Abstract
Surgery had been and remains a mainstay in the treatment of gastric cancer. The Japanese surgical oncologists employed surgery-first approach to treat gastric cancer because of the widespread use of D2 lymph node dissection and the high incidence of oncologically resectable cancer, and early attempts at the multimodality treatment strategy featured surgery followed by postoperative chemotherapy. Although evidence to treat Stage II/III gastric cancer with this strategy is now abundant in the Far East, poor compliance of the post-gastrectomy patients to intense combination chemotherapies has been a limitation associated with this strategy. Evidence in support of neoadjuvant chemotherapy in the West and in various other types of cancer prompted the Japan Clinical Oncology Group (JCOG) researchers to explore this strategy, primarily for a selected population of locally advanced cancer that could either be unresectable by the surgery-first approach or is known to suffer from a poor prognosis; cancers with bulky lymph node metastases or those with a scirrhous phenotype. Encouraged by some promising results from these neoadjuvant trials and taking into account the aforementioned limitations associated with postoperative chemotherapy, the JCOG researchers decided to embark on a phase III trial to explore neoadjuvant chemotherapy among patients with clinically Stage III cancer. This review describes the development of the neoadjuvant strategy for gastric cancer in Japan, mainly by going through a series of clinical trials conducted by the JCOG.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Surgery remains a mainstay in the treatment of gastric cancer. However, a surgeon can dissect little more than what can be recognizable as cancer, and is incapable of seeing or coping with micrometastases and isolated tumor cells that may be scattered before or during surgery. On the other hand, several experimental studies and clinical trials to show the benefits of adjuvant chemotherapy suggest that micrometastases could be vulnerable to chemotherapy, whereas tumors that have grown to be macroscopically recognizable are no longer curable [1, 2].
Due to an abundance of advanced, but technically resectable gastric cancer, the lack of anticancer drugs with high objective response rates, and the relatively favorable prognosis achieved by surgery alone, the Japanese investigators have for a long time preferred surgery-first strategy and were reluctant to administer preoperative treatment for fear of disease progression during the treatment period. Consequently, they were late to join the global trend of exploring neoadjuvant or perioperative chemotherapy.
This review describes how neoadjuvant chemotherapy was first introduced into the treatment of advanced gastric cancer in Japan, and through what process, the concept came to be acknowledged as potentially applicable even for resectable gastric cancer.
Brief history of adjuvant therapy for gastric cancer
Several bodies of evidence both from the West and the East currently recommend that some form of adjuvant therapy should be delivered in addition to surgery in patients with potentially curable advanced gastric cancer. Despite long standing efforts, the Japanese were not the first to generate evidence in this regard. The administration of intravenous mitomycin and/or oral fluoropyrimidines became the community standard in Japan after the positive result of a phase III trial conducted between 1964 and 1973 with surgery alone as a control [3]. However, what had been considered as a pivotal study was later found to be of insufficient quality to merit international recognition. Consequently, all phase III trial evidences accumulated in the 1970s and 80s in Japan lost value, since they had been conducted with an assumption that postoperative adjuvant chemotherapy with intravenous mitomycin is effective and can serve as a control arm. Meanwhile, postoperative adjuvant chemotherapy with mitomycin as a key drug continued to be administered outside of clinical trials throughout the country. In the late 1980s, the Japan Clinical Oncology Group (JCOG), a study group funded by the government and participated by a limited number of centers of excellence, restarted randomized adjuvant chemotherapy trials with surgery alone as a control. Unfortunately, first few attempts by the JCOG failed to prove a survival benefit of chemotherapy arguably due to the inadequate eligibility criteria [4], small sample size [5], and lack of effective anticancer drugs or poor compliance to the chemotherapy [6]. Thus, a long and fruitless period persisted in Japan during which the standard treatment after R0 resection for advanced gastric cancer had been observation alone in centers of excellence, while a large proportion of patients treated in community hospitals were still given intravenous mitomycin.
In the meantime, the first evidence that proved a superiority in overall survival of adding adjuvant therapy over treatment with surgery alone was reported from the USA (Intergroup 0116 study) [7]. The treatment strategy explored in that study was postoperative chemoradiotherapy, and the median overall survival was 35 months for chemoradiotherapy and 26 months for surgery alone [hazard ratio (HR): 0.76; 95% confidence interval (CI): 0.62–0.93, P = 0.005]. Fifty-four % of patients underwent less than D1 dissection, and D2 dissection was performed only in 10% of the patients. Local recurrence was observed in 51 of 177 patients with relapse (29%) when treated by surgery alone, whereas chemoradiation reduced the incidence to 23 out of 120 (19%). The next evidence in support of adjuvant therapy emerged from the UK (the MAGIC trial) and took the form of intensive perioperative chemotherapy (three cycles of a combination therapy with epirubicin (50 mg/m2 on day 1), cisplatin (60 mg/m2 on day 1), and infusional 5FU (200 mg/m2/day for 21 days) given before and after surgery) [8]. The 5-year survival rate was 36% for patients treated by perioperative chemotherapy and 23% for those treated by surgery alone (HR: 0.75; 95% CI 0.60–0.93, P = 0.009). The survival of patients in the surgery alone arms in both these trials had been dismal, thus implying that gastric cancer in the Western countries was often found as more advanced disease compared with those in the Far East, and was often treated by D0 or D1 dissection which is suboptimal at least by the Asian standards. In Japan, a screening program has enabled detection of gastric cancer at a less advanced stage. In addition, the routine use of extended lymphadenectomy after more elaborate verification of the resection margin through meticulous preoperative endoscopy and intraoperative frozen sections has resulted in a low incidence of locoregional recurrence. Adjuvant therapy in that setting would need to focus on the management of distant micrometastases rather than locoregional residual disease, and chemoradiation would seem unlikely to meet this requirement. As for the neoadjuvant strategy, a lack of effective drug combinations at the time led the Japanese surgeons to adhere to the surgery-first strategy for cancer that is technically resectable.
The first hard evidence in support of the postoperative adjuvant strategy was reported from the ACTS-GC study conducted in Japan in which a treatment with S-1, an oral fluoropyrimidine (40 mg/mg2 twice daily on days 1–28 every 42 days, to be continued for 12 months) was found to improve the overall survival of Stage II/III gastric cancer (HR for death: 0.669; 95% CI 0.540–0.828, P = 0.003) [9]. This was followed by another phase III trial conducted by Korea and other countries in the Far East which proved survival benefit over surgery alone in Stage II/III gastric cancer patients of a combination of oral capecitabine (1000 mg/m2 twice daily on days 1–14 every 21 days) and intravenous oxaliplatin (130 mg/m2 on day 1) given postoperatively for 6 months (HR for death: 0.66; 95% CI 0.51–0.85, P = 0.0015) [10]. Although whether to use S-1 or the capecitabine/oxaliplatin combination remains an unsolved issue, the fact that similar results with almost identical hazard ratios were obtained from the two independently conducted phase III trials indicates that the benefit of postoperative chemotherapy following D2 dissection is now substantial for patients with advanced gastric cancer treated with D2 dissection.
Rationale for the neoadjuvant chemotherapy
In the 1990s, a greater number of cases with marked tumor shrinkage began to be witnessed in metastatic gastric cancer through advent of chemotherapeutic regimens using various combinations of modern antineoplastic agents [11] when compared with the era of 5FU and mitomycin. This fact and experience in other types of cancer prompted investigators to explore the concept of delivering chemotherapy preoperatively for gastric cancer [12]. The ultimate aim of neoadjuvant chemotherapy is to achieve an improvement in the long-term survival rate rather than the tumor shrinkage per se. Given that chemotherapy is usually incapable of curing bulky solid cancer, an improvement in survival could in theory be achieved only through exterminating micrometastases, so that resection of the remaining macroscopic lesions is sufficient for obtaining a cure. Accordingly, the target of the neoadjuvant chemotherapy, after all, is the same as that of postoperative adjuvant chemotherapy with the exception of relatively infrequent situations in which marked tumor shrinkage is mandatory for macroscopically complete resection.
The MAGIC trial, a UK-based phase III trial in which the superiority of perioperative chemotherapy over treatment with surgery alone was proven, is arguably the first evidence in support of neoadjuvant chemotherapy [8]. Actually, only 55% of the patients assigned to the perioperative chemotherapy group subsequently began to receive postoperative chemotherapy, suggesting that preoperative chemotherapy was the chief driving force behind success of this multimodality treatment strategy. There was another European study exploring perioperative chemotherapy in which a combination of 5FU and cisplatin was used [13]. This study generated further evidence in support of perioperative chemotherapy over surgery alone, with the 5-year overall survival rate of 38% in the chemotherapy group versus 24% in the surgery alone group (HR: 0.69; 95% CI 0.48–0.89, P = 0.003). Again, the completion rate of the whole perioperative chemotherapy was only 48%, whereas the completion rate of preoperative chemotherapy phase was 97%, implicating greater role of the neoadjuvant or preoperative chemotherapy. The potential benefit of delivering neoadjuvant chemotherapy could be summarized as follows: (1) chemotherapy-induced tumor downstaging may enhance resectability; (2) patients receive systemic chemotherapy without waiting for surgery and postoperative recovery; (3) treatment is administered while there is measurable disease present to assess response; and (4) during preoperative chemotherapy, patients with rapidly progressive disease can be identified and spared a futile gastrectomy [12].
Evolution of the neoadjuvant strategy in Japan
Looking from another angle, the last sentence in the previous paragraph could be rewritten as follows and reflect weakness of this strategy: during preoperative chemotherapy, resectable disease could progress to unresectable/metastatic disease, thus depriving the patient of a chance to be cured. Since surgery had for a long time been the only treatment modality to cure gastric cancer, and a relatively low proportion of advanced cancer with no distant metastasis was actually found to be technically unresectable, the Japanese surgeons took this weakness rather seriously and spared neoadjuvant chemotherapy for specific subsets of patients who either needed tumor shrinkage for R0 resection or those who were known to suffer from an extremely poor prognosis even after R0 resection. Accordingly, the JCOG investigators decided to explore neoadjuvant chemotherapy in the following two subsets: (1) gastric cancer with “bulky N” status and (2) Borrmann type IV and large type III gastric cancer.
Neoadjuvant strategy for the “bulky N” disease
A bulky N2 subset defined by the JCOG consisted of patients with at least three second tier lymph nodes measuring ≥1.5 cm or a bulky mass measuring ≥3 cm that consists of two or more second tier lymph nodes. For this subgroup of patients, surgery could be challenging and tumor shrinkage may serve to facilitate resection. On the other hand, cancer with metastatic lymph node in the para-aortic region is classified into Stage IV and is usually considered oncologically unresectable. In addition, the prognostic value of prophylactic super-extended lymph node dissection that includes systemic dissection of the para-aortic nodes (No. 16b1 and No. 16a2) among patients without lymphadenopathy in those regions had already been denied by a randomized phase III trial [14]. However, past retrospective studies have shown that some long-term survivors do exist among patients who underwent treatment consisting of para-aortic LN dissection and postoperative adjuvant chemotherapy and were found histopathologically to harbor metastases in the para-aortic region [15, 16]. Consequently, the JCOG decided to explore neoadjuvant chemotherapy with a mixed population of patients either with at least one enlarged (≥1 cm) lymph node in the No. 16b1–No. 16a2 regions (N3) or with the bulky N2 status, collectively termed “bulky N”. A series of phase II trials was conducted for this population, testing a strategy of 2–3 courses of the most promising combination chemotherapy at each time point followed by D2 plus para-aortic lymph node dissection (Table 1). The multimodality treatment was delivered only after confirming the P0 (no peritoneal seeding)/CY0 (negative for the peritoneal washing cytology) status by staging laparoscopy.
A combination of irinotecan (70 mg/m2 on days 1 and 15, every 28 days) and cisplatin (80 mg/m2 on day 1) [17] was used in the first of these attempts (JCOG0001). In this trial, barium swallow, endoscopy and computerized tomography (CT) had to be conducted after every cycle to evaluate the response, so that a patient with any sign of progressive disease in the locoregional region could immediately proceed to salvage surgery. Such considerations reflect the extremely discreet attitude taken by surgical oncologists at the time when deciding not to employ the more familiar surgery-first approach. Two cycles of irinotecan/cisplatin were scheduled, and another cycle was allowed only when further tumor shrinkage was considered preferable for R0 resection. Although the planned sample size was 60, the trial was closed prematurely after enrolment of 55 cases when the third case of treatment-related death ascertained that the mortality rate would exceed the preplanned safety limit of 5%. Although the trial thus failed to prove the safety of the strategy using this combination therapy, the clinical response rate and R0 resection rate were promising at 55 and 65%, respectively. Moreover, the 3-year survival rate was 27%, and the lower limit of the 95% CI at 15.2% barely exceeded the threshold of 15%.
A combination of S-1 (40 mg/m2 twice daily for days 1–21 of a 28-day cycle) and cisplatin (60 mg/m2 on day 1), which eventually became the standard in the first-line treatment of advanced/metastatic gastric cancer in Japan, was the regimen explored in the second JCOG attempt to treat the “bulky N” population with neoadjuvant chemotherapy (JCOG0405) [18]. Fifty-three patients were accrued, and, this time, no treatment-related death was observed. The R0 resection rate was 82% and the 3-year and 5-year survival rates were unexpectedly high at 59 and 53%, respectively. Surgical morbidity observed in this trial (pancreatic fistula 20%, leakage 6%) was higher than that in a JCOG phase III trial in which para-aortic lymph node dissection was conducted [14], most likely due to more frequent application of splenectomy (55 vs 37%) and pancreatectomy (10 vs 5%). Due to the overwhelmingly prolonged OS when compared with data from the previous phase II study and historical data from the participating institutions, neoadjuvant chemotherapy with S-1 and CDDP followed by radical surgery is now considered the tentative standard of care in this specific population [19].
JCOG1002, the most recent attempt in the series of neoadjuvant trial for the “bulky N” disease, explored the DCS regimen, a triplet containing docetaxel (40 mg/m2 on day 1), cisplatin (60 mg/m2 on day 1), and S-1 (40 mg/m2 twice daily for days 1–14 of a 28-day cycle), with a response rate as a primary endpoint [20]. Unfortunately, the response rate at 58% did not reach the expected value of 80%, and S-1/CDDP will remain the current standard. Since JCOG1002 was the only neoadjuvant trial for bulky N disease that was conducted after the results of the ACTS-GC trial [9] were published, it became the only trial among this series of trials to incorporate postoperative adjuvant chemotherapy with S-1. Whether the postoperative chemotherapy confers any additional survival benefit to neoadjuvant chemotherapy alone remains unknown, and the long-term follow-up data of this trial are awaited for comparison with those of the previous trials. One could argue that the JCOG1002 actually explored perioperative chemotherapy strategy in which the combination regimen used in the neoadjuvant phase was replaced by an evidence-based postoperative monotherapy to cope with the well-documented poor treatment compliance during the postoperative phase [21, 22]. In fact, whether the postoperative chemotherapy is necessary after neoadjuvant chemotherapy followed by R0 resection is one of important clinical questions in the era of neoadjuvant chemotherapy that may remain unanswered for some time.
Neoadjuvant strategy for scirrhous-type gastric cancer
Scirrhous-type gastric cancer has been feared particularly for its propensity to develop into peritoneal carcinomatosis. Based on in-house data at National Cancer Center Tokyo that Borrmann type III cancer with >8 cm diameter suffer from dismal prognosis similar to Borrmann type IV cancer, the concept of “Borrmann type IV and large type III cancer” was eventually established as a category representing the scirrhous-type gastric cancer and it was rendered eligible for another series of JCOG neoadjuvant chemotherapy trials.
After a single institution phase II trial at National Cancer East in which neoadjuvant chemotherapy with sequential high-dose methotrexate and fluorouracil combined with doxorubicin (FAMTX) was found to have no survival benefits for the scirrhous-type (mostly Borrmann type IV) cancer [23], Kinoshita et al. proposed another neoadjuvant study with the same population using S-1, an upcoming new drug at the time, and proceeded to conduct the study as a multi-institutional trial to accelerate the patient accrual. Patients with the scirrhous-type gastric cancer who were deemed resectable by a series of preoperative examinations that included staging laparoscopy were eligible. Patients who were positive for the peritoneal lavage cytology in the absence of macroscopic peritoneal deposits were also eligible for this trial. Two cycles of single agent S-1 (40 mg/m2 twice daily for days 1–28 of a 42-day cycle) was tolerated by 52 of 55 patients, 52 patients underwent laparotomy, and R0 resection was achieved in 36 patients with acceptable morbidity and no mortality. Unfortunately, the 2-year survival rate, the primary endpoint, was 59% and was marginally below the expected level at 60%. In addition, the R0 resection rate was not higher than the historical control [24].
In the subsequent attempt (JCOG0210), the feasibility of the combination of S-1 and cisplatin, identical with the regimen that was explored with the bulky N disease (JCOG0405), was tested [25]. The aforementioned concept of “Borrmann type IV and large type III cancer” was actually established while planning for this trial, so as to expand the target population. Staging laparoscopy was not mandated in this trial. The primary endpoint was completion of the protocol treatment that included two cycles of S-1/cisplatin followed by R0/R1 resection (R1 resection was counted only when it was due only to positivity in the peritoneal lavage cytology) by D2 dissection, and 50 patients were deemed necessary by SWOG’s two-stage design based on the expected completion rate of 60% and the threshold of 45%. Six of 49 eligible patients failed to complete two cycles of the treatment due to disease progression in 2, adverse events in 3, including one treatment-related death, and patient refusal in one. Of the 47 patients who underwent surgery, R0 resection was performed in 31, R1 resection due to positive cytology status in 6, and R2 resection in 10. These added up to R0/R1 resection rate of 75.5% (95% CI 61.1–86.7%, 37 out of 49). The primary endpoint was met, since 36 patients (73.5%; 95% CI 63.7–81.7%) had completed two cycles of neoadjuvant chemotherapy before achieving R0/R1 resection. No surgical mortality was reported. The median survival time of all 49 patients was 17.3 months, and the 3-year survival was 24.5% (95% CI 13.6–37.1%), in which the lower limit was slightly short of the threshold value of 15%.
Since safety and feasibility were confirmed in this trial, the JCOG proceeded to the next step of generating hard evidence through a phase III trial, JCOG0501, with overall survival as the primary endpoint. This trial started as a comparison of neoadjuvant S-1/cisplatin versus surgery alone for the “Borrmann type IV and large type III cancer” in which, again, staging laparoscopy was not mandatory. After initiation of this trial, a randomized phase III study to look at survival benefit of reduction surgery was planned and started (JCOG0705 or the REGATTA trial). In that trial, technically resectable Stage IV gastric cancer with either para-aortic lymphadenopathy exceeding the No. 16a2/b1 region, 2–4 metastatic nodules in the liver, or moderate peritoneal metastases was randomized to receive either palliative D1 dissection followed by chemotherapy with S-1/cisplatin or chemotherapy alone [26]. Staging laparoscopy was mandatory to select patients who were appropriate for entry into that trial. A majority of patients belonging to the type IV and large type III category also became candidates for that trial and began to undergo staging laparoscopy. Consequently, more strict criteria were needed as to which patients are to be eligible for JCOG0501 and which are more suitable for JCOG0705. After elaborate discussion at group meetings, final agreements were reached so as to register patients with cytology-positive status only and those with small number of peritoneal deposits adjacent to the stomach to the JCOG0501 trial, whereas those with more extensive peritoneal metastasis are deemed eligible for the JCOG0705. In addition, following the evidence of ACTS-GC trial obtained, while the patient accrual for JCOG0501 was in progress, the protocol was amended, so that all patients registered thereafter received 12 months of postoperative adjuvant chemotherapy with S-1. Eventually, accrual of 316 patients was completed in July 2013 and final data after preplanned follow-up for 4 years and 4 months will be available by 2018.
Changing attitudes reflecting results of the previous trials and efficacy of the evolving chemotherapeutic regimens
Through conducting neoadjuvant trials with advanced and particularly aggressive gastric cancers as the targets, the Japanese researchers accumulated experience with this strategy and found that disease progression during 2–3 cycles of chemotherapy is not observed as frequently as they had initially feared. The long-term outcome data from the JCOG0405 study were particularly compelling, and some members of the JCOG even considered conducting a randomized trial to confirm non-inferiority of omitting the postoperative S-1 during the future neoadjuvant trials. These positive impressions acquired through the neoadjuvant studies produced two new streams in the development of neoadjuvant chemotherapy. One stream was to prolong the duration of neoadjuvant chemotherapy. Ultimate targets of the adjuvant chemotherapy are micrometastases that had not been detected preoperatively and were, therefore, destined to be left in situ after gastrectomy (Fig. 1). Whether the effect of two cycles of combination chemotherapy, such as S-1/cisplatin, is sufficient for complete elimination of the micrometastases is currently unknown. The duration of the neoadjuvant therapy could be prolonged to ensure more detrimental effects to the micrometastases while wishing for greater shrinkage of the visible lesions. Yoshikawa et al. conducted a series of randomized phase II trials employing a 2 by 2 factorial design to gain greater insight into this issue. In the first trial named COMPASS study [27], they compared four types of neoadjuvant chemotherapy: two cycles of S-1/cisplatin, four cycles of S-1/cisplatin, two cycles of a combination of paclitaxel (80 mg/m2 on days 1, 8, and 15 every 28 days) and cisplatin (25 mg/m2 on days 1, 8, and 15) [28], and four cycles of paclitaxel/cisplatin for patients with a mixed population of advanced gastric cancer (“bulky N disease”, “Borrmann type IV or large type III cancer” and advanced junctional adenocarcinoma). All patients were given 6 months of S-1 monotherapy after surgery. The investigators were delighted to find that all four cases of a pathological complete response (pCR) of the primary lesion were observed in the four-cycle treatment groups [27], because the pathological response in the primary had been reported to translate into a prolongation of survival. However, they were eventually disappointed to find no difference in survival between the patients who received two cycles and those who received four cycles [29]. Another randomized phase II trial named COMPASS-D study which compares two cycles of S-1/cisplatin, four cycles of S-1/cisplatin, two cycles of DCS (the triplet consisting of docetaxel, cisplatin, and S-1 used in the JCOG1002 trial), and four cycles of DCS was launched soon after registration for the COMPASS study was closed, and further evidence on the duration of preoperative chemotherapy will be available in due time.
Concept of adjuvant chemotherapy when the oncological effects to the primary plus regional nodes (resectable components) and micrometastases (unresectable components) are depicted independent of each other. In the postoperative adjuvant chemotherapy strategy (left), surgery is performed after a waiting time which depends on the availability of relevant resources, and treatment against micrometastases is invariably delayed, sometimes pronouncedly due to surgical complications or post-gastrectomy feeding/nutritional disorders. Several adverse effects due to surgical stress will also have to be taken into consideration. In the neoadjuvant strategy (right), both the primary and micrometastases are treated heavily by intense chemotherapy without overt waiting time, although the timing of surgical treatment will be delayed. In either of the strategies, compliance for the postoperative chemotherapy will be compromised due to the detrimental effects of gastrectomy
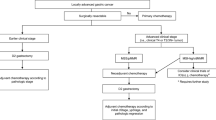
Another stream was to expand the eligibility criteria for neoadjuvant studies. Although postoperative S-1 is the current standard of care for curatively resected Stage II/III gastric cancer, several patients with Stage III disease still suffer from recurrences. Since satisfactory survival data were obtained in the series of neoadjuvant studies with bulky N disease, while attempts to deliver S-1/cisplatin postoperatively resulted in poor relative dose intensities and completion rates [21, 22], the JCOG investigators considered that the time is ripe to expand the indication for neoadjuvant strategy and include clinically Stage III cancer. One major concern when planning the trial was the accuracy of preoperative staging. To clarify this issue, a preparatory prospective trial was conducted to evaluate the accuracy of the preoperative staging using multi-detector computerized tomography and endoscopy (JCOG1302-A). In this study, 1000 patients with estimated T category of ≥T2, excluding M1 disease and the “bulky N” and “Borrmann type IV/large type III” populations, were registered and preoperative diagnosis of the T and N categories were submitted, followed by subsequent pathological reports of the resected specimens. Consequently, the optimal preoperative combination of T and N categories that had a reasonably high possibility of identifying the Stage III cancer while ruling out the Stage I cancer was determined to be T3–T4/N+. In JCOG0509, a randomized phase III study which was launched in September 2016, patients with the preoperative diagnosis of T3–T4/N+/M0 are randomized to receive either a set of neoadjuvant chemotherapy, D2 dissection and postoperative S-1 or a combination of D2 dissection and postoperative S-1 only (Fig. 2). Relevance of adding another treatment arm consisting of neoadjuvant chemotherapy and D2 dissection with no postoperative chemotherapy was seriously discussed, but the estimated sample size for the three arm trial was considered too large for the study group for prompt accrual. Reflecting recent approval of oxaliplatin, clinical non-inferiority of the SOX regimen (S-1 40 mg/m2 twice daily for days 1–14, oxaliplatin 130 mg/m2 on days 1, every 21 days) to the standard S-1/cisplatin combination in the advanced/metastatic setting [30], and feasibility of this regimen in the neoadjuvant setting [31], neoadjuvant chemotherapy with three courses of SOX (the duration being 9 weeks as opposed to 8–10 weeks for delivering two courses of S-1/cisplatin) was selected for this trial. Safety of surgery after the neoadjuvant chemotherapy will be evaluated according to the new classification of surgical complications compiled by the JCOG [32].
Concept of JCOG1509, the latest phase III trial to explore neoadjuvant chemotherapy for clinically Stage III gastric cancer. Asterisk the eligibility criteria were determined as patients with diagnosis of cT3–4N + M0 by endoscopy and contrast-enhanced computerized tomography. Double asterisk ANOTHER trial to explore novel postoperative adjuvant chemotherapy COULD theoretically be proposed for pathologically confirmed stage III gastric cancer that had not met the criteria of cT3–4 N + M0
As for the HER2 positive cancer, the use of trastuzumab in the adjuvant setting has not been approved by the government. A retrospective analysis by immunostaining and fluorescence in situ hybridization of 89 resected specimens from participants of the JCOG neoadjuvant studies for bulky N disease (JCOG0001 and JCOG0405) revealed that 27% (24 cases) of the specimens were HER2 positive, and 41% if confined to the differentiated type cancer [33]. Thus, there is a possibility that HER2 status should not be ignored when treating bulky N disease with neoadjuvant chemotherapy. Patients with regional lymph node of ≥15 mm diameter in the short axis in addition to the patients with bulky N status are eligible for the JCOG1301 study in which the patients are randomized to receive 2–3 courses of neoadjuvant chemotherapy either by S-1/cisplatin/trastuzumab (S-1: 40 mg/m2 twice a day on days 1–14, cisplatin: 60 mg/m2 on day 1, every 21 days) or S-1/cisplatin, followed by surgery and postoperative S-1. Since the use of trastuzumab in the adjuvant setting has not been approved by the social insurance, off-label use in this study was reviewed and approved by the Ministry of Health, Labor and Welfare to be conducted within the advanced medical treatment scheme. It is of note that neoadjuvant trials for HER2 positive have been conducted more extensively outside of Japan.
Conclusions
The current standard of care for resectable Stage II/III gastric cancer in Japan is D2 dissection followed by postoperative adjuvant chemotherapy. After a long struggle and some compelling results with the JCOG phase II studies with bulky N disease, the Japanese researchers reached a stage where survival benefit of adding neoadjuvant chemotherapy to the standard treatment consisting of D2 dissection and postoperative chemotherapy for resectable gastric cancer will be explored in a phase III trial. Further insight regarding the neoadjuvant strategy may be available soon through final survival analysis of a randomized trial testing the strategy for scirrhous-type cancer. These trials could lead to an extensive revision in the algorithm for the treatment of gastric cancer in the near future.
References
Yokoyama H, Nakanishi H, Kodera Y, Ikehara Y, Ohashi N, Ito S, et al. Biological significance of isolated tumor cells and micrometastasis in lymph nodes evaluated using a green fluorescent protein-tagged human gastric cancer cell line. Clin Cancer Res. 2006;12:361–8.
The GASTRIC (Global Advanced/Adjuvant Stomach Tumor Research International Collaboration) Group, Paoletti X, Oba K, Burzykowski T, Michiels S, Ohashi Y, et al. Benefit of adjuvant chemotherapy for resectable gastric cancer. A meta-analysis. JAMA. 2010; 303:1729–37.
Imanaga H, Nakazato H. Results of surgery for gastric cancer and effect of adjuvant mitomycin C on cancer recurrence. World J Surg. 1977;1:213–21.
Nakajima T, Nashimoto A, Kitamura M, Kito T, Iwanaga T, Okabayashi K, Goto M. Adjuvant mitomycin and fluorouracil followed by oral uracil plus tegafur in serosa-negative gastric cancer: a randomised trial. Gastric Cancer Surgical Study Group. Lancet. 1999;354:273–7.
Nashimoto A, Nakajima T, Furukawa H, Kitamura M, Kinoshita T, Yamamura Y, et al. Randomized trial of adjuvant chemotherapy with mitomycin, fluorouracil, and cytosine arabinoside followed by oral fluorouracil in serosa-negative gastric cancer: Japan Clinical Oncology Group 9206-2. J Clin Oncol. 2003;21:2282–7.
Miyashiro I, Furukawa H, Sasako M, Yamamoto S, Nashimoto A, Nakajima T, et al. Randomized clinical trial of adjuvant chemotherapy with intraperitoneal and intravenous cisplatin followed by oral fluorouracil (UFT) in serosa-positive gastric cancer versus curative resection alone: final results of the Japan Clinical Oncology Group trial JCOG9206-2. Gastric Cancer. 2011;14:212–8.
Macdonald JS, Smalley SR, Benedetti J, Hundahl SA, Estes NC, Stemmermann GN, et al. Chemoradiotherapy after surgery compared with surgery alone for adenocarcinoma of the stomach or gastroesophageal junction. N Engl J Med. 2001;345:725–30.
Cunningham D, Allum WH, Stenning SP, Thompson JN, Van de Velde CJ, Nicolson M, et al. Perioperative chemotherapy versus surgery alone for resectable gastroesophageal cancer. N Engl J Med. 2006;355:11–20.
Sasako M, Sakuramoto S, Katai H, Kinoshita T, Furukawa H, Yamaguchi T, et al. Five-year outcomes of a randomized phase III trial comparing adjuvant chemotherapy with S-1 versus surgery alone in stage II or III gastric cancer. J Clin Oncol. 2011;29:4387–93.
Noh SH, Park SR, Yang H-K, Chung HC, Chung I-J, Kim S-W, et al. Adjuvant capecitabine plus oxaliplatin for gastric ancer after D2 gastrectomy (CLASSIC): 5-year follow-up of an open-label, randomised phase 3 trial. Lancet Oncol. 2014;15:1389–96.
Webb A, Cunningham D, Scarffe JH, Harper P, Norman A, Joffe JK, et al. Randomized trial comparing epirubicin, cisplatin, and fluorouracil versus fluorouracil, doxorubicin, and methotrexate in advanced esophagogastric cancer. J Clin Oncol. 1997;15:261–7.
Lowy AM, Mansfield PF, Leach SD, Pazdur R, Dumas P, Ajani JA. Response to noeadjuvant chemotherapy best predicts survival after curative resection of gastric cancer. Ann Surg. 1999;229:303–8.
Ychou M, Boige V, Pignon JP, Conroy T Bouche O, Lebreton G, et al. Perioperative chemotherapy compared with surgery alone for resectable gastroesophageal adenocarcinoma: and FNCLCC and FFCF multicenter phase III trial. J Clin Oncol. 2011;29:1715–21.
Sasako M, Sano T, Yamamoto S, Kurokawa Y, Nashimoto A, Kurita A, et al. D2 lymphadenectomy alone or with para-aortic nodal dissection for gastric cancer. N Engl J Med. 2008;359:453–62.
Kunisaki C, Akiyama H, Nomura M, Matsuda G, Otsuka Y, Ono H, et al. Comparison of surgical results of D2 versus D3 gastrectomy (para-aortic lymph node dissection) for advanced gastric carcinoma: a multi-institutional study. Ann Surg Oncol. 2006;13:659–67.
Fujimura T, Nakamura K, Oyama K, Funaki H, Fujita H, Kinami S, et al. Selective lymphadenectomy of para-aortic lymph nodes for advanced gastric cancer. Oncol Rep. 2009;22:509–14.
Yoshikawa T, Sasako M, Yamamoto S, Sano T, Imamura H, Fujitani K, et al. Phase II study of neoadjuvant chemotherapy and extended surgery for locally advanced gastric cancer. Br J Surg. 2009;96:1015–22.
Tsuburaya A, Mizusawa J, Tanaka Y, Fukushima N, Nashimoto A, Sasako M, et al. Neoadjuvant chemotherapy with S-1 and cisplatin followed by D2 gastrectomy with para-aortic lymph node dissection for gastric cancer with extensive lymph node metastasis. Br J Surg. 2014;101:653–60.
Japan Gastric Cancer Association. Japanese gastric cancer treatment guidelines 2014 (ver. 4). Gastric Cancer. 2016 (in press).
Ito S, Sano T, Mizusawa J, Takahari D, Katayama H, Katai H, et al. A phase II study of preoperative chemotherapy with docetaxel, cisplatin, and S-1 followed by gastrectomy with D2 plus para-aortic lymph node dissection for gastric cancer with extensive lymph node metastasis: JCOG1002. Gastric Cancer. 2016 (in press).
Kodera Y, Ishiyama A, Yoshikawa T, Kinoshita T, Ito S, Yokoyama H, et al. A feasibility study of postoperative chemotherapy with S-1 and cisplatin (CDDP) for gastric carcinoma (CCOG0703). Gastric Cancer. 2010;13:197–203.
Kurimoto K, Ishigure K, Mochizuki Y, Ishiyama A, Matsui T, Ito S, et al. A feasibility study of postoperative chemotherapy with S-1 and cisplatin (CDDP) for stage III/IV gastric cancer (CCOG1106). Gastric Cancer. 2015;18:354–9.
Takahashi S, Kinoshita M, Konishi M, Nakagohri T, Inoue K, Ono M, et al. Phase II study of sequential high-dose methotrexate and fluorouracil combined with doxorubicin as a neoadjuvant chemotherapy for scirrhous gastric cancer. Gastric Cancer. 2001;4:192–7.
Kinoshita T, Sasako M, Sano T, Katai H, Furukawa H, Tsuruburaya A, et al. Phase II trial of S-1 for neoadjuvant chemotherapy against scirrhous gastric cancer (JCOG0002). Gastric Cancer. 2009;12:37–42.
Iwasaki Y, Sasako M, Yamamoto S, Nakamura K, Sano T, Katai H, et al. Phase II study of preoperative chemotherapy with S-1 and cisplatin followed by gastrectomy for clinically resectable type 4 and large type 3 gastric cancer (JCOG0201). J Surg Oncol. 2013;107:741–5.
Fujitani K, Yang HK, Mizusawa J, Kim YW, Terashima M, Han SU, et al. Gastrectomy plus chemotherapy versus chemotherapy alone for advanced gastric cancer with a single non-curative factor (REGATTA): a phase 3, randomised controlled trial. Lancet Oncol. 2016;17:309–18.
Nagata N, Kimura M, Hirabayashi N, Tsuburaya A, Murata T, Kondo K, et al. Phase II study of weekly paclitaxel and cisplatin combination therapy for advanced or recurrent gastric cancer. Hepatogatroenterology. 2008;55:1846–50.
Yoshikawa T, Tanabe K, Nishikawa K, Ito Y, Matsui T, Kimura Y, et al. Induction of a pathological complete response by four courses of neoadjuvant chemotherapy for gastric cancer: early results of the randomized phase II COMPASS trial. Ann Surg Oncol. 2014;21:213–9.
Yoshikawa T, Morita S, Tanabe K, Nishikawa K, Ito Y, Matsui T, et al. Survival results of a randomised two-by-two factorial phase II trial comparing neoadjuvant chemotherapy with two and four course of S-1 plus cisplatin (SC) and paclitaxel plus cisplatin (PC) followed by D2 gastrectomy for resectable advanced gastric cancer. Eur J Cancer. 2016;62:103–11.
Yamada Y, Higuchi K, Nishikawa K, Gotoh M, Fuse N, Sugimoto N, et al. Phase III study comparing oxaliplatin plus S-1 with cisplatin plus S-1 in chemotherapy-naïve patients with advanced gastric cancer. Ann Oncol. 2015;26:141–8.
Honma Y, Yamada Y, Terazawa T, Takashima A, Iwasa S, Kato K, et al. Feasibility of neoadjuvant S-1 and oxaliplatin followed by surgery for resectable advanced gastric adenocarcinoma. Surg Today. 2016;46:1076–82.
Katayama H, Kurokawa Y, Nakamura K, Ito H, Kanemitsu Y, Masuda N, et al. Extended Clavien-Dindo classification of surgical complications: Japan Clinical Oncology Group postoperative complications criteria. Surg Today. 2016;46:668–85.
Matsumoto T, Sasako M, Mizusawa J, Hirota S, Ochiai A, Kushima R, et al. HER2 expression in locally advanced gastric cancer with extensive lymph node (bulky N2 or paraaortic) metastasis (JCOG1005-A trial). Gastric Cancer. 2015;18:467–75.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Kodera, Y. Neoadjuvant chemotherapy for gastric adenocarcinoma in Japan. Surg Today 47, 899–907 (2017). https://doi.org/10.1007/s00595-017-1473-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00595-017-1473-2