Abstract
Purpose
The neutrophil–lymphocyte ratio (NLR) is a biochemical marker of the systemic inflammatory response and has been associated with prognosis for various types of cancer. This retrospective study investigates the relationship between the pre- and postoperative NLR and the prognosis of gastric cancer patients.
Methods
The subjects were 280 patients who underwent curative surgery for histopathologically diagnosed gastric adenocarcinoma.
Results
The preoperative NLR was significantly correlated with tumor size, tumor depth, lymphatic invasion, venous invasion, and disease stage. In contrast, there was no correlation between the postoperative NLR and the various clinicopathological variables. Prognosis was significantly worse for patients with a high preoperative NLR than for those with a low preoperative NLR. Prognosis was also significantly worse for patients with a high postoperative NLR than for those with a low postoperative NLR. Furthermore, the prognosis was worse for gastric cancer patients whose pre- and postoperative NLRs were both high. Multivariate analysis indicated that a high pre- and postoperative NLR was an independent prognostic indicator.
Conclusions
The combination of pre- and postoperative NLRs appears to be useful for predicting the prognosis of gastric cancer patients.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
In 2008, there were an estimated 989,600 new cases of gastric cancer worldwide and 738,000 deaths, accounting for 8 and 10% of total cancer cases and cancer deaths, respectively [1]. As such, establishing postoperative prognostic factors for gastric cancer patients is important. Several studies have indicated that depth of invasion and lymph node metastasis are the most important prognostic factors in gastric cancer [2, 3]. Recent advances in histochemical and molecular biological techniques have identified different prognostic factors [4,5,6,7,8,9]; however, they are complicated and unsuitable for the routine clinical setting. Moreover, long-term studies are needed to address whether these markers can be used in treatment strategies. In contrast, serum markers are easy to measure and useful for diagnosis, predicting survival rates, and monitoring recurrence following surgery [10, 11].
Systemic inflammatory response plays an important role in cancer development and progression. Markers of systemic inflammation have been demonstrated as independent prognostic factors for survival in several malignancies including colon, lung, pancreatic, hepatobiliary, and gastric cancer [12,13,14,15,16,17,18,19,20]. The neutrophil–lymphocyte ratio (NLR) is a commonly used marker that has been reported as a prognostic factor [18,19,20]. While most studies have focused on the preoperative NLR, the postoperative NLR may be representative of systemic inflammatory response after tumor removal. To date, there have been no reports on the relationship between the postoperative NLR and the prognosis of gastric cancer patients.
Some gastric cancer patients suffer disease recurrence after R0 resection because of micrometastasis. Since systemic inflammatory response plays an important role in cancer progression, a high postoperative NLR may support the development of micrometastasis in gastric cancer patients and contribute to disease recurrence following curative gastrectomy. Therefore, the aim of this study was to establish the prognostic significance of pre- and postoperative NLRs in patients with gastric cancer.
Materials and methods
Patients
This study enrolled 280 patients with a histopathological diagnosis of gastric adenocarcinoma, who underwent curative surgery at Tottori University Hospital between 2001 and 2013. Data were collected retrospectively. Clinicopathological findings were established according to the 14th edition of the Japanese Classification of Gastric Carcinoma [21]. All patients underwent distal partial, proximal partial, or total gastrectomy with regional lymph node dissection. The postoperative NLR was measured 1 month after surgery and the Clavien–Dindo (CD) system was used to classify each patient’s postoperative complications [22, 23].
Among the 280 patients included in the current study, 62 received adjuvant chemotherapy, but none received preoperative chemotherapy. Nine patients were treated with uracil–tegafur (UFT, TAIHO Co, Japan) and 53 were treated with S-1 (TAIHO Co, Japan). These patients received 200–400 mg of UFT, 2–3× daily orally, or 80 mg/m2/day oral S-1. These regimens were administered for 6–12 months postoperatively in principle. Patients were checked periodically for early recurrence by diagnostic imaging, including chest X-ray, double-contrast barium meal study, upper gastrointestinal fiberscopy, ultrasonography, and computed tomography. Causes of death and patterns of recurrence were established by reviewing medical records, including laboratory data, ultrasonography, computed tomography, scintigrams, peritoneal punctures, and laparotomies, or by direct inquiry with family members. In some cases, postmortems were undertaken to confirm the cause of death. Institutional review board approval was obtained, and the informed consent requirement was waived for this study.
Statistical analysis
For statistical analyses, Chi square and Fisher’s exact probability tests were used to compare the distribution of individual variables between patient groups. Differences between the two groups were evaluated using the Mann–Whitney U test. Survival curves were calculated according to the Kaplan–Meier method. Differences between survival curves were examined with the log-rank test. We used multivariate analysis of factors considered prognostic of overall survival (OS), with Cox’s proportional hazards model and a stepwise procedure. P < 0.05 was considered significant. GraphPad Prism (GraphPad Software, Inc., La Jolla, CA, USA) and Stat View (Abacus Concepts, Inc., Berkeley, CA, USA) software were used for statistical analyses.
Results
The mean pre- and postoperative NLRs were 2.32 (range 0.36–8.7) and 1.99 (range 0.46–12.3), respectively. There was a significant correlation between the pre- and postoperative NLRs (r = 0.38; P < 0.0001) (Fig. 1). Table 1 shows the correlations between the pre- and postoperative NLRs and clinicopathological variables in the gastric cancer patients. There were significant correlations between a high preoperative NLR and tumor size (P = 0.0006), depth of invasion (P = 0.0003), lymphatic vessel invasion (P = 0.0184), venous invasion (P = 0.0181), and stage of disease (P = 0.0119). In contrast, there was no correlation between the postoperative NLR and clinicopathological variables in patients with gastric cancer (Table 1). In the current study, 62 patients underwent adjuvant chemotherapy. The postoperative NLR was 1.7 ± 0.82 and 2.1 ± 1.4 in patients who received vs. those who did not receive adjuvant chemotherapy, respectively. This difference was not significant (P = 0.11). Of the 280 patients, 177 and 103 underwent open and laparoscopic surgery, respectively. The postoperative NLR was 2.0 ± 1.4 vs. 2.0 ± 1.0 in patients who underwent open surgery vs. those who underwent laparoscopic surgery, respectively. This difference was not significant (P = 0.13). Furthermore, 66 patients (23.6%) suffered Clavien–Dindo grade II or higher complications (Table 2). The postoperative NLR was 1.6 ± 0.79 vs. 2.1 ± 1.4 in patents without vs. those with Clavien–Dindo grade II or higher complications, respectively, and this difference was significant (P < 0.0001). The postoperative NLR was 2.2 ± 1.4 vs. 2.2 ± 1.2 in patients with infectious complications vs. those with non-infectious complications, respectively. This difference was not significant (P = 0.68).
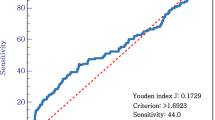
Next, we investigated the prognostic significance of the pre- and postoperative NLRs in patients with gastric cancer. Receiver operating characteristic (ROC) analysis showed the optimal cutoff values of the pre- and postoperative NLRs to be 2.7 (area under the curve (AUC) 0.57, P = 0.12) and 1.8 (AUC 0.58, P = 0.08), respectively (Fig. 2). Based on these results, patients were divided as follows: preNLR ≥2.7 (preNLRHigh, n = 84), preNLR <2.7 (preNLRLow, n = 196), postNLR ≥1.8 (postNLRHigh, n = 114), and postNLR <1.8 (postNLRLow, n = 166). The 5-year survival rates were significantly related to the preNLR (preNLRLow, 83.0%; preNLRHigh, 63.2%; P = 0.03; Fig. 3a) and the postNLR (postNLRLow, 82.7%; postNLRHigh, 68.5%; P = 0.0018; Fig. 3b).
a Survival curves according to the preoperative NLR. The 5-year survival rate was significantly worse for patients with preNLRHigh than for those with preNLRLow (63.2 vs. 83.0%, P = 0.03). b Survival curves according to the postoperative NLR. The 5-year survival rate was significantly worse for patients with postNLRHigh than for those with postNLRLow (82.7 vs. 68.5%, P = 0.0018)
Among the 280 patients in the current study, 51 were both preNLRHigh and postNLRHigh; 96 were either preNLRHigh or postNLRHigh; and 133 were both preNLRLow and postNLRLow. The patients with both preNLRLow and postNLRLow, those with either preNLRHigh or postNLRHigh, and those with preNLRHigh and postNLRHigh were assigned 0, 1, and 2, respectively. An ROC analysis indicated that the AUC was 0.61, which was higher than that for the individual use of either the preNLR or the postNLR (Fig. 4). The 5-year survival rates of patients with both preNLRHigh and postNLRHigh, those with either preNLRHigh or postNLRHigh, and those with both preNLRLow and postNLRLow were 58.1, 75.1, and 92.8%, respectively. The prognosis of the preNLRHigh/postNLRHigh group was significantly worse than that of those who were either preNLRHigh or postNLRHigh, or preNLRLow/preNLRLow (Fig. 5). Finally, multivariate analysis indicated that the combination of pre- and postoperative NLRs was an independent prognostic indicator (Table 3).
Survival curves according to the combination of pre and postoperative NLRs. The 5-year survival rates were 58.1, 75.1, and 85.7% of patients with both preNLRHigh and postNLRHigh, either preNLRHigh or postNLRHigh, and both preNLRLow and postNLRLow, respectively, and the differences were significant (P = 0.0014)
Regarding the correlation between the cause of death and the NLR, recurrence was found significantly more often in patients with both preNLRHigh and postNLRHigh and in patients with either preNLRHigh or postNLRHigh, than in patients with both preNLRLow and postNLRLow (Fig. 4). The other disease was more frequent in patients with both preNLRHigh and postNLRHigh than in those with either preNLRHigh or postNLRHigh or those with both preNLRLow and postNLRLow, but the differences were not significant (Fig. 6).
Discussion
The outcome of patients with cancer is largely determined not only by tumor-related factors, but also by patient-related factors, including inflammation, malnutrition, and immune status [12,13,14,15,16,17,18,19,20, 24, 25]. In the current study, we demonstrated that preNLRHigh was significantly associated with the poor prognosis of gastric cancer patients. This finding is consistent with that of a previous report [19]. Although previous studies have highlighted preoperative NLR, the dynamics of NLR after treatment can better reflect the balance between pro-tumor inflammatory status and anti-tumor immune status [26, 27]. Therefore, we hypothesized that post-treatment NLR may also be a significant prognostic factor predictive of the survival outcome for gastric cancer patients. Our results demonstrated that the prognosis of patients with postNLRHigh was significantly worse than that of those with postNLRLow, indicating that postoperative NLR, as well as preoperative NLR, is useful for predicting the prognosis of gastric cancer patients. In the current study, 51 patients with preNLRHigh and 63 patients with preNLRLow were postNLRHigh, even 1 month after R0 surgery. Although it is unclear why a high NLR was observed in those patients, three mechanisms have been proposed. One is the effect of residual tumor cells, which secrete various pro-inflammatory cytokines, and negative immune modulators, which trigger relative neutrophilia and lymphocytopenia. Another possible mechanism is the effect of postoperative complications. In fact, the postoperative NLR was significantly higher in patients with postoperative complications than in those without postoperative complications in the current study. The NLR increases not only neutrophilia induced by inflammation, but also lymphopenia induced by malnutrition, which is caused by anastomotic stenosis, lymphorrhea, and bleeding, as observed in the current study. Therefore, there was no significant difference in the postoperative NLR between patients with infectious complications and those with non-infectious complications. Recent studies have demonstrated that postoperative complications are closely associated with poor prognosis in gastric cancer patients [28, 29]. It is possible that prolonged inflammation and malnutrition caused by postoperative complications impair cell-mediated immunity, which results in a poor prognosis for gastric cancer patients. Therefore, the poor prognosis of patients with postNLRHigh in the current study might be due in part to their postoperative complications. The other possible mechanism is surgical stress, which was found to induce neutrophilia and lymphocytopenia, resulting in a high NLR. However, this is unlikely because we demonstrated previously that both neutrophilia and lymphocytopenia induced by surgical stress recover within 1 month after surgery [30]. On the other hand, 62 patients received postoperative adjuvant chemotherapy in the current study. As chemotherapy induces neutropenia, it is possible that postoperative adjuvant chemotherapy is associated with postoperative NLR. In this regard, there was no significant difference in the postoperative NLR between patients who received and those who did not receive postoperative adjuvant chemotherapy. The postoperative NLR was measured 1 month after surgery in this study. However, as postoperative adjuvant chemotherapy was initiated 4–6 weeks after surgery in principle, the effect of postoperative adjuvant chemotherapy on postoperative NLR was probably limited.
The precise mechanisms underlying the association between an increased NLR and the adverse outcomes of cancer patients remain unclear. Ock et al. reported recently that the NLR is mainly associated with osteopontin and interleukin-6 (IL-6) in gastric cancer patients. Osteopontin and IL-6 are well-known chemotactic factors for neutrophils [31, 32]. High levels of osteopontin and IL-6 have been reported to be associated with a poor prognosis for most tumor types, including gastric cancer [33,34,35]. Osteopontin can modulate extracellular remodeling to promote epithelial–mesenchymal transition and angiogenesis [36, 37]. IL-6 can activate the signal transducer and activator of the transcription signaling pathway, which induces cancer progression [32, 35]. A high NLR also reflects a decreased lymphocyte count, as well as an increased neutrophil count. Because lymphocytes can act as a T cell-mediated immune surveillance system, decreased circulating lymphocytes indicate significant impairment of the immune defense against cancer [38]. Findings show that elevated lymphocyte counts are significantly associated with a good prognosis and that the recovery of lymphocytopenia improves the survival outcomes of patients with various malignancies [24]. Recently, Choi et al. demonstrated that within the tumor microenvironment, NLR was associated with the density of CD4+ T cells, which leads to prognostic values of systemic inflammation in gastric cancer [39]. Taken together, the NLR is a risk factor of systematic inflammation, which reflects the balance between pro-tumor inflammatory status (neutrophilia) and anti-tumor immune status (lymphopenia).
Our results suggest that the correlation between the preoperative and postoperative NLRs was weak. This is reasonable because the mechanisms for a high NLR are different before and after surgery. Therefore, we hypothesized that the combination of the pre- and postoperative NLR might be more useful to predict the prognosis of gastric cancer patients than either the preoperative NLR or the postoperative NLR. In fact, the AUC of the combination of the pre- and postoperative NLR was higher than that of the individual use of either the preoperative or postoperative NLR, indicating that the combination of pre- and postoperative NLRs was more useful to predict the prognosis of gastric cancer patients than either the preoperative NLR or the postoperative NLR. In fact, the prognosis of patients with either preNLRHigh or postNLRHigh was significantly better than that of those with both preNLRHigh and postNLRHigh and worse than that of those with both preNLRLow and postNLRLow. Furthermore, multivariate analysis indicated that the combination of pre- and postoperative NLR was an independent prognostic indicator.
This study had some limitations. First, because it was retrospective, there was some bias. Second, we measured the NLR 1 month after surgery and used this as the postoperative NLR; however, the best timing to measure postoperative NLR remains unclear. Third, the number of patients in the current study was small; therefore, a large-scale, prospective, randomized, controlled trial is needed to confirm the results.
In conclusion, the combination of pre- and postoperative NLR appears to be useful for predicting the prognosis of gastric cancer patients. Because a peripheral blood cell count is a quick, easy, and non-invasive assay, measuring the pre- and postoperative NLR may be a useful biological marker in the routine clinical setting.
References
Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D. Global cancer statistics. CA Cancer J Clin. 2011;61:69–90.
Bozzetti F, Bonfanti G, Morabito A, Bufalino R, Menotti V, Andreola S, et al. A multifactorial approach for the prognosis of patients with carcinoma of the stomach after curative resection. Surg Gynecol Obstet. 1986;162:229–34.
Maruyama K. The most important prognostic factors for gastric cancer patients. Scand J Gastroenterol. 1987;22:63–8.
Fuse N, Kuboki Y, Kuwata T, Nishina T, Kadowaki S, Shinozaki E, et al. Prognostic impact of HER2, EGFR, and c-MET status on overall survival of advanced gastric cancer patients. Gastric Cancer. 2016;19:183–91.
Higaki E, Kuwata T, Nagatsuma AK, Nishida Y, Kinoshita T, Aizawa M, et al. Gene copy number gain of EGFR is a poor prognostic biomarker in gastric cancer: evaluation of 855 patients with bright-field dual in situ hybridization (DISH) method. Gastric Cancer. 2016;19:63–73.
Okugawa Y, Toiyama Y, Hur K, Toden S, Saigusa S, Tanaka K, et al. Metastasis-associated long non-coding RNA drives gastric cancer development and promotes peritoneal metastasis. Carcinogenesis. 2014;35:2731–9.
Wu JG, Wang JJ, Jiang X, Lan JP, He XJ, Wang HJ, et al. MiR-125b promotes cell migration and invasion by targeting PPP1CA-Rb signal pathways in gastric cancer, resulting in a poor prognosis. Gastric Cancer. 2015;18:729–39.
Kurokawa Y, Matsuura N, Kimura Y, Adachi S, Fujita J, Imamura H, et al. Multicenter large-scale study of prognostic impact of HER2 expression in patients with resectable gastric cancer. Gastric Cancer. 2015;18:691–7.
Zhang H, Wang X, Shen Z, Xu J, Qin J, Sun Y. Infiltration of diametrically polarized macrophages predicts overall survival of patients with gastric cancer after surgical resection. Gastric Cancer. 2015;18:740–50.
Park HJ, Ahn JY, Jung HY, Lim H, Lee JH, Choi KS, et al. Clinical characteristics and outcomes for gastric cancer patients aged 18–30 years. Gastric Cancer. 2014;17:649–60.
Nam DH, Lee YK, Park JC, Lee H, Shin SK, Lee SK, et al. Prognostic value of early postoperative tumor marker response in gastric cancer. Ann Surg Oncol. 2013;20:3905–11.
Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011;144:646–74.
Lee S, Oh SY, Kim SH, Lee JH, Kim MC, Kim KH, et al. Prognostic significance of neutrophil lymphocyte ratio and platelet lymphocyte ratio in advanced gastric cancer patients treated with FOLFOX chemotherapy. BMC Cancer. 2013;13:350.
Templeton AJ, McNamara MG, Seruga B, Vera-Badillo FE, Aneja P, Ocana A, et al. Prognostic role of neutrophil-to-lymphocyte ratio in solid tumors: a systematic review and meta-analysis. J Natl Cancer Inst. 2014;106:dju124.
Proctor MJ, Morrison DS, Talwar D, Balmer SM, Fletcher CD, O’Reilly DS, et al. A comparison of inflammation-based prognostic scores in patients with cancer. A Glasgow Inflammation Outcome Study. Eur J Cancer. 2011;47:2633–41.
Pinato DJ, North BV, Sharma R. A novel, externally validated inflammation-based prognostic algorithm in hepatocellular carcinoma: the prognostic nutritional index (PNI). Br J Cancer. 2012;106:1439–45.
Fox P, Hudson M, Brown C, Lord S, Gebski V, De Souza P, et al. Markers of systemic inflammation predict survival in patients with advanced renal cell cancer. Br J Cancer. 2013;109:147–53.
Stotz M, Gerger A, Eisner F, Szkandera J, Loibner H, Ress AL, et al. Increased neutrophil–lymphocyte ratio is a poor prognostic factor in patients with primary operable and inoperable pancreatic cancer. Br J Cancer. 2013;109:416–21.
Kim JH, Han DS, Bang HY, Kim PS, Lee KY. Preoperative neutrophil-to-lymphocyte ratio is a prognostic factor for overall survival in patients with gastric cancer. Ann Surg Treat Res. 2015;89:81–6.
Kumar R, Geuna E, Michalarea V, Guardascione M, Naumann U, Lorente D, et al. The neutrophil–lymphocyte ratio and its utilisation for the management of cancer patients in early clinical trials. Br J Cancer. 2015;112:1157–65.
Japanese Gastric Cancer Association. Japanese classification of gastric carcinoma: 3rd English edition. Gastric Cancer. 2011;14:101–12.
Clavien PA, Barkun J, de Oliveira ML, Vauthey JN, Dindo D, Schulick RD, et al. The Clavien–Dindo classification of surgical complications: five-year experience. Ann Surg. 2009;250:187–96.
Katayama H, Kurokawa Y, Nakamura K, Ito H, Kanemitsu Y, Masuda N, et al. Extended Clavien–Dindo classification of surgical complications: Japan Clinical Oncology Group postoperative complications criteria. Surg Today. 2016;46:668–85.
Langsenlehner T, Thurner EM, Krenn-Pilko S, Langsenlehner U, Stojakovic T, Gerger A, et al. Validation of the neutrophil-to-lymphocyte ratio as a prognostic factor in a cohort of European prostate cancer patients. World J Urol. 2015;33:1661–7.
Yamada D, Eguchi H, Asaoka T, Tomihara H, Noda T, Wada H, et al. The basal nutritional state of PDAC patients is the dominant factor for completing adjuvant chemotherapy. Surg Today. 2017;. doi:10.1007/s00595-017-1522-x.
Dan J, Zhang Y, Peng Z, Huang J, Gao H, Xu L, et al. Postoperative neutrophil-to-lymphocyte ratio change predicts survival of patients with small hepatocellular carcinoma undergoing radiofrequency ablation. PLoS One. 2013;8:e58184.
Peng W, Li C, Wen TF, Yan LN, Li B, Wang WT, et al. Neutrophil to lymphocyte ratio changes predict small hepatocellular carcinoma survival. J Surg Res. 2014;192:402–8.
Tokunaga M, Tanizawa Y, Bando E, Kawamura T, Terashima M. Poor survival rate in patients with postoperative intra-abdominal infectious complications following curative gastrectomy for gastric cancer. Ann Surg Oncol. 2013;20:1575–83.
Hayashi T, Yoshikawa T, Aoyama T, Hasegawa S, Yamada T, Tsuchida K, et al. Impact of infectious complications on gastric cancer recurrence. Gastric Cancer. 2015;18:368–74.
Takaya S, Saito H, Ikeguchi M. Upregulation of immune checkpoint molecules, PD-1 and LAG-3, on CD4+ and CD8+ T cells after gastric cancer surgery. Yonago Acta Medica. 2015;58:39–44.
Wang KX, Denhardt DT. Osteopontin: role in immune regulation and stress responses. Cytokine Growth Factor Rev. 2008;19:333–45.
Rose-John S, Waetzig GH, Scheller J, Grotzinger J, Seegert D. The IL-6/sIL-6R complex as a novel target for therapeutic approaches. Expert Opin Ther Targets. 2007;11:613–24.
Wu CY, Wu MS, Chiang EP, Wu CC, Chen YJ, Chen CJ, et al. Elevated plasma osteopontin associated with gastric cancer development, invasion and survival. Gut. 2007;56:782–9.
Rittling SR, Chambers AF. Role of osteopontin in tumour progression. Br J Cancer. 2004;90:1877–81.
Middleton K, Jones J, Lwin Z, Coward JI. Interleukin-6: an angiogenic target in solid tumours. Crit Rev Oncol Hematol. 2014;89:129–39.
Rangaswami H, Bulbule A, Kundu GC. Osteopontin: role in cell signaling and cancer progression. Trends Cell Biol. 2006;16:79–87.
Denhardt DT, Noda M, O’Regan AW, Pavlin D, Berman JS. Osteopontin as a means to cope with environmental insults: regulation of inflammation, tissue remodeling, and cell survival. J Clin Investig. 2001;107:1055–61.
Vesely MD, Schreiber RD. Cancer immunoediting: antigens, mechanisms, and implications to cancer immunotherapy. Ann N Y Acad Sci. 2013;1284:1–5.
Choi Y, Kim JW, Nam KH, Han SH, Kim JW, Ahn SH, et al. Systemic inflammation is associated with the density of immune cells in the tumor microenvironment of gastric cancer. Gastric Cancer. 2017;20:602–11.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
We declare no conflicts of interest.
Ethical approval
All procedures performed in studies involving human participants were done in accordance with the ethical standards of the institutional research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Rights and permissions
About this article
Cite this article
Miyatani, K., Saito, H., Kono, Y. et al. Combined analysis of the pre- and postoperative neutrophil–lymphocyte ratio predicts the outcomes of patients with gastric cancer. Surg Today 48, 300–307 (2018). https://doi.org/10.1007/s00595-017-1587-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00595-017-1587-6