the histological response to preoperative chemotherapy, there was no significant difference in the RFS between patients with low and high Foxp3 + lymphocyte counts. A pathological CR (pCR) after CRT for rectal cancer was reported to predict a good prognosis [42]. In the present study, almost all patients with a good histological response had a grade 2 histological response, and only 2 patients (3.1%) had a grade 3 histological response, which is equivalent to a pCR. Because the pCR rate was low in patients with low Foxp3 + lymphocyte counts, low Foxp3 + lymphocyte infiltration might not have contributed to the improved prognosis. Although NACT has been reported to be associated with lower pCR rates than NACRT [43], NACT may be expected to shrink tumors, as was observed in this study. Tumor shrinkage can help improve R0 resection rates and reduce the risk of local recurrence. Further studies will be needed to investigate the relationship between Foxp3 + lymphocytes after preoperative chemotherapy and the prognosis.
Several limitations associated with the present study warrant mention. First, as we were using standard immunohistochemistry, our staining method was unable to differentiate Tregs from other Foxp3-expressing cell subsets. Second, the present study was retrospective in nature and included fewer than 100 patients. Third, the issue concerning the heterogeneity of TILs in the tumor was not resolved. To reduce the effect of this heterogeneity, we assessed the average number of TILs in three different areas. Fourth, we did not investigate the microsatellite instability (MSI) status in this study. Unlike microsatellite stable (MSS) cancers, MSI-positive cancers are characterized by the presence of an inflammatory reaction in the form of TILs [44]. The frequency of MSI in stage IV colorectal cancer was reported to be about 1.9–3.7% in Japan [45] and about 4–8% in Western countries [46]. As the frequency of MSI was not shown to be high in Japan, the MSI status was unlikely to have differed in the present study from that in previous studies. Finally, whether or not the density of TILs at the surface of the tumor reflects not only the antitumor immune status of the whole tumor but also the density of TILs in the ST and CC areas is unclear. We previously reported that a low infiltration of Tregs in peritumoral areas but not in intratumoral areas was a significantly negative prognostic predictor in patients undergoing resection for colorectal cancer liver metastases [30]. Therefore, we speculated that Tregs in the ST areas were more influential than those in the CC areas.
In conclusion, we showed that a low stromal Foxp3 + Treg infiltration after NACT was associated with the tumor reduction rate in LARC. Our data suggest that Tregs may inhibit the response to NACT and may represent a therapeutic target in rectal cancer.
References
Bosset JF, Collette L, Calais G, et al. Chemotherapy with preoperative radiotherapy in rectal cancer. N Engl J Med. 2006;355:1114–23.
Gerard JP, Conroy T, Bonnetain F, et al. Preoperative radiotherapy with or without concurrent fluorouracil and leucovorin in T3–4 rectal cancer: results of FFCD 9203. J Clin Oncol. 2006;24:4620–5.
Sauer R, Liersch T, Merkel S, et al. Preoperative versus postoperative chemoradiotherapy for locally advanced rectal cancer: results of the German CAO/ARO/AIO-94 randomized phase III trial after a median follow-up of 11 years. J Clin Oncol. 2012;30:1926–33.
Peeters KC, van de Velde CJ, Leer JW, et al. Late side effects of short-course preoperative radiotherapy combined with total mesorectal excision for rectal cancer: increased bowel dysfunction in irradiated patients—a Dutch colorectal cancer group study. J Clin Oncol. 2005;23:6199–206.
Pollack J, Holm T, Cedermark B, et al. Long-term effect of preoperative radiation therapy on anorectal function. Dis Colon Rectum. 2006;49:345–52.
Birginsson H, Pahlman L, Gunnarsson U, et al. Adverse effects of preoperative radiation therapy for rectal cancer: long-term follow-up of the Swedish Rectal Cancer Trial. J Clin Oncol. 2005;23:8697–705.
Marijnen CA, van de Velde CJ, Putter H, et al. Impact of short-term preoperative radiotherapy on health-related quality of life and sexual functioning in primary rectal cancer: report of a multicenter randomized trial. J Clin Oncol. 2005;23:1847–58.
Stephens RJ, Thompson LC, Quirke P, et al. Impact of short-course preoperative radiotherapy for rectal cancer on patients’ quality of life: date from the Medical Research Council CR07 National Cancer Institute of Canada Clinical Trials Group C016 randomized clinical trial. J Clin Oncol. 2010;28:4233–9.
Cho Y, Miyamoto M, Kato K, et al. CD4+ and CD8+ T cells cooperate to improve prognosis of patients with esophageal squamous cell carcinoma. Cancer Res. 2003;63:1555–9.
Hiraoka K, Miyamoto M, Cho Y, et al. Concurrent infiltration by CD8+ T cells and CD4+ T cells is favourable prognostic factor in non-small-cell lung carcinoma. Br J Cancer. 2006;94:275–80.
Loi S, Sirtaine N, Piette F, et al. Prognostic and predictive value of tumor-infiltrating lymphocytes in a phase III randomized adjuvant breast cancer trial in node-positive breast cancer comparing the addition of docetaxel to doxorubicin with doxorubicin-based chemotherapy: BIG 02–98. J Clin Oncol. 2013;31:860–7.
Zhang L, Conejo-Garcia JR, Katsaris D, et al. Intratumoral T cells, recurrence, and survival in epithelial ovarian cancer. N Engl J Med. 2003;348:203–13.
Mei Z, Liu Y, Liu C, et al. Tumour-infiltrating inflammation and prognosis in colorectal cancer: systematic review and meta-analysis. Br J Cancer. 2014;110:1595–605.
Curiel TJ, Coukos G, Zou L, et al. Specific recruitment of regulatory T cells in ovarian carcinoma fosters immune privilege and predicts reduced survival. Nat Med. 2004;10:942–9.
deLeeuw RJ, Kost SE, Kakal JA, et al. The prognostic value of FoxP3+ tumor-infiltrating lymphocytes in cancer: a critical review of the literature. Clin Cancer Res. 2012;18:3022–9.
Salama P, Phillips M, Grieu F, et al. Tumor-infiltrating FOXP3+ T regulatory cells show strong prognostic significance in colorectal cancer. J Clin Oncol. 2009;27:186–92.
Nowak AK, Lake RA, Marzo AL, et al. Induction of tumor cell apoptosis in vivo increases tumor antigen cross-presentation, cross-priming rather than cross-tolerizing host tumor-specific CD8 T cells. J Immunol. 2003;170:4905–13.
Ghiringhelli F, Menard C, Puig PE, et al. Metronomic cyclophosphamide regimen selectively depletes CD4+CD25+ regulatory T cells and restores T and NK effector functions in end stage cancer patients. Cancer Immunol Immunother. 2007;56:641–8.
Zhang B, Bowerman NA, Salama JK, et al. Induced sensitization of tumor stroma leads to eradication of established cancer by T cells. J Exp Med. 2007;204:49–55.
Ramakrishman R, Assudani D, Nagaraj S, et al. Chemotherapy enhances tumor cell susceptibility to CTL-mediated killing during cancer immunotherapy in mice. J Clin invest. 2010;120:1111–24.
Burnette BC, Liang H, Lee Y, et al. The efficacy of radiotherapy relies upon induction of type I interferon-dependent innate and adaptive immunity. Cancer Res. 2011;71:2488–96.
Gupta A, Probst HC, Vuong V, et al. Radiotherapy promotes tumor-specific effector CD8+ T cells via dendritic cell activation. J Immunol. 2012;189:558–66.
Kim S, Ramakrishman R, Lavilla-Alonso S, et al. Radiation-induced autophagy potentiates immunotherapy of cancer via up-regulation of mannose 6-phosphate receptor on tumor cells in mice. Cancer Immunol Immunother. 2014;63:1009–21.
Frey B, Stache C, Rubner Y, et al. Combined treatment of human colorectal tumor cell lines with chemotherapeutic agents and ionizing irradiation can in vitro induce tumor cell death forms with immunogenic potential. J Immunotoxicol. 2012;9:301–13.
Maeda K, Hazama S, Tokuno K, et al. Impact of chemotherapy for colorectal cancer on regulatory T-cells and tumor immunity. Anticancer Res. 2011;31:4569–74.
Ladoire S, Arnould L, Apetoh L, et al. Pathological complete response to neoadjuvant chemotherapy of breast carcinoma is associated with the disappearance of tumor-infiltrating foxp3+ regulatory T cells. Clin Cancer Res. 2008;14:2413–20.
McCoy MJ, Hemmings C, Anyaegbu CC, et al. Tumour-infiltrating regulatory T cell density before neoadjuvant chemoradiotherapy for rectal cancer does not predict treatment response. Oncotarget. 2017;8:19803–13.
Eisenhauer EA, Therasse P, Bogaerts J, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer. 2009;45(2):228–47.
Homma Y, Taniguchi K, Murakami T, et al. Immunological impact of neoadjuvant chemoradiotherapy in patients with borderline resectable pancreatic ductal adenocarcinoma. Ann Surg Oncol. 2014;21(2):670–6.
Nakagawa K, Tanaka K, Homma Y, et al. Low Infiltration of peritumoral regulatory T cell predicts worse outcome following resection of colorectal liver metastases. Ann Surg Oncol. 2015;22(1):180–6.
McCoy MJ, Hemmings C, Miller TJ, et al. Low stromal Foxp3+ regulatory T-cell density is associated with complete response to neoadjuvant chemoradiotherapy in rectal cancer. Br J Cancer. 2015;113:1677–86.
Teng F, Meng X, Kong L, et al. Tumor-infiltrating lymphocytes, forkhead box P3, programmed death ligand-1, and cytotoxic T lymphocyte-associated antigen-4 expressions before and after neoadjuvant chemoradiation in rectal cancer. Transl Res. 2015;166:721–32.
Matsutani S, Shibutani M, Maeda K, et al. Significance of tumor-infiltrating lymphocytes before and after neoadjuvant therapy for rectal cancer. Cancer Sci. 2018;109:966–79.
Roxburgh CS, Shia J, Vakiani E, Daniel T, Weiser MR. Potential immune priming of the tumor microenvironment with FOLFOX chemotherapy in locally advanced rectal cancer. Oncoimminology. 2018;22:e1435227.
Rettig L, Seidenberg S, Parvanova I, et al. Gemcitabine depletes regulatory T-cells in human and mice and enhances triggering of vaccine-specific cytotoxic T-cells. Int J Cancer. 2011;129:832–8.
van der Most RG, Currie AJ, Mahendran S, et al. Tumor eradication after cyclophosphamide depends on concurrent depletion of regulatory T cells: a role for cycling TNFR2-expressing effector-suppressor T cells in limiting effective chemotherapy. Cancer Immunol Immunother. 2009;58:1219–28.
Frey B, Rubner Y, Kulzer L, et al. Antitumor immune responses induced by ionizing irradiation and further immune stimulation. Cancer Immunol Immunother. 2014;63:29–36.
Rizzo S, Bronte G, Fanale D, et al. Prognostic vs. predictive molecular biomarkers in colorectal cancer: is KRAS and BRAF wild type status required for anti-EGFR therapy? Cancer Treat Rev. 2010;36(3):S56-61.
Vakiani E, Solit DB. KRAS and BRAF: drug targets and predictive biomarkers. J Pathol. 2011;223:219–29.
Granville CA, Memmott RM, Balogh A, et al. A central role for Foxp+ regulatory T cell in K-Ras-driven lung tumorigenesis. PLoS ONE. 2009;4:5061.
Zdanov S, Mandapathil M, Abu Eid R, et al. Mutant KRAS conversion of conventional T cells into regulatory T cells. Cancer Immunol Res. 2016;4:354–65.
Rödel C, Thomas P, Papadoupolos T, et al. Prognostic significance of tumor regression after preoperative chemoradiotherapy for rectal cancer. J Clin Oncol. 2005;23:8688–96.
Sclafani F, Cunningham D. Neoadjuvant chemotherapy without radiotherapy for locally advanced rectal cancer. Fut Oncol. 2014;10:2243–57.
Michael-Robinson JM, Biemer-Hüttmann A, Purdie DM, et al. Tumour infiltrating lymphocytes and apoptosis are independent features in colorectal cancer stratified according to microsatellite instability status. Gut. 2001;48:360–6.
Yoshino T, Arnold D, Taniguchi H, et al. Pan-Asian adapted ESMO consensus guidelines for management of patients with metastatic colorectal cancer: a JSMO-ESMO initiative endorsed by CSCO, KACO, MOS, SSO and TOS. Ann oncol. 2018;29:44–70.
Van Cutsem E, Cervantes A, Adam R, et al. ESMO consensus guidelines for the management of patients with metastatic colorectal cancer. Ann Oncol. 2016;27:1386–422.
Acknowledgements
We would like to give many thanks to H. Sakurada for performing skillful laboratory work.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflicts of interest for this article.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Sekizawa, K., Nakagawa, K., Ichikawa, Y. et al. Relationship between stromal regulatory T cells and the response to neoadjuvant chemotherapy for locally advanced rectal cancer. Surg Today 52, 198–206 (2022). https://doi.org/10.1007/s00595-021-02311-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00595-021-02311-8