Abstract
Purposes
To evaluate vertebral artery (VA) dominancy and the risk of brain infarction in T4 lung cancer patients with tumor invasion into the subclavian artery.
Methods
We reconstructed the subclavian artery in 10 patients with T4 non-small cell lung cancer. The histological stages were IIIA in eight patients and IIIB in two patients. We evaluated the VA dominancy by performing a four-vessel study preoperatively and investigated the relationship between the methods of VA treatment and postoperative brain complications, retrospectively.
Results
Seven patients had a superior sulcus tumor (SST) and three had direct invasion into the mediastinum. Based on the tumor location, a transmanublial approach was used in five patients and a posterolateral hook incision was used in the other five. All subclavian artery (SA) reconstructions were done using an artificial woven graft. Preoperative angiography of the VA revealed poor development of the contralateral side in two patients. One of these patients suffered a severe brain infarction on postoperative day 2, which proved fatal. In the other patient, the VA was connected to the left SA graft by a side-to-end anastomosis and there was no postoperative brain complication.
Conclusions
Preoperative SA and VA angiography is mandatory for identifying the need for VA reconstruction in lung cancer patients with major arterial invasion.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Resecting a superior sulcus tumor (SST) with invasion of the great arteries in the thorax is one of the most challenging operations for T4 lung cancer [1]. This is because the thoracic inlet (TI) is difficult to access surgically; vascular reconstruction requires a sophisticated surgical technique, and lymph node involvement and postoperative local recurrence are common. In fact, involvement of the great vessels, such as the subclavian artery (SA), carotid artery, and aortic arch, often means the tumor is inoperable. However, selected T4 patients without N2 disease can be operative candidates, with acceptable morbidity and mortality, in highly specialized centers [2,3,4]. Lahon et al. reported that T4 lung cancer with SA invasion can be safely resected and reconstructed with good long-term survival, although they did not mention whether the vertebral artery (VA) can be sacrificed [2]. While it is generally accepted that one VA can be sacrificed, although the contralateral VA should be investigated before the operation, there is little evidence to support this recommendation. In the present study, we investigated which patients are suitable candidates for VA reconstruction.
Patients and methods
This study was approved by the institutional review boards of Tokyo Women’s Medical University and the collaborating hospitals. Informed consent was waived because it was a retrospective analysis. Between November 1999 and March 2013, we performed subclavian artery reconstruction for T4 non-small cell lung cancer in ten patients. The patients’ medical records were reviewed retrospectively for collection of the following data: clinical assessment, magnetic resonance imaging (MRI) angiography or conventional X-ray angiography of the SA and VA on both sides and the cerebral arterial circle; operative methods; use of extracorporeal perfusion; methods of reconstruction of the SA; and surgical outcomes.
Information on the clinical stage, functional evaluation, and operative indication were obtained by functional testing (ECG, spirometry, echocardiography), bronchoscopy, brain MRI, and 18F-fluorodeoxyglucose positron emission tomography. Contraindications to surgical resection were substantial involvement of the esophagus, tracheal or brachial plexus higher than the C8 route, distant metastasis, and clinical N2 or N3 disease [5].
Evaluation of tumor involvement and blood flow in the SA, VA, and cerebral arterial circle
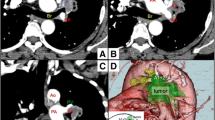
SA invasion was diagnosed by contrast-enhanced computed tomography (CT) and angiography. Blood flow in the SA, VA (Fig. 1) and cerebral arterial circle (Fig. 2a) was also evaluated by MRI angiography or conventional X-ray angiography.
Magnetic resonance imaging (MRI) angiography or enhanced computed tomography (CT) of the vertebral artery in Patients #8, 9, and 10. a Normal development of the bilateral vertebral artery in Patient #8. b, c Development of the left vertebral artery was predominant over that of the right vertebral artery in Patients #9 and 10
Surgical technique
All patients underwent complete resection with extended removal of the SA and adjacent structures. A posterolateral hook skin incision was used in patients with SST when removal of the posterior portion of the first rib was necessary, whereas a median sternotomy with transmanubrial approach was used in patients with SST or direct mediastinal invasion when resection of the aortic arch or its three associated branches or the anterior portion of the first rib was necessary. Partial extracorporeal circulation of the lower half of the body was utilized during aortic reconstruction. When the tumor invasion was close to the bifurcation of the SA and VA and if angiography confirmed that the VA on the contralateral side was well developed (Fig. 1a), the VA was transected. When SA reconstruction is done via a posterolateral incision, the VA is difficult to see because it is concealed behind the SA at the level of the eighth cervical or first thoracic vertebra. Therefore, we always ensure identification of the VA by twisting the SA lightly and transecting it at the bifurcation. Since it is impossible to reconstruct the VA via a posterolateral incision, a transmanubrial approach is recommended. The VA was reconstructed in only one of our patients, using an end-to-side anastomosis to suture the remaining VA to the SA graft (Fig. 3). The subclavian vein was frequently resected without reconstruction. The preferred SA reconstruction was direct end-to-end anastomosis with a woven vascular graft. The aortic arch was also reconstructed using a woven vascular graft. Postoperative anticoagulation therapy was not given. Finally, enhanced CT was performed to assess the patency of the reconstructed arteries.
We resected the cervical lymph nodes close to the tumor for sampling, but not for systematic lymph node resection. Since the cervical lymphatic system is complicated and systematic cervical lymph node resection carries a high risk of neurological complications, this was not performed routinely.
Postoperative major complications and long-term outcomes
We evaluated the postoperative major cardiopulmonary complications including pneumonia, bronchial stump dehiscence, empyema, acute lung injury or lung edema, prolonged arrhythmia requiring medical treatment, heart failure, ischemic heart disease and prolonged ventilatory support. We also evaluated the brain complications including brain infarction, neurological impediment and paralysis. Finally, long-term outcomes, including survival time and cause of death were investigated.
Results
Patient characteristics
Tables 1 and 2 summarize the patients’ clinical data. All patients were men, with a mean age of 55.9 ± 8.8 years (range 37–66 years). Five patients had adenocarcinoma, three had squamous cell carcinoma, and two had large cell carcinoma. The pathological stage was IIIA in eight patients and IIIB in two patients. Seven patients had SST and three had direct mediastinal invasion of the tumor. Angiography of the great arteries showed normal development of the VA in eight patients and predominance of the left VA over the right VA in two. Six patients underwent partial lung resection because most of the tumor was in the extrapleural region and extended into the mediastinum or thoracic inlet. Four patients underwent complete lobectomy. One patient received neoadjuvant chemotherapy, four received postoperative radiotherapy, two received postoperative chemoradiotherapy, and two did not receive any adjuvant or neoadjuvant therapy. The most recent two patients received curative concurrent chemoradiotherapy at another hospital and were referred to our hospital for management of local recurrence without lymph node or distant metastases 1 year after curative treatment.
Surgical resection and arterial reconstruction
The operative approach, based on the tumor location, was via a posterolateral hook incision in five patients and via median sternotomy or a transmanubrial approach via a fourth intercostal thoracotomy in five patients. Three patients were given extracorporeal perfusion for reconstruction of the aorta. Chest wall resection including the first rib was performed in seven patients. The SA was reconstructed by interposition of a woven vascular graft, using an end-to-end anastomosis, in eight patients. A woven vascular Y-graft was used in two patients and a 4-branch vascular graft for total arch reconstruction was used in one patient (Patient #8) [6]. The VA was transected in seven patients and preserved in two patients. In Patient #10, the VA was revascularized to the SA graft by a side-to-end anastomosis (Fig. 3).
The definitive pathology examination revealed complete R0 resection in six patients and R1 resection in four patients, all of whom received postoperative 60 Gray radiation therapy. When complete resection cannot be achieved, there is no surgical indication. We always aimed to perform R0 resection, but a cytological remnant at the brachial plexus was confirmed by pathology in four patients. Pathological involvement of the VA was not found in any patient, but if SA reconstruction was necessary, transection or reconstruction of the VA was frequently required. The VA was able to be preserved in only one patient because the tumor was not located at the bifurcation from the SA.
Morbidity and mortality
None of the patients suffered major postoperative cardiopulmonary complications. Patient #3, who underwent VA transection, had temporary cerebellar symptoms that improved within 1 month after the operation. He had normal development of the VA on both sides. Patient #9 suffered severe brain infarction on postoperative day 2 (Fig. 2b) and died on postoperative day 5. He had undergone SA reconstruction with VA transection without postoperative anticoagulation therapy despite poor development of the contralateral VA and poor connection between the right VA and cerebral arterial circle (Figs. 1b, 2a). Patient #10, who underwent VA reconstruction because of poor development of the contralateral VA (Fig. 1c), had no brain complications and had good circulation through the left VA and the cerebral arterial circle was maintained postoperatively (Fig. 4).
Long-term follow-up
Seven patients died of cancer recurrence 17–30 months after their operation (mean ± SD 22.1 ± 4.7 months). One patient (#6) survived without recurrence for 12 months and another patient (#10) survived with bone metastasis for 13 months. The 1-, 2-, and 3-year survival rates were 90, 25.7, and 0%, respectively.
Discussion
We performed SA reconstruction in ten patients and confirmed the necessity of VA reconstruction for those with poor development of the contralateral VA. Therefore, preoperative SA and VA angiography is mandatory in case there is involvement of both the SA and VA. The Japan Clinical Oncology Group (JCOG) established the postoperative complication (PC) criteria and listed the surgical adverse event (AE) terms and gradings [7]. Thrombus/embolism is included in this list and postoperative brain infarction caused by VA thrombus is one of the most serious complications.
Since the cerebral arterial circle circulation is supplied from both sides, transection of the VA on one side is generally permissible; however, when MRI angiography clearly shows poor development of the contralateral VA, the VA on the operative side should be revascularized. Matula et al. studied the VA in 402 patients and found hypoplasia (defined as a diameter <3.5 mm) in 16 patients (7.0%): of the right VA in 11 (4.8%) patients and of the left VA in 5 (2.2%) [8].
We explain the events leading to the brain infarction in Patient #9 in the following way (Fig. 5): in the normal development of VA on both sides, retrograde blood flow from the contralateral VA supplies the operative side as a collateral pathway after VA transection. However, if there is poor development of the contralateral VA, both the collateral pathway and blood flow via the posterior communicating artery will be insufficient. This poor circulation would cause a thrombus in the basilar artery leading to brain infarction. VA-carotid artery transposition provides a surgical option for revascularization of the VA with relatively low long-term complication and restenosis rates [9]. A few methods of VA reconstruction in lung cancer surgery have been reported. Ishibashi et al. reported performing an end-to-side anastomosis between the left VA and left carotid artery at the C6 level using a microscope [10]. During the anastomosis, regional cerebral oxygen saturation was monitored using INVOS™ (Edwards Lifescience, CA, USA). There was minimal restenosis after this procedure and few serious postoperative sequelae [11]. Since the INVOS™ was introduced, we have been using it to monitor regional cerebral oxygen saturation (rSO2); however, the rSO2 did not decrease during the operation so it may be difficult to predict postoperative brain infarction. Watanabe et al. reported performing a second-look operation for lung cancer in a patient with left SST [8]. First, they placed a saphenous vein graft between the left common carotid artery and the left VA and then they performed lung resection 3 weeks later. Both operations were carried out by skilled neurosurgeons. In contrast, we used an intrathoracic bypass approach because the tumor invasion into the bifurcation of the VA and the SA resulted in insufficient length of the VA for direct anastomosis with the SA artery graft. However, this technique may be difficult because of the restricted operating field.
Anatomy of the vertebral artery and subclavian artery to explain the brain infarction in Patient #9. Narrowing of the right VA resulted in insufficient collateral pathway and blood flow via the posterior communicating artery. This poor circulation resulted in a thrombus in the basilar artery leading to brain infarction
Six patients underwent partial lung resection because the tumor was localized mainly in the mediastinum with a small amount in the lung. These patients had no hilar or ipsilateral mediastinal lymph node metastasis. Therefore, we expected that tumor extension would be mainly direct invasion to the mediastinum and that lymphatic metastasis via the intra-pulmonary lymphatic route might not exist. In fact, there was no ipsilateral hilar lymph node metastasis in these patients, whose recurrence was distant metastasis or local recurrence in the mediastinum. This selection might affect the survival.
Induction chemoradiotherapy followed by surgery became a standard approach for superior sulcus tumors (SSTs) in the 2000s, despite the absence of a large-scale randomized study [12, 13]. The value of induction chemoradiotherapy has also been recognized for T4 lung cancers with great vessel invasion, other than SSTs. In general, selected patients with SSTs have acceptable long-term survival after complete resection. Yildizeli et al. reported the following overall 5-year survival rates for four subgroups within the T4 category: 36.6% for SSTs, 42.5% for those with carinal involvement, 29.4% for those with SVC invasion, and 61.2% for those with mediastinal involvement [5]. However, they also reported that involvement of the SA was independently associated with lower long-term survival rates after surgical resection of T4 malignancies [5]. The reason for the poor prognosis associated with SA invasion may be residual tumor cells in the brachial plexus and adjacent tissues, and the tendency for malignant cells to metastasize into the cervical lymph nodes.
In our series, only one patient (Patient #7) had induction chemotherapy, but not chemoradiotherapy, while the others had no induction treatment. This may also account for the poor long-term survival. Our surgical series comprised extremely severe and complicated cases of T4 lung cancer due to direct invasion of the great arteries. Most may have been contraindicated for surgery. Patients #1 to #6 underwent surgery between 1999 and 2003. We then became circumspect in deciding on the operative indication. In 2009, Patient #7 received postoperative chemoradiotherapy and Patient #8 received induction chemotherapy. However, since long-term survival was still poor, we fundamentally withdrew T4 lung cancer with SA invasion from the operative indications. Therefore, Patients #9 and #10 underwent salvage surgery.
In conclusion, the operative indication for T4 lung cancer with tumor invasion of the great arteries should be considered cautiously because of the poor long-term prognosis. Preoperative SA and VA angiography is mandatory for identifying the need for VA reconstruction after resection of lung cancer with major arterial invasion. VA reconstruction is recommended for patients with poor development of the contralateral VA, even though transection of the VA on one side is generally permissible.
References
Reardon ES, Schrump DS. Extended resections of non-small cell lung cancers invading the aorta, pulmonary artery, left atrium, or esophagus: can they be justified? Thorac Surg Clin. 2014;24:457–64.
Lahon B, Mercier O, Fadel E, Mussot S, Fabre D, Hamdi S, et al. Subclavian artery resection and reconstruction for thoracic inlet cancer: 25 years of experience. Ann Thorac Surg. 2013;96:983–8.
Spaggiari L, Tessitore A, Casiraghi M, Guarize J, Solli P, Borri A, et al. Survival after extended resection for mediastinal advanced lung cancer: lessons learned on 167 consecutive cases. Ann Thorac Surg. 2013;95:1717–25.
Klepetko W, Wisser W, Bîrsan T, Mares P, Taghavi S, Kupilik N, et al. T4 lung tumors with infiltration of the thoracic aorta: is an operation reasonable? Ann Thorac Surg. 1999;67:340–4.
Yildizeli B, Dartevelle PG, Fadel E, Mussot S, Chapelier A. Results of primary surgery with T4 non-small cell lung cancer during a 25-year period in a single center: the benefit is worth the risk. Ann Thorac Surg. 2008;86:1065–75.
Suzuki H, Sekine Y, Ko E, Sunazawa T, Iida H, Kishi H, et al. Permanent cerebral bypass approach for lung cancer resection with aortic arch invasion. Thorac Cardiovasc Surg. 2011;59:378–80.
Katayama H, Kurokawa Y, Nakamura K, et al. Extended Clavien–Dindo classification of surgical complications: Japan Clinical Oncology Group postoperative complications criteria. Surg Today. 2016;46:668.
Watanabe T, Okada Y, Sakurada A, Sado T, Matsuda Y, Shimizu H, et al. Resection of apical lung carcinoma involving the vertebral artery. Ann Thorac Surg. 2010;90:302–3.
Rangel-Castilla L, Kalani MY, Cronk K, Zabramski JM, Russin JJ, Spetzler RF. Vertebral artery transposition for revascularization of the posterior circulation: a critical assessment of temporary and permanent complications and outcomes. J Neurosurg. 2015;122:671–7.
Ishibashi N, Endo C, Hoshikawa Y, Noda M, Saiki Y, Motoyoshi N, et al. Completely resected superior sulcus tumor and vascular reconstruction of vertebral and subclavian arteries. Gen Thorac Cardiovasc Surg. 2012;60:777–80.
Edwards WH, Mulherin JL Jr. The surgical reconstruction of the proximal subclavian and vertebral artery. J Vasc Surg. 1985;2:634–42.
Kunitoh H, Kato H, Tsuboi M, Shibata T, Asamura H, Ichinose Y, Katakami N, Nagai K, Mitsudomi T, Matsumura A, Nakagawa K, Tada H, Saijo N, Japan Clinical Oncology Group. Phase II trial of preoperative chemoradiotherapy followed by surgical resection in patients with superior sulcus non-small-cell lung cancers: report of Japan Clinical Oncology Group trial 9806. J Clin Oncol. 2008;26:644–9.
Shien K, Toyooka S, Kiura K, Matsuo K, Soh J, Yamane M, Oto T, Takemoto M, Date H, Miyoshi S. Induction chemoradiotherapy followed by surgical resection for clinical T3 or T4 locally advanced non-small cell lung cancer. Ann Surg Oncol. 2012;19:2685–92.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
We declare no conflicts of interest.
Rights and permissions
About this article
Cite this article
Sekine, Y., Saitoh, Y., Yoshino, M. et al. Evaluating vertebral artery dominancy before T4 lung cancer surgery requiring subclavian artery reconstruction. Surg Today 48, 158–166 (2018). https://doi.org/10.1007/s00595-017-1573-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00595-017-1573-z