Abstract
Objective
We sought to develop a nomogram for the prediction of tumor recurrence after resection of hepatocellular carcinoma (HCC) within the Milan criteria.
Method
Consecutive HCC patients admitted for hepatectomy between 1994 and 2014 were enrolled in this study. Patients were excluded if they had recurrent HCC or tumors beyond the Milan criteria. Patients were randomized and assigned to the derivation and validation sets in a 1:1 ratio. Independent factors for disease-free survival were identified using the Cox regression model. A nomogram was derived and validated with the receiver-operating characteristic (ROC) and calibration curves.
Results
There were 617 eligible patients included in the analysis. The median age was 59 years, 481 were male, and 87.8% of the patients were hepatitis B virus carriers. The median follow-up was 68.7 months. The 5-year overall survival rate was 73.3% and HCC recurrence was detected in 55% of the patients. In the derivation set, a nomogram was constructed based on the seven independent factors for disease-free survival: age, alpha-fetoprotein, preoperative prothrombin time, magnitude of hepatectomy, postoperative complication, number of tumor nodules, and presence of microvascular invasion. A satisfactory discrimination ability was observed in both the derivation and validation sets (c-stat 0.672 and 0.665, respectively). The calibration plot yielded agreement between the predicted and observed outcomes, using the derived nomogram.
Conclusion
A validated nomogram quantifies the risk of recurrence after hepatectomy for HCC within the Milan criteria, and assists with the planning of individual postoperative surveillance protocols.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Hepatocellular carcinoma (HCC) is a prevalent and lethal malignancy, accounting for about one million deaths worldwide each year [1]. Only 20–37% of HCCs are amendable to hepatectomy [2, 3] despite improved HCC screening protocols in the predisposed populations [4]. Guidelines recommend hepatectomy as the first-line surgical treatment for early HCC [5, 6]. For the fortunate minority with resectable disease, a 5-year overall survival rate of 60–70% is commonly achievable; however, the HCC recurrence rate is equally high, at about 50% 5 years after surgery [7, 8]. Vigorous biochemical and radiological surveillance in terms of contrasted computed tomography (CT) is essential to detect early recurrence so that repeat resection or salvage liver transplantation may be possible. However, “over-screening” of low-risk patients not only dissipates limited medical resources, but unnecessary exposure to radiation poses potential carcinogenic risks [9]. To streamline the surveillance protocol, there is a need to stratify the risks of recurrence in post-hepatectomy HCC patients.
Only a few scoring systems have been proposed for the prediction of post-hepatectomy HCC recurrence, most of which are not validated and lack discriminatory power [10,11,12]. To our knowledge, this is the first study to derive a validated prognostic nomogram for disease-free survival after curative resection of HCC within the Milan criteria, which in turn can serve as a reference for the individualization of post-resection surveillance protocols.
Method
Patient selection
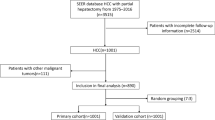
Data on consecutive patients admitted to our center for treatment of HCC between 1994 and 2014 were extracted from a prospectively maintained database. All adult patients (aged 18 years or older) with a pathological diagnosis of HCC within the Milan criteria were recruited. Patients with one or more of the following conditions were excluded: uncertain diagnosis, positive margin status, mixed cholangiohepatocellular carcinoma, recurrent HCC after curative treatment (tumor ablation, resection or liver transplantation), preoperative portal vein embolization, or Associating Liver Partition and Portal vein Ligation for Staged hepatectomy (ALPPS). We reviewed the medical records of all the eligible patients. Demographic characteristics, preoperative investigational details, intraoperative data and postoperative outcomes were extracted for analysis.
Preoperative assessment
HCC was diagnosed when two contrasted cross-sectional imaging results showed an arterially enhancing lesion, 1 cm or larger in diameter, with portal venous washout. One set of typical imaging was sufficient for patients with cirrhosis. An elevated alpha-fetoprotein (AFP) level was not a pre-requisite for HCC diagnosis. Antiviral treatment (Entecavir) was given to patients with hepatitis B virus, once a working diagnosis of HCC was made. In the preoperative assessment, complete blood count, liver renal function and clotting profile were checked, to establish the baseline hepatic function. All patients scheduled to undergo hepatectomy had an indocyanine green (ICG) clearance test [4, 13] done with pulse spectrophotometry. Adequate liver function was defined as ICG retention of less than 20% [14, 15]. Volumetric assessment was performed before major hepatectomy (resection of more than three Couinaud segments). For patients with a non-cirrhotic liver, hepatectomy was contraindicated if the future liver remnant was less than 30% (by the HKU formula) of the estimated standard liver volume [2, 16].
Surgical technique and postoperative surveillance
Our surgical techniques were described previously [17]. Briefly, most major hepatectomies were performed via a right subcostal incision with a midline sternal extension. Other incisions, such as bilateral subcostal incisions, a Mercedes incision, or a midline incision were used sometimes, depending on the location and size of the tumor. Intraoperative ultrasonography was performed for topographic screening of the tumor and mapping of the transection line. After hilar dissection and individual ligation of the ipsilateral hepatic artery and portal vein, transection was carried out using a Cavitron ultrasonic surgical aspirator (CUSA) along the line of demarcation. Whether the anterior or conventional approach was used was at the operating surgeon’s discretion [18]. Transection was concluded by division of the hepatic vein and bile duct using a stapling device. The cystic duct was cannulated and bile leak was checked by a methylene blue injection.
After discharge, the patients were followed up every 3 months in the first 2 years and 6 monthly thereafter. The AFP level was checked before every follow-up visit. Contrasted imaging was done 3 months after surgery and then 6 monthly thereafter. Disease recurrence was defined by radiological evidence of intrahepatic or extrahepatic tumors.
Statistical methods
This was a retrospective analysis of prospectively collected data on consecutive patients who underwent hepatectomy for HCC at our center. The study period was from 1994 to 2014 and patients with recurrent HCC or HCC beyond the Milan criteria [19] were excluded. Eligible patients were allocated randomly to the derivation and validation groups in a 1:1 ratio [20]. In the derivation group, univariate analysis was performed and factors with a P value of less than 0.1 were selected for multivariate analysis. The Cox regression model using the forward condition method was applied. Independent factors associated with HCC recurrence were hence identified. P values of 0.05 or below were considered significant. A predictive nomogram was constructed using the R software, version 3.4.1. Discrimination of the derived nomogram was assessed by the receiver-operating characteristic curve with respect to the presence of HCC recurrence upon follow-up. A calibration plot was used to assess the discrepancy between the nomogram-predicted probability and the genuine recurrence rate. Nomogram discrimination and calibration were repeated using validation set data. An area under the curve value of over 0.6 was considered satisfactory prediction. Disease-free survival of the patients was analyzed using the Kaplan–Meier curve. Statistical calculation was performed using SPSS 24.0.
Results
Characteristics of the whole study population
A total of 617 patients were recruited. The median age was 59 years and most (78%) were male. Hepatitis B surface antigen was detected in 87.8% of the patients and comorbidity (defined as any chronic medical illness) was present in 46.4%. The median AFP level was 38. Most patients had relatively preserved liver function, as reflected by a low Child score, ICG retention rate at 15 min, and albumin–bilirubin index. The median tumor size, number of tumor nodules, and resection margin width were 3 cm, 1 cm, and 1 cm, respectively. Major resection was required in 28.4% of the patients and the median operation time and blood loss were 299 min and 515 ml, respectively. On pathological examination, 33.1% of the patients had microvascular permeation and 16.5% had poor tumor cell differentiation. The median follow-up duration was 68.8 months and HCC recurrence was detected in 55% of the patients.
All patients were randomized and allocated to the derivation and validation groups in a 1:1 ratio by computer software. Apart from the clinically insignificant differences in white cell count and ALT, there were no significant differences between the groups. Table 1 summarizes the clinical characteristics of the patients.
Nomogram derivation and validation
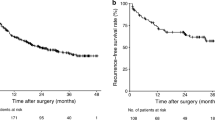
There were 291 patients allocated to the derivation group, and 11 factors were found to be associated with disease-free survival. On multivariate analysis, seven independent factors were identified, namely age, prothrombin time, AFP, magnitude of hepatectomy, postoperative complication, number of tumor nodules, and the presence of lymphovascular invasion (Table 2). With these independent factors, a prognostic nomogram for the prediction of 2- and 5-year disease-free survival was derived (Fig. 1a, b). This nomogram demonstrated good discrimination ability in the derivation and validation sets (c-stat 0.672 and 0.665, respectively) (Fig. 2a, b). Calibration plot of the nomogram suggested agreement between the predicted and observed disease-free survival probability (Fig. 3a, b).
Discussion
Through this retrospective study of over 600 patients with early HCC, a nomogram composed of seven independent factors was derived, validated, and shown to be accurate in the prediction of disease-free survival by discrimination and calibration tests.
HCC is known to be associated with a high recurrence rate, even after curative resection. Recurrence develops within 2 years of resection in most patients. Repeat resection, repeated ablation or even salvage transplantation are often possible for early recurrence. This underscores the importance of a reliable recurrence predictive model which can help to individualize the surveillance strategy for patients after curative hepatectomy. Through improvements in health consciousness and availability of effective screening tools, many HCCs are being detected at a relatively early stage. In general, patients with HCC within the Milan criteria are considered to have earlier stage disease than those with large HCC, multifocal HCC, or HCC with major vascular invasion. A significant difference in tumor biology between early and advanced disease is expected from accumulation of tumor mutation in the course of disease progression. To minimize the confounding effect of analyzing early and advanced HCC together, this study recruited patients with HCC within the Milan criteria and derived a nomogram suitable for this patient subgroup only.
An elevated AFP level is a known factor for HCC recurrence after hepatectomy. Zhou et al. performed a multivariate analysis of a series of 247 HCC patients after curative resection and found that an AFP level > 400 ng/ml, vascular invasion, multiple tumors, and postoperative complications were the independent factors for early HCC recurrence [21]. Similar findings were reported by other studies [22,23,24,25] as well as ours. Our study also identified major hepatectomy as an independent factor for disease-free survival. Since HCC tends to invade the portal vein, resulting in intrahepatic spread, theoretically major hepatectomy should be a protective factor as the tumor-bearing sectors are removed together with the portal pedicle. The oncological impact of major/anatomical resection has been described in retrospective series [26, 27] and a meta-analysis [28]. In our study, advanced age was also found to have a detrimental effect on disease-free survival, although this is not unequivocal in the literature [29,30,31,32]. Associated comorbidities and limited tolerance of physiological stress would contribute to poorer survival of the aged population.
Few validated predictive models for HCC recurrence are documented in the literature. A novel inflammation-based score (IBS) was derived and validated by Fu et al., using the data of more than 1000 patients; however, the concordance index of their nomogram in predicting recurrence-free survival in the validation set was 0.621, suggesting a moderate discriminatory power only [33]. Another nomogram incorporating an inflammatory index derived by Shen et al. demonstrated a high concordance index (c-index over 0.7), but the small patient number (159 patients) in the validation set led to potential bias. A nomogram of 2-year disease-free survival, including patient sex, log of calculated tumor volume, albumin level, platelet count, and microvascular invasion was proposed and validated with a good concordance index (i.e. 0.66) by the Asan group, using data from a large number of patients. However, the high AFP level and large tumor size in their study population might limit the application of their findings to patients with early HCC [34]. More recently, a clinical risk score (CRS) was derived [35] and validated [36], composed of only three parameters: disease status beyond the Milan criteria, multiple tumor nodules, and microvascular invasion. However, other potential predictive factors such as AFP were not analyzed and the results cannot be applied to patients with HCC within the Milan criteria [36].
This study had some limitations. First, its retrospective nature is associated with unavoidable biases. Second, the patients were recruited over a long period of time, so substantial changes in perioperative care and operative technique could have influenced the surgical and oncological outcomes. Third, inflammatory indices such as neutrophil/lymphocyte ratio and other markers associated with HCC recurrence, such as PIVKA-II, were not analyzed. Fourth, operative and pathological data were required for the calculation of disease-free survival, negating their use in the preoperative setting. Nevertheless, the current study demonstrated the derivation and validation of a nomogram for the calculation of disease-free survival in a specific group of HCC patients (within the Milan Criteria) using a reasonably large patient population. This nomogram should act as a reference in formulating surveillance strategies for patients with early HCC.
Conclusion
A validated nomogram will help to predict the post-hepatectomy disease-free survival of patients with HCC within the Milan criteria and an individualized surveillance protocol can be formulated accordingly.
Abbreviations
- ABLI:
-
Albumin–bilirubin index
- AFP:
-
Alpha-fetal protein
- AFP_Ln:
-
Natural log of AFP
- ALT:
-
Alanine transferase
- ALPPS:
-
Associating liver partition with portal vein ligation for staged hepatectomy
- AST:
-
Aspartate transaminase
- AUC:
-
Area under curve
- CTP:
-
Child–Turcotte–Pugh
- CRS:
-
Clinical risk score
- CUSA:
-
Cavitron ultrasonic surgical aspirator
- ESLV:
-
Estimated standard liver volume
- HCC:
-
Hepatocellular carcinoma
- HKU:
-
The University of Hong Kong
- IBS:
-
Inflammation-based score
- ICG:
-
Indocyanine green
- IOUS:
-
Intraoperative ultrasonography
- INR:
-
International normalization ratio
- LVI:
-
Lymphovascular invasion
- PIVKA-II:
-
Protein-induced vitamin K antagonist II
- ROC:
-
Receiver-operating characteristic
- TACE:
-
Trans-arterial chemoembolization
References
El-Serag HB. Hepatocellular carcinoma: an epidemiologic view. J Clin Gastroenterol. 2002;35(5):72-S8.
Fan ST, Lo CM, Liu CL, Lam CM, Yuen WK, Yeung C, et al. Hepatectomy for hepatocellular carcinoma: toward zero hospital deaths. Ann Surg. 1999;229(3):322–30.
Fong Y, Sun RL, Jarnagin W, Blumgart LH. An analysis of 412 cases of hepatocellular carcinoma at a Western center. Ann Surg. 1999;229(6):790–9 (discussion 9-800).
Ma KW, Cheung TT. Surgical resection of localized hepatocellular carcinoma: patient selection and special consideration. J Hepatocell Carcinoma. 2017;4:1–9.
Llovet JM, Ducreux M, Lencioni R, Di Bisceglie AM, Galle PR, Dufour JF, Greten TF, Raymond E, Roskams T, De Baere T, Ducreux M, Mazzaferro V, Bernardi M. EASL-EORTC clinical practice guidelines: management of hepatocellular carcinoma. J Hepatol. 2012;56(4):908–43.
Yau T, Tang VY, Yao T-J, Fan S-T, Lo C-M, Poon RT. Development of Hong Kong liver cancer staging system with treatment stratification for patients with hepatocellular carcinoma. Gastroenterology. 2014;146(7):1691–700.e3.
Poon RT, Fan ST, Lo CM, Liu CL, Wong J. Long-term survival and pattern of recurrence after resection of small hepatocellular carcinoma in patients with preserved liver function: implications for a strategy of salvage transplantation. Ann Surg. 2002;235(3):373–82.
Bhangui P, Allard MA, Vibert E, Cherqui D, Pelletier G, Cunha AS, et al. Salvage versus primary liver transplantation for early hepatocellular carcinoma: do both strategies yield similar outcomes? Ann Surg. 2016;264(1):155–63.
Furlow B. Radiation dose in computed tomography. Radiol Technol. 2010;81(5):437–50.
Umeda Y, Matsuda H, Sadamori H, Matsukawa H, Yagi T, Fujiwara T. A prognostic model and treatment strategy for intrahepatic recurrence of hepatocellular carcinoma after curative resection. World J Surg. 2011;35(1):170–7.
Li Y, Ruan DY, Yi HM, Wang GY, Yang Y, Jiang N. A three-factor preoperative scoring model predicts risk of recurrence after liver resection or transplantation in hepatocellular carcinoma patients with preserved liver function. Hepatobiliary Pancreat Dis Int. 2015;14(5):477–84.
Nakagawa S, Hayashi H, Nitta H, Okabe H, Sakamoto K, Higashi T, et al. Scoring system based on tumor markers and Child-Pugh classification for HCC patients who underwent liver resection. Anticancer Res. 2015;35(4):2157–63.
Lam CM, Fan ST, Lo CM, Wong J. Major hepatectomy for hepatocellular carcinoma in patients with an unsatisfactory indocyanine green clearance test. Br J Surg. 1999;86(8):1012–7.
Poon RT, Fan ST, Lo CM, Liu CL, Lam CM, Yuen WK, et al. Improving perioperative outcome expands the role of hepatectomy in management of benign and malignant hepatobiliary diseases: analysis of 1222 consecutive patients from a prospective database. Ann Surg. 2004;240(4):698–708 (discussion—10).
Cheung TT, Chan SC, Chok KS, Chan AC, Yu WC, Poon RT, et al. Rapid measurement of indocyanine green retention by pulse spectrophotometry: a validation study in 70 patients with Child-Pugh A cirrhosis before hepatectomy for hepatocellular carcinoma. Hepatobiliary Pancreat Dis Int. 2012;11(3):267–71.
Chan SC, Liu CL, Lo CM, Lam BK, Lee EW, Wong Y, et al. Estimating liver weight of adults by body weight and gender. World J Gastroenterol. 2006;12(14):2217–22.
Fan ST, Mau Lo C, Poon RT, Yeung C, Leung Liu C, Yuen WK, et al. Continuous improvement of survival outcomes of resection of hepatocellular carcinoma: a 20-year experience. Ann Surg. 2011;253(4):745–58.
Liu CL, Fan ST, Cheung ST, Lo CM, Ng IO, Wong J. Anterior approach versus conventional approach right hepatic resection for large hepatocellular carcinoma: a prospective randomized controlled study. Ann Surg. 2006;244(2):194–203.
Mazzaferro V, Regalia E, Doci R, Andreola S, Pulvirenti A, Bozzetti F, et al. Liver transplantation for the treatment of small hepatocellular carcinomas in patients with cirrhosis. N Engl J Med. 1996;334(11):693–700.
Steyerberg EW, Harrell FE Jr, Borsboom GJ, Eijkemans MJ, Vergouwe Y, Habbema JD. Internal validation of predictive models: efficiency of some procedures for logistic regression analysis. J Clin Epidemiol. 2001;54(8):774–81.
Zhou YM, Zhang XF, Li B, Sui CJ, Yang JM. Postoperative complications affect early recurrence of hepatocellular carcinoma after curative resection. BMC Cancer. 2015;15:689.
Shah SA, Cleary SP, Wei AC, Yang I, Taylor BR, Hemming AW, et al. Recurrence after liver resection for hepatocellular carcinoma: risk factors, treatment, and outcomes. Surgery. 2007;141(3):330–9.
Cha C, Fong Y, Jarnagin WR, Blumgart LH, DeMatteo RP. Predictors and patterns of recurrence after resection of hepatocellular carcinoma. J Am Coll Surg. 2003;197(5):753–8.
Cheng Z, Yang P, Qu S, Zhou J, Yang J, Yang X, et al. Risk factors and management for early and late intrahepatic recurrence of solitary hepatocellular carcinoma after curative resection. HPB. 2015;17(5):422–7.
Li T, Fan J, Qin LX, Zhou J, Sun HC, Qiu SJ, et al. Risk factors, prognosis, and management of early and late intrahepatic recurrence after resection of primary clear cell carcinoma of the liver. Ann Surg Oncol. 2011;18(7):1955–63.
Wong TCL, Cheung TT, Chok KSH, Chan ACY, Dai WC, Chan SC, et al. Treatment strategy to improve long-term survival for hepatocellular carcinoma smaller than 5 cm: major hepatectomy vs minor hepatectomy. World J Surg. 2014;38(9):2386–94.
Zhao H, Chen C, Gu S, Yan X, Jia W, Mao L, et al. Anatomical versus non-anatomical resection for solitary hepatocellular carcinoma without macroscopic vascular invasion: a propensity score matching analysis. J Gastroenterol Hepatol. 2017;32(4):870–8.
Zhou Y, Xu D, Wu L, Li B. Meta-analysis of anatomic resection versus nonanatomic resection for hepatocellular carcinoma. Langenbeck’s Arch Surg. 2011;396(7):1109–17.
Motoyama H, Kobayashi A, Yokoyama T, Shimizu A, Sakai H, Furusawa N, et al. Impact of advanced age on the short-and long-term outcomes in patients undergoing hepatectomy for hepatocellular carcinoma: a single-center analysis over a 20-year period. Am J Surg. 2015;209(4):733–41.
Kaibori M, Yoshii K, Yokota I, Hasegawa K, Nagashima F, Kubo S, et al. Impact of advanced age on survival in patients undergoing resection of hepatocellular carcinoma: report of a Japanese Nationwide Survey. Ann Surg. 2017. https://doi.org/10.1097/SLA.0000000000002526.
Mirici-Cappa F, Gramenzi A, Santi V, Zambruni A, Di Micoli A, Frigerio M, et al. Treatments for hepatocellular carcinoma in elderly patients are as effective as in younger patients: a 20-year multicentre experience. Gut. 2010;59(3):387–96.
Liu P-H, Hsu C-Y, Lee Y-H, Hsia C-Y, Huang Y-H, Su C-W, et al. Uncompromised treatment efficacy in elderly patients with hepatocellular carcinoma: a propensity score analysis. Medicine. 2014;93(28).
Fu YP, Ni XC, Yi Y, Cai XY, He HW, Wang JX, et al. A novel and validated inflammation-based score (IBS) predicts survival in patients with hepatocellular carcinoma following curative surgical resection: A STROBE-compliant article. Medicine. 2016;95(7):e2784.
Shim JH, Jun MJ, Han S, Lee YJ, Lee SG, Kim KM, et al. Prognostic nomograms for prediction of recurrence and survival after curative liver resection for hepatocellular carcinoma. Ann Surg. 2015;261(5):939–46.
Lee SY, Konstantinidis IT, Eaton AA, Gonen M, Kingham TP, D’Angelica MI, et al. Predicting recurrence patterns after resection of hepatocellular cancer. HPB. 2014;16(10):943–53.
Zheng J, Chou JF, Gonen M, Vachharajani N, Chapman WC, Majella Doyle MB, et al. Prediction of hepatocellular carcinoma recurrence beyond milan criteria after resection: validation of a clinical risk score in an international cohort. Ann Surg. 2017;266(4):693–701.
Acknowledgements
We thank Mr. Yuen Ho Kam, Kim Bsc (Comp&stat) for data management and analysis.
Funding
This study received no support in the form of equipment, drugs, grants or funding.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
We have no conflicts of interest to declare in relation to this study.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Ma, K.W., She, W.H., Cheung, T.T. et al. Validated nomogram for the prediction of disease-free survival after hepatectomy for hepatocellular carcinoma within the Milan criteria: individualizing a surveillance strategy. Surg Today 49, 521–528 (2019). https://doi.org/10.1007/s00595-019-1764-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00595-019-1764-x