Abstract
Purpose
To identify the possible roles of carcinoembryonic antigen (CEA) testing after liver resection for synchronous colorectal liver metastasis (CLM).
Methods
The subjects of this retrospective study were patients who underwent complete resection of primary tumors and synchronous CLM between 1997 and 2007 at 20 institutions in Japan. We studied the associations between perioperative CEA levels and the characteristics of recurrence.
Results
Recurrence was detected during the median follow-up time of 52 months in 445 (73.7%) of the total 604 patients analyzed. A postoperative CEA level >5 ng/ml was an independent predictor, with the highest hazard ratio (2.25, 95% confidence interval 1.29–3.91, P = 0.004). A postoperative CEA level >5 ng/ml had a specificity of 86.2% and a positive predictive value of 84.2% for recurrence. Patients with a high postoperative CEA level had a significantly higher recurrence rate, with a shorter time until recurrence and a higher frequency of multiple metastatic sites than those with a low postoperative CEA level. Among the patients with recurrence, 173 (52.7%) had an elevated CEA level (>5 ng/ml) when recurrence was detected.
Conclusions
A postoperative CEA level >5 ng/ml was an independent predictor of recurrence; however, CEA testing was not a reliable surveillance tool to identity recurrence after liver resection.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Surgical resection of colorectal liver metastasis (CLM) is accepted as the only potentially curative treatment. However, despite advances in the management of CLM, recurrence is found in the liver remnant or at other sites in 50–75% of patients who undergo liver resection for CLM [1,2,3,4]. It is thought that the high frequency of recurrence after liver resection is due to occult metastases derived from the primary colorectal cancer (CRC) and residual lesions scattered from CLM [5, 6].
The serum levels of carcinoembryonic antigen (CEA), a glycoprotein, frequently increase in patients with various types of cancer. Thus, CEA testing is widely used in the management of CRC patients, especially as a surveillance tool after the resection of primary stages I–III CRC tumors, as an early detector of recurrence [7,8,9,10]. Moreover, many studies have shown that perioperative CEA is a predictive marker of recurrence after complete (R0) resection for metastatic CRC [11,12,13,14]. However, it is unclear how perioperative CEA should be used in the management of patients with CLM and the efficacy of CEA surveillance after surgery for CLM has not been fully investigated. If the serum CEA level after liver resection reflects the existence of residual occult tumors, an elevated CEA level could be not only a predictor of recurrence, but also a good surveillance tool after liver resection. We conducted this study to define the association of perioperative CEA levels and the characteristics of recurrence after liver resection and to identify the possible roles of CEA testing in the management of patients after R0 resection for primary tumors and then CLM.
Patients and methods
Study design
We reviewed medical records collected from the 20 institutes participating in the Japanese Study Group for Postoperative Follow-up of Colorectal Cancer (JFUP-CRC). The JFUP-CRC is a joint study group, established in 2001 to propose the most adaptive and effective follow-up program for the management of CRC patients (the investigators in this group are listed in Acknowledgements).
To assess the value of CEA as a predictor of recurrence or as a surveillance tool after liver resection, we reviewed the collected data and analyzed the association between perioperative CEA levels and the characteristics of recurrence after liver resection for synchronous CLM. All patients enrolled in this study underwent surgical procedures that were covered by the Japanese national health insurance. This study was exempt from institutional ethical review because patient information could not be identified from the data.
Data collection
The subjects of this retrospective analysis were patients who underwent R0 resection for both primary tumors and synchronous CLM between January, 1997, and December, 2007. Patients with extrahepatic metastases were excluded. We collected data on the characteristics of the primary tumor and liver metastases, perioperative chemotherapy, recurrence, and survival, as well as the preoperative and postoperative CEA levels, defined as levels measured within 1 month before and within 3 months after liver resection, respectively. The CEA level at the time of recurrence was also measured. Patients whose CEA levels had not been measured were excluded from the study population. Patients who received any chemotherapy for more than 6 months after liver resection were regarded as having received adjuvant chemotherapy. All other patients were grouped as having received ‘no adjuvant chemotherapy.’
Statistical analysis
Categorical variables were compared using the Chi-square test. Multivariate analysis was completed for factors with a P value <0.10 on univariate analysis using a logistic regression model. To evaluate the survival time and the time until recurrence, the Kaplan–Meier method was used and a comparison was made using the log-rank test. Differences with a P value of <0.05 were considered significant. All statistical analyses were performed using SPSS version 13.0 (SPSS Inc., Chicago, IL, USA).
Results
Patient characteristics and clinical outcomes
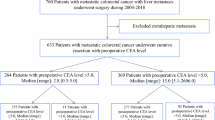
We reviewed the records of 604 patients with synchronous CLM who underwent resection of primary tumors and CLM between 1997 and 2007. The median follow-up after liver resection was 52 months (range 3–199 months). Table 1 summarizes the clinical and pathological data of the 604 patients. There were 383 (63.4%) men and 221 (36.6%) women, with a mean age of 63 years (range 29–91 years). The primary tumors were advanced with regard to invasion [T3 and T4, n = 408 (67.5%)] and nodal status [node positive, n = 431 (71.4%)], and 40.7% of tumors were located in the rectum. The median diameter of the largest metastasis was 25 mm (range 4–170 mm), and the median number of metastases was 2 (range 1–32). Among the 604 patients, 470 (77.8%) underwent liver resection simultaneously to the primary tumor resection, and the remaining patients underwent metachronous liver resection after resection of the primary tumor. Fifty (8.3%) patients received neoadjuvant chemotherapy, and 364 (60.3%) received adjuvant chemotherapy. Recurrence was detected in 445 (73.7%) patients during the median follow-up time of 52 months and within 3 years after liver resection in 91.0%. The median survival time after liver resection was 66 months (range 3–199 months), and the 5-year cumulative overall survival rate was 51.9%.
CEA as a predictor of recurrence
To assess the predictive factor of recurrence, clinicopathological variables were compared between patients with and without recurrence (Fig. 1). We investigated which of the following four CEA variables was the strongest predictor of recurrence: a high preoperative CEA level; a high postoperative CEA level; a high relative CEA level, being the ratio of postoperative CEA against preoperative CEA; and both high preoperative and postoperative CEA levels. The cutoff levels for preoperative, postoperative, and relative CEA were calculated using receiver operating characteristic (ROC) curves (Supplementary Fig. 1). The most appropriate cutoff levels were 90 ng/ml for preoperative CEA, 5 ng/ml for postoperative CEA, and 0.75 for relative CEA, respectively. Those four variables were entered individually into the univariate analysis (a preoperative CEA level > or ≤90; a postoperative CEA level > or ≤5; a relative CEA level > or ≤0.75; both a preoperative CEA level >5 and a postoperative CEA level >5 or not), and each one was entered separately into the multivariate analysis with the other predictors (Table 1). A postoperative CEA level >5 ng/ml was identified as an independent predictor of recurrence with the highest HR (2.25, 95% confidence interval (CI) 1.29–3.91, P = 0.004). The recurrence rates were 84.2% for patients with a postoperative CEA level >5 ng/ml and 70.5% for those with a postoperative CEA level ≤5 ng/ml (P = 0.001). For the prediction of recurrence, a postoperative CEA level >5 ng/ml had the highest specificity (86.2%) and the highest positive predictive value (PPV 84.2%), while the sensitivity was 26.3%.
Characteristics of recurrence according to postoperative CEA
The 445 patients with recurrence were divided into two groups according to their postoperative CEA level: a high-CEA group with a postoperative CEA level >5 ng/ml (n = 117) and a low-CEA group with a postoperative CEA level ≤5 ng/ml (n = 328; Fig. 1). We compared the time to recurrence after liver resection, the sites of recurrence, and the CEA level at the time of recurrence between the groups.
Time to recurrence after liver resection
The median time until recurrence was significantly shorter in the high-CEA group than in the low-CEA group (5.0 vs. 9.1 months, P < 0.0001). Recurrence was found within 6 months of resection in 55.9% of patients in the high-CEA group vs. 27% of patients in the low-CEA group (Fig. 2).
Sites and distribution of recurrence
Table 2 shows the sites and distribution of recurrence in each group. More patients in the high-CEA group had metastases at multiple sites than those in the low-CEA group (36.8 vs. 21.6%, P = 0.001). The liver remnant was the most common site of recurrence in both groups (68.4 and 68.0%). Lung and lymph node metastases were more frequent in the high-CEA group than in the low-CEA group (40.1 vs. 28.4%; P = 0.018, and 17.1 vs. 10.4%; P = 0.056, respectively). There were no differences in recurrence rates at other sites.
CEA level at the time of recurrence
A CEA level of >5 ng/ml was found at the time of recurrence in 52.7% of the patients in the low-CEA group. This means that the sensitivity of CEA testing to detect actual recurrence is 52.7%. We also measured the ratio of CEA at the time of recurrence vs. the postoperative CEA level in the high-CEA group. The median ratio was 1.25, with 41% of patients having a ratio of more than 1.5 and 52.1% having a ratio of more than 1.2 (Table 3). The specificity and accuracy of CEA testing to diagnosis actual recurrence are not available due to the lack of corresponding CEA levels in the patients without recurrence.
Discussion
In accordance with the findings of previous studies that postoperative CEA is a predictive marker of recurrence [12, 13], the patients in this series with a postoperative CEA level >5 ng/ml were at a significantly higher risk of recurrence, with a shorter time until recurrence and a higher frequency of multiple metastatic sites. Our findings support the consensus that the serum CEA level after liver resection reflects the existence of residual occult tumors in the liver remnant or other organs.
An intensive adjuvant chemotherapy regimen after liver resection would be recommended for patients with a high recurrence risk. Although many studies [15,16,17,18] have investigated the efficacy of adjuvant chemotherapy after curative resection of CLM, effective regimens and indications for adjuvant chemotherapy have not yet been established. Since a postoperative CEA level >5 ng/ml had a high specificity and PPV to predict recurrence, this cutoff level could be used to stratify candidates who would benefit from adjuvant chemotherapy. From the biological viewpoint that a high postoperative CEA level may indicate residual occult tumors, this variable would be reasonable to use in selecting those candidates. However, the sensitivity of a postoperative CEA level >5 ng/ml was low, and even patients with a low postoperative CEA level (≤5 ng/ml) had a high recurrence rate of 70.5%. Therefore, additional criteria for selecting candidates for adjuvant chemotherapy will be necessary. Nodal metastases and a larger number of liver metastases, which were also independent predictors of recurrence with a high hazard ratio in this study, could be helpful.
An adequate and effective follow-up program to detect recurrent tumors earlier is important. Several meta-analyses [19,20,21] have provided evidence that intensive follow-up programs after curative surgery improve the outcome of patients with non-metastatic CRC. In particular, CEA testing, as well as imaging modalities, contributes to improving outcomes [22]. However, the efficacy of CEA testing as a surveillance tool after resection of metastatic CRC has not been fully investigated. Some guidelines recommend follow-up programs comprised of CEA testing and CT for patients who have undergone R0 resection for metastatic CRC. The ESMO Clinical Practice Guidelines [23] recommend a follow-up program with CEA testing and CT at intervals of 3–6 months during the first 3 years. Similarly, the NCCN Guidelines (Version 2. 2015) recommend a follow-up program of CEA testing and CT scans every 3–6 months for the first 2 years, then CEA testing every 6 months and CT scans every 6–12 months for the following 3 years. In the present study, almost half of the patients with a low postoperative CEA level did not have elevated CEA levels at the time of recurrence, suggesting that CEA monitoring is not useful in follow-up. Accordingly, intensive imaging studies as well as CEA monitoring would be necessary for earlier and more accurate detection of recurrent tumors.
This study was limited by its retrospective design; therefore, further prospective studies are warranted to confirm our findings. More data on chronological change in CEA levels after liver resection are also needed, and other factors that influence CEA elevation, such as smoking, should be taken into consideration, to clarify the role of CEA testing.
In conclusion, a postoperative CEA level >5 ng/ml was an independent and strong predictor of recurrence, which could assist in decisions about treatment after liver resection. However, since CEA monitoring was not reliable as a surveillance tool, intensive imaging studies based on the characteristics of recurrence would be necessary for optimal follow-up after liver resection for CLM.
References
de Jong MC, Mayo SC, Pulitano C, Lanella S, Ribero D, Strub J, et al. Repeat curative intent liver surgery is safe and effective for recurrent colorectal liver metastasis: results from an international multi-institutional analysis. J Gastrointest Surg. 2009;13(12):2141–51.
Chua TC, Saxena A, Chu F, Zhao J, Morris DL. Predictors of cure after hepatic resection of colorectal liver metastases: an analysis of actual 5- and 10-year survivors. J Surg Oncol. 2011;103(8):796–800.
Mayo SC, Pawlik TM. Current management of colorectal hepatic metastasis. Expert Rev Gastroenterol Hepatol. 2009;3(2):131–44.
Sa Cunha A, Laurent C, Rault A, Couderc P, Rullier E, Saric J. A second liver resection due to recurrent colorectal liver metastases. Arch Surg. 2007;142(12):1144–9 (discussion 50).
Nanko M, Shimada H, Yamaoka H, Tanaka K, Masui H, Matsuo K, et al. Micrometastatic colorectal cancer lesions in the liver. Surg Today. 1998;28(7):707–13.
Hayashi M, Inoue Y, Komeda K, Shimizu T, Asakuma M, Hirokawa F, et al. Clinicopathological analysis of recurrence patterns and prognostic factors for survival after hepatectomy for colorectal liver metastasis. BMC Surg. 2010;10:27.
Wood CB, Ratcliffe JG, Burt RW, Malcolm AJ, Blumgart LH. The clinical significance of the pattern of elevated serum carcinoembryonic antigen (CEA) levels in recurrent colorectal cancer. Br J Surg. 1980;67(1):46–8.
Wichmann MW, Müller C, Lau-Werner U, Strauss T, Lang RA, Hornung HM, et al. The role of carcinoembryonic antigen for the detection of recurrent disease following curative resection of large-bowel cancer. Langenbecks Arch Surg. 2000;385(4):271–5.
Chau I, Allen MJ, Cunningham D, Norman AR, Brown G, Ford HE, et al. The value of routine serum carcino-embryonic antigen measurement and computed tomography in the surveillance of patients after adjuvant chemotherapy for colorectal cancer. J Clin Oncol. 2004;22(8):1420–9.
McCall JL, Black RB, Rich CA, Harvey JR, Baker RA, Watts JM, et al. The value of serum carcinoembryonic antigen in predicting recurrent disease following curative resection of colorectal cancer. Dis Colon Rectum. 1994;37(9):875–81.
Oussoultzoglou E, Rosso E, Fuchshuber P, Stefanescu V, Diop B, Giraudo G, et al. Perioperative carcinoembryonic antigen measurements to predict curability after liver resection for colorectal metastases: a prospective study. Arch Surg. 2008;143(12):1150–8 (discussion 8-9).
Bredt LC, Rachid AF. Predictors of recurrence after a first hepatectomy for colorectal cancer liver metastases: a retrospective analysis. World J Surg Oncol. 2014;12:391.
Araujo RL, Gönen M, Allen P, DeMatteo R, Kingham P, Jarnagin W, et al. Positive postoperative CEA is a strong predictor of recurrence for patients after resection for colorectal liver metastases. Ann Surg Oncol. 2015;22(9):3087–93.
Verberne CJ, Wiggers T, Vermeulen KM, de Jong KP. Detection of recurrences during follow-up after liver surgery for colorectal metastases: both carcinoembryonic antigen (CEA) and imaging are important. Ann Surg Oncol. 2013;20(2):457–63.
Portier G, Elias D, Bouche O, Rougier P, Bosset JF, Saric J, et al. Multicenter randomized trial of adjuvant fluorouracil and folinic acid compared with surgery alone after resection of colorectal liver metastases: FFCD ACHBTH AURC 9002 trial. J Clin Oncol. 2006;24(31):4976–82.
Mitry E, Fields AL, Bleiberg H, Labianca R, Portier G, Tu D, et al. Adjuvant chemotherapy after potentially curative resection of metastases from colorectal cancer: a pooled analysis of two randomized trials. J Clin Oncol. 2008;26(30):4906–11.
Ychou M, Hohenberger W, Thezenas S, Navarro M, Maurel J, Bokemeyer C, et al. A randomized phase III study comparing adjuvant 5-fluorouracil/folinic acid with FOLFIRI in patients following complete resection of liver metastases from colorectal cancer. Ann Oncol. 2009;20(12):1964–70.
Kanemitsu Y, Kato T, Shimizu Y, Inaba Y, Shimada Y, Nakamura K, et al. A randomized phase II/III trial comparing hepatectomy followed by mFOLFOX6 with hepatectomy alone as treatment for liver metastasis from colorectal cancer: Japan Clinical Oncology Group Study JCOG0603. Jpn J Clin Oncol. 2009;39(6):406–9.
Pita-Fernández S, Alhayek-Aí M, González-Martín C, López-Calviño B, Seoane-Pillado T, Pértega-Díaz S. Intensive follow-up strategies improve outcomes in nonmetastatic colorectal cancer patients after curative surgery: a systematic review and meta-analysis. Ann Oncol. 2015;26(4):644–56.
Tjandra JJ, Chan MK. Follow-up after curative resection of colorectal cancer: a meta-analysis. Dis Colon Rectum. 2007;50(11):1783–99.
Renehan AG, Egger M, Saunders MP, O’Dwyer ST. Impact on survival of intensive follow up after curative resection for colorectal cancer: systematic review and meta-analysis of randomised trials. BMJ. 2002;324(7341):813.
Primrose JN, Perera R, Gray A, Rose P, Fuller A, Corkhill A, et al. Effect of 3 to 5 years of scheduled CEA and CT follow-up to detect recurrence of colorectal cancer: the FACS randomized clinical trial. JAMA. 2014;311(3):263–70.
Van Cutsem E, Cervantes A, Nordlinger B, Arnold D, Group EGW. Metastatic colorectal cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2014;25(Suppl 3):1–9.
Acknowledgements
There were no financial support and funding for this study. We completed this study in collaboration with the following investigators: K. Hirata (Sapporo Medical University), A. Murata (Hirosaki University), K. Hatakeyama (Niigata University), K. Hase (National Defense Medical College), K. Kotake (Tochigi Cancer Center), T. Watanabe (Tokyo University), K. Takahashi (Tokyo Metropolitan Cancer and Infectious diseases Center Komagome Hospital), Y. Kanemitsu (National Cancer Center Hospital), S. Kameoka (Tokyo Women’s Medical University), H. Yano (National Center for Global Health and Medicine), K. Sugihara (Tokyo Medical and Dental University), H. Hasegawa (Keio University), Y. Hashiguchi (Teikyo University), T. Masaki (Kyorin University), M. Watanabe (Kitazato University), K. Maeda (Fujita Health University), K. Komori (Aichi Cancer Center Hospital), Y. Sakai (Kyoto University), M.Ohue (Osaka Medical Center for Cancer and Cardiovascular Diseases), K. Shirouzu (Kurume University).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
We have no potential conflicts of interest to disclose.
Electronic supplementary material
Below is the link to the electronic supplementary material.
595_2017_1530_MOESM1_ESM.tif
Supplementary material 1 (TIFF 1949 kb) Supplementary Fig. 1 Sensitivity and specificity of each carcinoembryonic antigen (CEA) variable: a Preoperative CEA; b Postoperative CEA; c Relative CEA
Rights and permissions
About this article
Cite this article
Okazaki, S., Baba, H., Iwata, N. et al. Carcinoembryonic antigen testing after curative liver resection for synchronous liver metastasis of colorectal cancer: a Japanese multicenter analysis. Surg Today 47, 1223–1229 (2017). https://doi.org/10.1007/s00595-017-1530-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00595-017-1530-x