Abstract
Purpose
Patients with hepatocellular carcinoma (HCC) and portal vein tumor thrombus (PVTT) invading the portal trunk (Vp4) are poor surgical candidates because of the technical difficulties involved. To overcome the limitations, we developed a technique of back-flow thrombectomy (BFT) based on the inherent portal hemodynamics and the macroscopic form of PVTT.
Methods
Forty-six patients with multiple HCC and Vp4 PVTT underwent hepatectomy with tumor thrombectomy. We used the BFT to treat 24 patients, 18 of whom had PVTT in the contralateral second portal branch. The form of PVTT was classified macroscopically into the floating and expansive types.
Results
The rate of complete removal by BFT of PVTT in the contralateral second portal branch was 89%. The patency rates at the thrombectomy site in all 46 patients and in the 24 BFT patients, 3 months after hepatectomy were 93 and 90%, respectively. The median OS of all 46 patients was 15 months, with 1- and 3-year OS rates of 58.5 and 17.1%, respectively. The median OS of the 24 patients treated with BFT vs. the 22 not treated with BFT was 14 and 15 months, respectively.
Conclusions
BFT can expand the therapeutic time window for patients with HCC and deep-seated PVTT and may improve their survival.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The incidence of hepatocellular carcinoma (HCC) developing secondary to chronic liver diseases is increasing worldwide. Although several factors contribute to the dismal prognosis associated with HCC, major vascular invasion, especially portal vein tumor thrombus (PVTT), represents one of the most important risk factors for widespread intra- and extra-hepatic diffusion of the disease [1, 2]. Furthermore, PVTT can extend into the main portal vein and aggravate portal vein hypertension, thus potentially causing a life-threatening sequence of events, such as massive ascites or variceal hemorrhage. These complications can hamper effective treatments. As a result, natural history data show that HCC with PVTT is uniformly fatal, with median survival ranging from only 2–3 months [3, 4].
The current European Association for the Study of the Liver (EASL)/American Association for the Study of the Liver (AASLD) guidelines [5] classify HCC with PVTT as Barcelona Clinical Liver Cancer (BCLC) stage C, and recommend systemic therapy with sorafenib based on the results of randomized controlled trials [6–8]. However, there is still controversy about treatment selection for patients with intermediate (BCLC stage B) and advanced (BCLC stage C) HCC. A recent large cohort study including more than 2000 HCC patients from ten hepatobiliary tertiary referral centers (three Asian centers, three American centers, and four European centers) found that about 50% of these patients underwent liver resection against EASL/AASLD guidelines [9]. Notably, more than 220 patients underwent liver resection, even though their disease was staged BCLC C, with a 38% 5-year survival rate. Most medical centers in Asian countries are not adherent to the EASL/AASLD guidelines, and believe that certain patients with BCLC stage C HCC could benefit from other treatment modalities rather than sorafenib monotherapy [10, 11]. These treatments include transcatheter arterial chemoembolization (TACE), hepatic arterial infusion chemotherapy, radiation therapy, and liver resection. Of these, liver resection has advantages because it can decrease portal vein pressure, improve liver function, and expand the therapeutic time window for further treatment because PVTT can also be mechanically removed.
PVTT within the ipsilateral portal branch can be resected easily, together with primary tumors, in an en bloc fashion at the time of a right or left hemi-hepatectomy. PVTT in the portal vein bifurcation or in the portal trunk is also resected easily using peeling-off techniques [12]. However, the clinical presentation of many HCC patients is complicated by more deep-seated PVTT at the time of diagnosis. The progression of PVTT beyond this stage remains a contraindication for surgical treatment in most countries. As a result, only 8.8% of Vp4 patients are treated with surgery in Japan [13].
To expand the indications for the surgical treatment of patients with HCC and PVTT, the development of a less invasive, simple and effective tumor thrombectomy technique for deep-seated PVTT is imperative. We propose a new macroscopic classification of PVTT, which has a high degree of relevance for planning thrombectomy. Based on this classification, we developed a new thrombectomy technique named “back-flow thrombectomy” (BFT). We describe the BFT procedure in detail and report the results of liver resection and thrombectomy performed using this new technique in HCC patients with PVTT invading the contralateral portal vein.
Materials and methods
Study population
Between January 2000 and December 2012, 461 patients with newly diagnosed HCC underwent liver resection at Kobe University Hospital. The subjects of this study were 46 patients with PVTT reaching either the portal trunk or the more distal contralateral portal branches (Vp4). All underwent PVTT removal at the time of liver resection. PVTT was identified by preoperative abdominal ultrasonography, computed tomography (CT), angiography, and intraoperative exploration. The diagnoses of HCC and PVTT were confirmed by histological examinations after surgery. We reviewed the clinical, pathological and preoperative laboratory data of all patients retrospectively.
Some surgical procedures not covered by health insurance, including percutaneous isolated hepatic perfusion (PIHP), were approved by the ethics committee at Kobe University Graduate School of Medicine (No. 54), and undertaken as medical treatments at the patient’s expense, under the authority of Kobe University Hospital. All patients gave written informed consent for the treatment.
HCC staging
HCC staging was done according to the Japan Liver Cancer Study Group Criteria, 5th edition [14], TNM classification [15], and the Barcelona Clinic Liver Cancer (BCLC) Group Criteria [16].
Macroscopic classification of PVTT
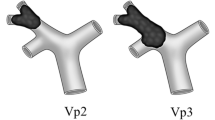
For this study, we used two macroscopic classifications of PVTT: one based on the extent of PVTT and one based on the form of PVTT. The extent of PVTT was assessed macroscopically according to the Japan Liver Cancer Study Group Criteria: Vp2 was considered to indicate PVTT with tips in the ipsilateral second portal branch; Vp3, to indicate PVTT with tips in the ipsilateral first portal branch; Vp4, to indicate PVTT with tips reaching either the portal trunk or the more distal contralateral portal branches.
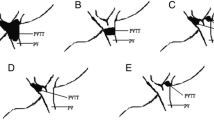
The type of PVTT was also classified macroscopically as either “floating” or “expansive” according to the new criteria described in this study (Fig. 1). The type of PVTT was decided at each point along the portal vein. Both types of PVTT may co-exist in the same individual. A portal vein with floating type PVTT maintains its original vascular caliber. This type of PVTT floats in the portal blood flow, which perfuses the distal part of the segment. It can be easily extracted by the BFT technique. On the other hand, the expansive type of PVTT is expanded and fixed in the portal vein and frequently extends into the tiny branches. Because of its expansive growth, the diameter of the portal vein becomes larger than the caliber of the original portal vein. Mechanical crushing is needed to extract this type of PVTT.
Classification of the macroscopic form of portal vein tumor thrombus (PVTT). PVTT in the right first branch and left first portal branch is defined as the “expansive” type. PVTT in the left second portal branch and the left third portal branch is defined as the “floating” type. The types of PVTT were determined at each point along the portal vein. Both types of PVTT can co-exist in the same individual. A portal vein with floating-type PVTT maintains its original vascular caliber. This type of PVTT is floating in the portal blood flow that perfuses the distal part of the segment. It can be easily extracted only by the BFT technique. On the other hand, the expansive type of PVTT is expanded and fixed in the portal vein. It frequently extends into the tiny branches of the portal vein. Because of its expansive growth, the diameter of the portal vein becomes much larger than the caliber of the original portal vein. Mechanical crushing is needed to extract this type of PVTT
Preoperative evaluations
All patients underwent hepatic and superior mesenteric angiographic CT scans to assess not only the intrahepatic tumor spread, but also the extent of PVTT. We also performed contrast-enhanced CT within a few days prior to liver resection routinely, to accurately visualize the tip and form of the PVTT.
Our selection criteria for liver resection for patients with HCC and Vp4 PVTT were based on liver function, the extent of fibrotic changes of the liver, the extent of PVTT, and the predicted liver remnant volume. The final decision was made at the time of laparotomy using intraoperative ultrasonography. These criteria included a fibrosis score of the non-cancerous liver ≤f3 as assessed by a zero-time fresh frozen biopsy, Child’s class A, a lack of esophageal varices, no ascites, a platelet count ≥ 100 × 106/mL, and an estimated remnant liver volume ≥35%. The indocyanine green retention rate at 15 min (ICGR 15) was used as a reference, because the values are profoundly affected by tumor-related portal circulatory disturbances in patients with major PVTT. In general, PVTT was considered resectable when its tip was the floating type, even in the contralateral third portal branches. In this situation, residual portal flow around the tip could be observed. On the other hand, an expansive PVTT was considered unresectable when its tip reached the contralateral third portal branches, indicating that the future remnant liver would be deprived of the portal blood flow.
Tumor thrombectomy procedures
In this study, we used two thrombectomy procedures for Vp4 PVTT, depending on the macroscopic extent and form of PVTT. One was the peeling-off technique, reported previously by Inoue et al. [12]. This technique was applied for PVTT in either the portal vein bifurcation or the contralateral shallow (proximal) first portal branch because it was conducted under direct vision using a pair of scissors. The other was the BFT technique, which we developed for deep-seated PVTT (Fig. 2). Usually, the BFT technique is used to remove PVTT in the contralateral distal first portal branch or second portal branch after removal of the PVTT from the portal trunk and contralateral shallow first portal branch using the peeling-off technique.
Schematic drawing of the back-flow thrombectomy (BFT) technique. The steps for performing the BFT technique for PVTT invading the contralateral (left) third portal branch when applied to a right hemihepatectomy are described in detail and shown in the video (Online Resource 1); a Hepatocellular carcinoma with a large main tumor in the right liver and portal vein tumor thrombus (PVTT) in the ipsilateral (right) first branch, contralateral first (left) branch, and contralateral second branch of the expansive type and a PVTT in the contralateral (left) third portal branch of the floating type. b Preparation for the BFT technique—the left portal vein, right portal vein and main portal trunk are fully exposed up to the bifurcation after routine cholecystectomy and division of the right hepatic artery. The portal trunk, including collaterals, is clamped at the superior border of the pancreas to stop the hepatopetal portal blood flow. c While clamping the portal trunk, the right portal branch is opened with a transverse venotomy at an appropriate site near the bifurcation. d The PVTT in the portal bifurcation and proximal contralateral (right) first portal branch is peeled off the portal intima using forceps. e, f The expansive PVTT in the distal contralateral (left) first portal branch and contralateral second portal branch are mechanically crushed and extracted by a suction device. g, h An expansive PVTT in the contralateral second portal branch (umbilical portion) that has been partially chipped away by the suction device, and a floating PVTT in the contralateral third portal branch are extracted by the retrograde blood flow of the portal vein under the conditions of BFT. (i) The opening of the right portal vein is closed using 6–0 monofilament nylon. The back-flow pressure in the portal system is maintained throughout the thrombectomy procedure to facilitate PVTT extraction. Peripheral portal vein clamping beyond the PVTT or the use of a Fogarty catheter is avoided to prevent migration of the PVTT into the future remnant liver
The BFT technique is a crushing, suctioning, and flashing method performed under the back-flow pressure of the portal system. These procedures were carried out consecutively and confirmed by intraoperative ultrasonic guidance. An expansive PVTT must be crushed to remove it because it is fixed to the portal vein wall by its expansive pressure. For this reason, expansive PVTT within reach of the suction device can be extracted by the BFT technique. Meanwhile, floating PVTT, even in the deeper portal branch, can be extracted by reversal of the portal blood flow alone.
Follow-up and assessments
Postoperative examinations and follow-up were uniform for all patients and performed by the same team of surgeons. The follow-up regimen included liver function tests every month during the first year and every 3 months thereafter. A contrast CT scan was performed within 2 weeks and 1 month after surgery, and every 3 months thereafter to check for residual tumor(s) and recurrence, and for portal vein patency. Success of thrombectomy was defined as evidence of the complete removal of thrombus from the thrombectomy site on the first contrast-enhanced CT scan after surgery. Patients with a residual tumor in the liver were treated with TACE or PIHP [17–21] within 3 months after the operation. PIHP was given as an adjuvant therapy if patients fulfilled the inclusion criteria for PIHP and chose medical treatment at their own expense. Best supportive care was given to patients with advanced disease, poor liver function, or poor general health status. Surgical morbidities were defined according to the Clavien–Dindo classification [22, 23]. Mortality was defined as death during the hospital stay after liver resection.
Data analysis
Statistical analysis was performed using the Statistical Package for the Social Sciences software program (SPSS software, version 2.0.0 for Mac, International Business Machines Corporation, Armonk, New York, USA). Survival rates were calculated by the Kaplan–Meier method and survival curves were compared using the log-rank test. A P value of <0.05 was considered significant.
Results
Patient characteristics
Table 1 summarizes the patient and tumor characteristics at the time of resection. Four of the 46 patients had uni-lobar lesions and the remaining 42 patients had bilobar tumors. Five patients had extrahepatic disease; as lung metastases (n = 3), lymph node metastases (n = 3), and adrenal metastasis (n = 1). These included multiple responses.
Liver resection, tumor spread and thrombectomy techniques
All 46 patients underwent major hepatectomies, including left lobectomy (n = 6), extended left lobectomy (n = 11; Fig. 3a, b), right lobectomy (n = 22; Fig. 3c, d) and extended right lobectomy (n = 7). Lymph node and adrenal metastases were also removed at the time of liver resection. No tumor was detected macroscopically in the remnant liver after hepatectomy in the four patients who had uni-lobar tumors. The mean operation time was 10.1 ± 2.4 h and the mean blood loss was 1965 ± 1229 ml.
Representative cases of hepatocellular carcinoma with portal vein tumor thrombus (PVTT) invading the contralateral second portal branch. a, b a case of HCC with PVTT invading the right portal vein from left lobe; a A contrast-enhanced computed tomography (CT) scan before the operation showed a large hepatocellular carcinoma in the left liver with an expansive PVTT that invaded the right portal vein as expansive type PVTT and into the posterior portal branch as floating-type PVTT (white arrow); b a contrast-enhanced CT scan after extended left hepatectomy and thrombectomy using the BFT technique showed the intact portal flow of the posterior branch (white arrow); c, d a case of HCC with PVTT invading the left portal vein from the right lobe; c Contrast-enhanced CT before the operation showed a large hepatocellular carcinoma in the right liver with an expansive PVTT that invaded the umbilical portion and a floating PVTT that invaded P2 (white arrow); d a contrast-enhanced CT scan after right hemi-hepatectomy and thrombectomy using the BFT technique showing intact portal flow in the umbilical portion and P2 (white arrow)
Seven patients were found to have extrahepatic metastases after hepatectomy. The recurrence sites were the lung (n = 5), lymph node (n = 3), bone (n = 2) and adrenal grand (n = 1); including multiple responses. In the contralateral first portal branch or portal trunk, 22 of the 46 patients had floating PVTT, while 24 had expansive PVTT. In the contralateral second portal branch, 9 patients had floating PVTT, while 9 had expansive PVTT. In the contralateral third portal branch, 5 patients had floating PVTT. This included multiple responses.
In 24 patients with PVTT invading the contralateral distal first portal branch or further, BFT was applied after removal of the PVTT from the contralateral proximal first portal branch by the peeling-off technique. The tips of the PVTT of these 24 patients were located in the contralateral distal first portal branch (n = 6), the contralateral second portal branch (n = 13), and the contralateral third portal branch (n = 5). In the remaining 22 patients, only the peeling-off technique was used for PVTT within the main portal vein or contralateral shallow (proximal) first portal branch.
Planned adjuvant therapy, including PIHP (n = 27) [17–21] or TACE (n = 2), for residual tumors in the remnant liver, was given to 29 patients with bilobar tumors. Adjuvant therapy was not given to 17 patients; at their request (n = 6, 13.0%), or because of inadequate liver function (n = 5, 10.9%), uncontrolled progressive disease (n = 4, 8.7%), tumors undetectable after hepatectomy (n = 4, 8.7%), extrahepatic metastases (n = 4, 8.7%), and the presence of another cancer (n = 1, 2.2%). This included multiple responses. Postoperative complications (Clavien–Dindo grade III or more) developed in 8 of the 46 patients (17.4%); as bile leakage (n = 5), ascites (n = 1), postoperative bleeding (n = 1), and pancreatic fistula (n = 1). Three patients died during hospitalization from post-hepatectomy liver failure (n = 1), bile leakage (n = 1), or pulmonary lymphangitis carcinomatosa (n = 1). The postoperative mortality rate was 6.5%.
Success rate of thrombectomy by BFT
Floating PVTTs were completely removed from the contralateral second portal branch by the BFT technique in all nine patients, with a success rate of 100%. Expansive PVTTs were completely removed from the contralateral second portal branch by the BFT technique in seven of the nine patients, with a success rate of 77.8%. In the remaining two patients, most PVTTs were removed and the portal blood flow was restored, but the intramural thrombus or tumor thrombus could not be removed. The success rate of thrombectomy for the five patients with floating PVTT in the contralateral third portal branch was 100%.
Portal vein patency at the thrombectomy site
The patency rates of the portal vein at the site of thrombectomy in the evaluable patients, 3 (n = 42) and 6 months (n = 34) after hepatectomy were 92.8 and 85.2%, respectively. The patency rates of the portal vein at the site of thrombectomy in the evaluable patients treated by BFT at 3 (n = 22) and 6 months (n = 17) after hepatectomy were 90.9 and 88.2%, respectively.
Overall survival
The median follow-up was 13 (range 1–119) months, and 6 of the 46 (13.0%) patients were alive at the last follow-up date. The median overall survival of the 46 patients was 15 months, and the actuarial 1-, 3-, and 5-year overall survival rates were 58.5, 17.9, and 12.8%, respectively. The median overall survival of 42 patients without extrahepatic metastases (Stage VI) was 17 months, and the actuarial 1-, 3-, and 5-year overall survival rates were 61.6, 19.7 and 14.1%, respectively.
The median overall survival of the 28 patients with PVTT within the contralateral first portal branch was 15 months, and the actuarial 1-, 3-, and 5-year overall survival rates were 53.6, 15.3 and 7.7%, respectively. The median overall survival of the 18 patients with PVTT extending into the contralateral second portal branch or further was 14 months, and the actuarial 1-, 3-, and 5-year overall survival rates were 66.7, 22.7 and 22.7%, respectively. There was no significant difference in overall survival rates between patients with PVTT within the contralateral first portal branch and patients with PVTT extending into the contralateral second portal branch or further.
The median overall survival of the 24 patients with BFT and the 22 patients without BFT were 14 and 15 months, respectively (Fig. 4). There was no difference in the overall survival curves between patients treated with vs. those not treated with BFT (p = 0.76). The median overall survival of the 29 patients who received planned adjuvant therapy was 18 months, and the actuarial 1-, 3-, and 5-year overall survival rates were 72.4, 21.8 and 14.5%, respectively. The median overall survival of the 17 patients who did not receive adjuvant therapy was only 4 months.
Discussion
The findings of the present study demonstrated that thrombectomy could be performed safely using the BFT technique, based on the macroscopic form of PVTT, in patients with deep-seated PVTT, even when the PVTT invaded the contralateral third portal branch. Moreover, regrowth of PVTT from the portal venous wall where it was attached was rarely found in the first 6 months after thrombectomy. Thus, thrombectomy using BFT expands the therapeutic window for patients with multiple bilobar HCC and Vp4 PVTT, who were previously considered untreatable.
Although the current recommended first-line treatment for patients with HCC and PVTT (BCLC stage C) is target therapy with sorafenib [6–8], accumulating data suggest that selected patients with this type of refractory HCC might benefit from surgical treatment [9–11, 24–30]. If radical resection of tumors and PVTT can be achieved macroscopically, the 3-year survival rates after complete resection range from 16 to 35%. An even better 3-year survival rate of 42% was reported with TACE followed by hepatectomy [26]. However, the subjects of those studies were patients with PVTT in the ipsilateral portal vein or the main portal trunk. For years, HCC patients with PVTT beyond this stage have been considered poor surgical candidates because there was no effective technique for safely removing deep-seated PVTT. Since introducing the BFT technique, we expanded the indications for surgical treatment to patients with PVTT in the contralateral second portal branch and possibly the third portal branch. As a result, 18 of the 46 patients in this study had PVTT in the contralateral second portal branch or third portal branch. To our knowledge, this is the first case series demonstrating surgical results for HCC patients with PVTT that has invaded the contralateral portal branch.
There are two classification systems for PVTT based on extent in the portal vein [14, 31]. These classifications allow us to predict the prognosis of patients with HCC and PVTT, but provide little information about planning for thrombectomy, especially in patients with PVTT invading the contralateral portal vein. According to these classifications, this type of refractory PVTT is categorized into the same stage. The new PVTT classification we propose has been established for the explicit purpose of performing thrombectomy for patients with PVTT invading the contralateral portal vein. In fact, we successfully removed the PVTT in approximately 90% of patients with PVTT in the contralateral second portal branch, using this new PVTT classification and the BFT technique.
Apart from the extent of PVTT, the presence of intrahepatic metastasis may also hamper the surgical treatment of patients with HCC and PVTT. Most surgeons regard the presence of PVTT and bilobar tumors as a contraindication to surgical treatment because curative resection cannot be performed even after hemi-hepatectomy and tumor thrombectomy. In contrast, 42 of the 46 patients in this study had multiple bilobar lesions at the time of liver resection. This is a fundamental difference between this study and previous studies [24–31] in which the patients were highly selected.
Our strategy is to resect the primary tumor and life-threatening PVTT, which are difficult to treat with loco-regional therapies that can be administered later to treat any residual tumor(s) in the remnant liver. Several investigators advocate the same strategy, which is generally called “reductive hepatectomy” or “mass reduction surgery” [32, 33] for multiple bilobar HCC, using TACE or transcatheter arterial infusion. In 2004, we reported encouraging results of reductive hepatectomy followed by PIHP for patients with multiple bilobar HCC [19]. After that initial report, we extended the use of this strategy to more aggressive HCC with major portal invasion [21]. Through this experience, we were able to investigate many surgical cases of HCC with Vp4 PVTT.
There are two types of surgical procedure used to resolve portal vein obstruction in patients with HCC and PVTT extending into the contralateral portal vein at the time of hemi-hepatectomy. One is tumor thrombectomy and the other is an en bloc technique involving resection of the tumor thrombus together with the portal vein wall [12]. Oncologically, the en bloc technique is better because it can reduce the potential risk of local recurrence at the thrombectomy site; however, we did not use this technique in the present study because the en bloc technique is difficult to perform in patients with PVTT invading the contralateral second portal branch. It is also a complicated procedure, requiring portal vein reconstruction, and sometimes leads to greater loss of liver parenchyma, blood loss, and a long operation time. The postoperative outcomes of patients undergoing this technique have been unsatisfactory, with higher mortality and morbidity rates than those after hepatectomy for HCC without PVTT [27, 34].
There are currently two tumor thrombectomy techniques for PVTT. One is the peeling-off technique [12] and the other is catheter thrombectomy. The peeling-off technique was selected for patients with PVTT within the main portal vein or contralateral shallow (proximal) first portal branch because it is performed under direct vision using a pair of scissors [12]. This makes it difficult to use for deep-seated PVTT. In fact, in Inoue’s pivotal study of the peeling-off technique, 6 of 49 patients had PVTT invading the main portal branch or contralateral branch. We speculate that these six patients did not have PVTT invading the contralateral distal first portal branch or second portal branch for the reasons given. The peeling-off technique has an oncological advantage over catheter thrombectomy because it makes it possible to check macroscopically for any residual PVTT attached to the portal vein wall or in the tiny portal branches by eversion of the portal vein wall. While catheter thrombectomy could theoretically be applied for a deep-seated PVTT, it is generally not good enough to allow complete removal of the PVTT and in fact, may increase the risk of dislodgement of the crushed piece of tumor thrombus, with resultant hepatic infarction.
To overcome the disadvantages of the previous thrombectomy methods, we developed the BFT technique to extract deep-seated PVTT. The BFT technique is a crushing, suctioning, and flashing method based on the inherent hemodynamics of the liver and the macroscopic form of PVTT. It minimizes the potential risk of migration of the floating PVTT into the future remnant liver, and allows for effective extraction of both micro- and macroscopic cancer nests liberated into the blood stream during procedures. In some previous reports [12, 26, 30, 35] on surgical treatment for PVTT, crushing, suctioning, or flashing procedures were mentioned as a part of their thrombectomy procedures. However, these reports included few patients with PVTT invading the contralateral portal branch. Therefore, the extraction limit of PVTT and the removal efficiency of their procedures were not fully elucidated. In this study, we theoretically optimized these procedures to extract deep-seated PVTT as the BFT technique and answered the above questions.
One of the potential drawbacks of the BFP technique is the risk of cancer cell residue on the portal venous wall where the PVTT is attached, because the portal venous wall cannot be directly checked during the procedure. Our aim, but not expection, is to extract all cancer cells by the BFT technique. There is still a possibility that there may be rapid regrowth of the PVTT from a residual lesion, which may again obstruct the portal venous flow shortly after thrombectomy. However, regrowth of the PVTT was rarely observed for at least 6 months after thrombectomy. Although we have no definitive answer as to why the residual cancer cells on the portal vein wall did not regrow rapidly after thrombectomy, this observation is in agreement with the findings of a previous report [12]. Another drawback of the BFP technique is its limited application for organized thrombus, which is too hard to be crushed by forceps or a suction device, and requires the peeling-off technique to be extracted. Fortunately, PVTT in the contralateral second portal branch or more, which is a good target for the BFT, is rarely of the organized type because of its rapid growth.
The main limitation of this study is that it was a retrospective single-arm analysis performed at one center. Therefore, it could not provide direct evidence of any survival benefit of our surgical multidisciplinary approach. The median survival time of 15 months in this series is superior to those reported in subgroup analyses of studies on sorafenib [8], TACE [36, 37], some surgical studies [30, 38, 39], and a Japanese nationwide survey over 9.1 years [40], but was inferior to those of other surgical studies [12, 26] on patients with HCC and PVTT. Caution must be exercised when interpreting these results, because there was substantial heterogeneity among the studies in terms of inclusion criteria, liver function, intrahepatic metastasis and extent of PVTT. Our patients did not meet the inclusion criteria of the previous surgical reports, [24–31] as these studies did not include patients with PVTT that invaded the contralateral portal branch, with or without bilobar metastasis. Our study and the subgroup of the sorafenib study [8] had similar patient populations, with Child A patients and patients with major vascular invasion; however, there is still a selection bias between the studies. Our patients may have had better liver function than those in the sorafenib study, because our patients could tolerate hemi-hepatectomy. To identify the best treatment for patients with HCC and deep-seated PVTT, a prospective randomized study of different treatment modalities should be undertaken.
In conclusion, the BFT technique enabled us to perform thrombectomy even for patients with PVTT that invaded the contralateral third portal branch, and a high patency rate of the portal vein at the thrombectomy site was noted for at least 6 months after hepatectomy. Thus, the BFT technique can expand the therapeutic time window for adjuvant treatment, and may improve the survival of patients with HCC and deep-seated PVTT.
References
Okuda K, Ohtsuki T, Obata H, Tomimatsu M, Okazaki N, Hasegawa H, et al. Natural history of hepatocellular carcinoma and prognosis in relation to treatment. Study of 850 patients. Cancer. 1985;56(4):918–28.
Park KW, Park JW, Choi JI, Kim TH, Kim SH, Park HS, et al. Survival analysis of 904 patients with hepatocellular carcinoma in a hepatitis B virus-endemic area. J Gastroenterol Hepatol. 2008;23(3):467–73.
Llovet JM, Bustamante J, Castells A, Vilana R, Ayuso Mdel C, Sala M, et al. Natural history of untreated nonsurgical hepatocellular carcinoma: rationale for the design and evaluation of therapeutic trials. Hepatology. 1999;29(1):62–7.
Villa E, Moles A, Ferretti I, Buttafoco P, Grottola A, Del Buono M, et al. Natural history of inoperable hepatocellular carcinoma: estrogen receptors’ status in the tumor is the strongest prognostic factor for survival. Hepatology. 2000;32(2):233–8.
Bruix J, Sherman M, American Association for the Study of Liver D. Management of hepatocellular carcinoma: an update. Hepatology. 2011;53(3):1020–2.
Llovet JM, Ricci S, Mazzaferro V, Hilgard P, Gane E, Blanc JF, et al. Sorafenib in advanced hepatocellular carcinoma. N Engl J Med. 2008;359(4):378–90.
Cheng AL, Guan Z, Chen Z, Tsao CJ, Qin S, Kim JS, et al. Efficacy and safety of sorafenib in patients with advanced hepatocellular carcinoma according to baseline status: subset analyses of the phase III Sorafenib Asia-Pacific trial. Eur J Cancer. 2012;48(10):1452–65.
Bruix J, Raoul JL, Sherman M, Mazzaferro V, Bolondi L, Craxi A, et al. Efficacy and safety of sorafenib in patients with advanced hepatocellular carcinoma: subanalyses of a phase III trial. J Hepatol. 2012;57(4):821–9.
Torzilli G, Belghiti J, Kokudo N, Takayama T, Capussotti L, Nuzzo G, et al. A snapshot of the effective indications and results of surgery for hepatocellular carcinoma in tertiary referral centers: is it adherent to the EASL/AASLD recommendations?: an observational study of the HCC East-West study group. Ann Surg. 2013;257(5):929–37.
Wang JH, Changchien CS, Hu TH, Lee CM, Kee KM, Lin CY, et al. The efficacy of treatment schedules according to Barcelona Clinic Liver Cancer staging for hepatocellular carcinoma—Survival analysis of 3892 patients. Eur J Cancer. 2008;44(7):1000–6.
Changchien CS, Chen CL, Yen YH, Wang JH, Hu TH, Lee CM, et al. Analysis of 6381 hepatocellular carcinoma patients in southern Taiwan: prognostic features, treatment outcome, and survival. J Gastroenterol. 2008;43(2):159–70.
Inoue Y, Hasegawa K, Ishizawa T, Aoki T, Sano K, Beck Y, et al. Is there any difference in survival according to the portal tumor thrombectomy method in patients with hepatocellular carcinoma? Surgery. 2009;145(1):9–19.
Ikai I, Kudo M, Arii S, Omata M, Kojiro M, Sakamoto M, et al. Report of the 18th follow-up survey of primary liver cancer in Japan. Hepatol Res Off J Jpn Soc Hepatol. 2010;40:1043–59.
General rules for the clinical and pathological study of primary liver cancer 3rd English editioned: Kanehara; 2010.
TNM classification of malignant tumours International Union Against Cancer. 7th ed. Oxford: Wiley-Blackwell; 2009.
Llovet JM, Bru C, Bruix J. Prognosis of hepatocellular carcinoma: the BCLC staging classification. Semin Liver Dis. 1999;19(3):329–38.
Ku Y, Fukumoto T, Tominaga M, Iwasaki T, Maeda I, Kusunoki N, et al. Single catheter technique of hepatic venous isolation and extracorporeal charcoal hemoperfusion for malignant liver tumors. Am J Surg. 1997;173(2):103–9.
Ku Y, Iwasaki T, Fukumoto T, Tominaga M, Muramatsu S, Kusunoki N, et al. Induction of long-term remission in advanced hepatocellular carcinoma with percutaneous isolated liver chemoperfusion. Ann Surg. 1998;227(4):519–26.
Ku Y, Iwasaki T, Tominaga M, Fukumoto T, Takahashi T, Kido M, et al. Reductive surgery plus percutaneous isolated hepatic perfusion for multiple advanced hepatocellular carcinoma. Ann Surg. 2004;239(1):53–60.
Ku Y, Fukumoto T, Iwasaki T, Tominaga M, Samizo M, Nishida T, et al. Clinical pilot study on high-dose intraarterial chemotherapy with direct hemoperfusion under hepatic venous isolation in patients with advanced hepatocellular carcinoma. Surgery. 1995;117(5):510–9.
Fukumoto T, Tominaga M, Kido M, Takebe A, Tanaka M, Kuramitsu K, et al. Long-term outcomes and prognostic factors with reductive hepatectomy and sequential percutaneous isolated hepatic perfusion for multiple bilobar hepatocellular carcinoma. Ann Surg Oncol. 2014;21(3):971–8.
Dindo D, Demartines N, Clavien PA. Classification of surgical complications: a new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann Surg. 2004;240(2):205–13.
Katayama H, Kurokawa Y, Nakamura K, Ito H, Kanemitsu Y, Masuda N, et al. Extended Clavien-Dindo classification of surgical complications: Japan Clinical Oncology Group postoperative complications criteria. Surg Today. 2016;46(6):668–85.
Zhou J, Tang ZY, Wu ZQ, Zhou XD, Ma ZC, Tan CJ, et al. Factors influencing survival in hepatocellular carcinoma patients with macroscopic portal vein tumor thrombosis after surgery, with special reference to time dependency: a single-center experience of 381 cases. Hepato-gastroenterology 2006;53(68):275–80.
Ohkubo T, Yamamoto J, Sugawara Y, Shimada K, Yamasaki S, Makuuchi M, et al. Surgical results for hepatocellular carcinoma with macroscopic portal vein tumor thrombosis. J Am Coll Surg. 2000;191(6):657–60.
Minagawa M, Makuuchi M, Takayama T, Ohtomo K. Selection criteria for hepatectomy in patients with hepatocellular carcinoma and portal vein tumor thrombus. Ann Surg. 2001;233(3):379–84.
Tanaka A, Morimoto T, Yamaoka Y. Implications of surgical treatment for advanced hepatocellular carcinoma with tumor thrombi in the portal vein. Hepato-gastroenterology 1996;43(9):637–43.
Konishi M, Ryu M, Kinoshita T, Inoue K. Surgical treatment of hepatocellular carcinoma with direct removal of the tumor thrombus in the main portal vein. Hepato-gastroenterology 2001;48(41):1421–4.
Ikai I, Hatano E, Hasegawa S, Fujii H, Taura K, Uyama N, et al. Prognostic index for patients with hepatocellular carcinoma combined with tumor thrombosis in the major portal vein. J Am Coll Surg. 2006;202(3):431–8.
Pawlik TM, Poon RT, Abdalla EK, Ikai I, Nagorney DM, Belghiti J, et al. Hepatectomy for hepatocellular carcinoma with major portal or hepatic vein invasion: results of a multicenter study. Surgery. 2005;137(4):403–10.
Shi J, Lai EC, Li N, Guo WX, Xue J, Lau WY, et al. A new classification for hepatocellular carcinoma with portal vein tumor thrombus. J Hepato biliary Pancreat Sci. 2011;18(1):74–80.
Yamamoto K, Takenaka K, Kawahara N, Shimada M, Shirabe K, Itasaka H, et al. Indications for palliative reduction surgery in advanced hepatocellular carcinoma. The use of a remnant tumor index. Arch Surg. 1997;132(2):120–3.
Wakabayashi H, Ushiyama T, Ishimura K, Izuishi K, Karasawa Y, Masaki T, et al. Significance of reduction surgery in multidisciplinary treatment of advanced hepatocellular carcinoma with multiple intrahepatic lesions. J Surg Oncol. 2003;82(2):98–103.
Yamaoka Y, Kumada K, Ino K, Takayasu T, Shimahara Y, Mori K, et al. Liver resection for hepatocellular carcinoma (HCC) with direct removal of tumor thrombi in the main portal vein. World J Surg. 1992;16(6):1172–6. (discussion 7).
Kumada K, Ozawa K, Okamoto R, Takayasu T, Yamaguchi M, Yamamoto Y, et al. Hepatic resection for advanced hepatocellular carcinoma with removal of portal vein tumor thrombi. Surgery. 1990;108(5):821–7.
Chung JW, Park JH, Han JK, Choi BI, Han MC. Hepatocellular carcinoma and portal vein invasion: results of treatment with transcatheter oily chemoembolization. AJR Am J Roentgenol. 1995;165(2):315–21.
Kim KM, Kim JH, Park IS, Ko GY, Yoon HK, Sung KB, et al. Reappraisal of repeated transarterial chemoembolization in the treatment of hepatocellular carcinoma with portal vein invasion. J Gastroenterol Hepatol. 2009;24(5):806–14.
Roayaie S, Jibara G, Taouli B, Schwartz M. Resection of hepatocellular carcinoma with macroscopic vascular invasion. Ann Surg Oncol. 2013;20(12):3754–60.
Tang QH, Li AJ, Yang GM, Lai EC, Zhou WP, Jiang ZH, et al. Surgical resection versus conformal radiotherapy combined with TACE for resectable hepatocellular carcinoma with portal vein tumor thrombus: a comparative study. World J Surg. 2013;37(6):1362–70.
Kokudo T, Hasegawa K, Matsuyama Y, Takayama T, Izumi N, Kadoya M, et al. Survival benefit of liver resection for hepatocellular carcinoma associated with portal vein invasion. J Hepatol. 2016;65(5):938–43.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
We have no conflicts of interest to declare.
Rights and permissions
About this article
Cite this article
Fukumoto, T., Kido, M., Takebe, A. et al. New macroscopic classification and back-flow thrombectomy for advanced hepatocellular carcinoma with portal vein tumor thrombus invading the contralateral second portal branch. Surg Today 47, 1094–1103 (2017). https://doi.org/10.1007/s00595-017-1507-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00595-017-1507-9