Abstract
Purpose
The role of surgery for patients with non-small cell lung cancer (NSCLC) with clinical mediastinal lymph node metastasis (N2) remains controversial. We specified 4 criteria for performing initial surgery in these patients (single-station N2, non-bulky N2, N2 with regional mode of spread, and N2 without N1) and examined the outcomes to validate the treatment options.
Methods
Between September 2002 and December 2010, of 1290 patients who underwent complete resection for NSCLC, 808 patients underwent initial standard resection, including 779 patients with cN0–1 and 29 with cN2. We compared the outcomes, and evaluated patients with cN2–pN2.
Results
The median follow-up was 45.5 months (3–119 months). Seventy (9.0 %) and 24 (82.8 %) patients had p-N2 in the cN0–1 and cN2 groups, respectively (p < 0.0001). The 5-year disease-free survival (DFS) rates in the cN0–1 and cN2 groups were 73.3 and 50.6 %, respectively (p = 0.0053), and the 5-year overall survival (OS) rates were 81.3 and 71.1 %, respectively (p = 0.051). The 5-year DFS and OS of patients with cN2–pN2 were 52.5 and 72.6 %, respectively.
Conclusions
Patients with clinical N2 disease based on our criteria represent a highly specific group with a favorable prognosis. Resection should therefore be the initial treatment for these patients.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Most patients with locally advanced non-small cell lung cancer (NSCLC) with mediastinal lymph node (N2) involvement develop distant or local relapse shortly after primary treatment [1, 2]. Surgery alone is not generally recommended for patients with NSCLC with clinically manifested mediastinal lymph node metastasis (cN2/pN2). Instead, induction chemotherapy or chemoradiotherapy followed by surgery is the currently accepted regimen for the treatment of patients with N2 disease. Although some studies [3–6] have reported the outcomes of chemotherapy or chemoradiotherapy followed by surgery in clinical trials, the role of surgery for the treatment of this disease remains controversial.
Furthermore, several studies have demonstrated that patients with single-station N2 disease have a better prognosis than those with multiple-station N2 disease [7–10]. However, those studies predominately investigated the prognosis of patients with pathological single-station N2 disease, and so the prognosis of patients with clinical single-station N2 disease has not yet been elucidated. Accordingly, the aim of this study was to examine the outcomes of initial surgery in patients with NSCLC with clinical N2 disease who met our specific 4 criteria (single-station N2 disease, non-bulky N2 disease, N2 disease with regional mode of spread, or N2 disease without N1 disease) and to validate the treatment options for these patients.
Methods
Patients
Between September 2002 and December 2010, 1290 patients underwent complete resection for NSCLC at the Shizuoka Cancer Center Hospital. Complete resection was defined as the removal of the primary tumor and all accessible hilar and mediastinal lymph nodes with no residual tumor (resection of the macroscopic tumor with tumor-free resection margins on microscopic analysis). Of these patients, 809 patients underwent standard resection (lobectomy, bilobectomy, or pneumonectomy). Any patients who underwent minor resection were excluded. Systematic lymph node dissection was routinely performed, but lobe-specific nodal dissection was performed in cases of co-morbidities [11]. We excluded 1 patient with pN2 who had received induction therapy prior to surgery. Accordingly, 808 patients were included in the analysis.
Prior to surgery, all patients with potentially resectable NSCLC underwent fluorodeoxyglucose-positron emission tomography (PET–CT). The criterion for lymph node positivity was a shortest nodal diameter of ≥10 mm on CT and a metabolic activity of nodes higher than that of adjacent normal mediastinal and soft tissue on PET–CT. Patients with N2 disease were diagnosed by thoracic surgeons and a pulmonary radiologist.
Originally, we specified the following 4 criteria for performing initial surgery for patients with NSCLC with clinical N2 disease: single-station N2 disease, non-bulky N2 disease, N2 disease with regional mode of spread, and N2 disease without N1 disease. Only patients who fulfilled all of these criteria underwent initial surgery. According to these criteria, 29 patients had clinical N2 disease with unknown pathological N2 status disease.
We compared these 29 patients with cN2 (cN2 group) to 779 patients with cN0–1 (cN0–1 group). Data on age, sex, smoking index, histology, carcinoembryonic antigen (ng/mL), pT status, pN status, surgical procedure, lymph node dissection, type of resection, disease-free survival (DFS), and overall survival (OS) were recorded and analyzed. Additionally, we evaluated the clinicopathological and prognostic patients with cN2–pN2 disease (cN2–pN2 group).
The indications for adjuvant therapy after surgery were as follows: an age of less than 75 years, a performance status 0–1, no major surgical complications, and no interstitial lung disease. The most common regimen of adjuvant therapy was cisplatin and vinorelbine.
Outcomes and statistical analyses
OS and DFS were used as the endpoints. OS was defined as the time between surgery and death from any cause. DFS was defined as the time between surgery and locoregional or distant relapse or death. Data on patients who died due to causes other than lung cancer were censored.
The associations between variables were analyzed using the χ 2 test and Student t test. Survival curves were generated using the Kaplan–Meier method, and differences between subgroups were examined using the log-rank test. A probability value of <0.05 was considered significant. All statistical calculations were performed using JMP for Windows (SAS Inc., Cary, NC, USA).
Results
The median follow-up time was 45.5 months (3–119 months). Table 1 shows the comparison of the patient characteristics between the cN2 group and the cN0–1 group. The cN2 and cN0–1 groups did not differ with regard to age, sex, smoking index, or histology. The presence of pathological T factor was significantly more frequently observed in the cN2 group than in the cN0–1 group (p = 0.0016; Table 1). There were 70 patients (9.0 %) with p-N2 in the cN0–1 group and 24 patients (82.8 %) with pN2 in the cN2 group (p < 0.0001). A significantly higher number of patients underwent ND2a-2 in the cN2 group than in the cN0–1 group. Only 1 patient in cN2 group underwent pneumonectomy.
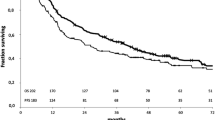
Figure 1 shows the 5-year DFS and OS. The 5-year DFS rates in the cN0–1 and cN2 groups were 73.3 and 50.6 %, respectively (p = 0.0053). The 5-year OS rates in the cN0–1 and cN2 groups were 81.3 and 71.1 %, respectively (p = 0.051).
The 5-year disease-free survival and overall survival rates of both groups. a The 5-year disease-free survival rates in the cN0–1 and cN2 groups were 73.3 and 50.6 %, respectively (p = 0.0053). b The 5-year overall survival rates in the cN0–1 and cN2 groups were 81.3 and 71.1 %, respectively (p = 0.051)
Table 2 shows the lymph node involvement in patients with clinical single-station N2 disease. Of those with clinical N2 disease, 24 patients had cN2–pN2 disease and 5 had cN2–pN0 disease (false-positive result).
Table 3 shows the patient characteristics of the cN2–pN2 group. There were only 4 patients with pN1 (hilar; #10 or #11) in the cN2–pN2 group. There were 16 patients (75 %) with single-station N2 and only 6 patients (25 %) with multiple-station N2 in the cN2–pN2 group. Eleven patients (45.8 %) in the cN2–pN2 group underwent adjuvant chemotherapy.
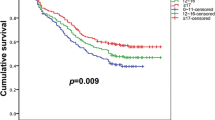
Figure 2 shows the 5-year DFS and OS of patients in the cN2–pN2 group. The 5-year DFS and OS rates of patients with cN2–pN2 were 52.5 and 72.6 %, respectively.
Table 4 shows the pattern of recurrence in patients with cN2–pN2. Of 24 patients with cN2–pN2 disease, 11 had recurrence. The pattern of recurrence was local and distant in 5 patients, and distant in 6 patients; none of the patients showed local recurrence only.
We examined the EGFR mutation status for 6 patients with recurrence and adenocarcinoma in the cN2–pN2 group. Three of these patients received tyrosine kinase inhibitors after recurrence.
Discussion
Survival after tumor resection is poor in patients with cN2 disease in whom surgical staging is determined by using CT or PET [1, 2, 8, 9]. Therefore, surgical resection for patients with cN2 disease does not seem to be justified. The current standard of care for stage IIIA NSCLC is concurrent chemoradiotherapy [12], with associated 5-year survival rates of approximately 15–20 % [13, 14]. Induction therapy followed by surgical resection is considered a reasonable alternative, although this has not yet been validated and it should, therefore, be investigated further in a clinical trial setting. Some studies have reported the outcomes of chemotherapy or chemoradiotherapy followed by surgery in a clinical trial setting [3–6]; however, the role of surgery in the treatment of this disease remains controversial.
Induction therapy has several potential advantages, such as an increase in the sensitivity of tumors in patients with early-stage disease, a decrease in the tumor volume to enable better local control after subsequent surgery, a quicker eradication of clinically undetected micrometastases, and better tolerance and compliance than post-surgical treatments [15]. However, induction therapy is also associated with the disadvantages of increasing the likelihood of post-operative complications or delaying surgery [5, 15]. These disadvantages may be negated, however, if initial surgery is performed for patients with cN2 disease.
Since the opening of the Shizuoka Cancer Center in September 2002, we performed initial surgery in patients with clinical N2 disease who met our specific criteria. Therefore, the sample of patients with cN2–pN2 disease who were evaluated in this study was smaller than that described in previous reports [7–10]. We only performed initial surgery for the small number of cN2–pN2 patients who fulfilled our predefined criteria.
The first criterion was single-station disease. Several studies have shown that among patients with pN2 disease, those with pathological single-station N2 disease have a better prognosis than those with pathological multiple-station N2 disease [7–10]. However, most of these studies have investigated the prognosis of patients with pathological single-station N2 disease and not the prognosis of patients with clinical single-station N2 disease. The sample of patients with pathological single-station N2 disease who were evaluated in this study was smaller than that in previous reports. Patients in the present study routinely received PET–CT before surgery, whereas patients in previous reports uncommonly received PET–CT. PET–CT is very useful when we assess metastasis of mediastinal lymph node [16, 17]. We believe the sample of patients with pathological single-station N2 disease who were evaluated in this study was smaller than that in previous reports because they routinely received PET–CT.
The second criterion for performing initial surgery in patients with NSCLC with N2 disease was the presence of non-bulky disease. Bulky N2 disease is generally defined as involvement of lymph nodes with greater than 2 cm short-axis diameter and implies extra-nodal involvement and multi-station nodal disease. It is thought that bulky N2 disease cannot be resected, and therefore, these patients have traditionally only been treated with conventional chemoradiotherapy [12].
Our third criterion for surgery was regional mode of spread. Several investigators [18–20] have reported the identification of distinct patterns of metastatic spread through the lymphatic system that are dependent on the location of the primary tumors. Asamura et al. [18] reported that tumors of the right lower lobe, with superior mediastinal metastasis, were associated with a particularly poor 5-year survival rate of 4.1 %. Aokage et al. [20] reported that upper lobe tumors, with subcarinal metastasis, were also associated with a poor 5-year survival of 9.1 %. We, therefore, consider patients who demonstrate N2 disease with non-regional mode spread to already have systemic disease.
The last criterion for surgery was N2 disease without N1 disease, so called “skip N2” disease. Previous reports have shown that, among patients with pN2 disease, those with “skip N2” disease have a better prognosis than those with N1 and N2 disease [21, 22]. Patients with pN1 (hilar) may require either sleeve resection or pneumonectomy. There were 4 patients (16.6 %) with pN1 (hilar) in the cN2–pN2 group, and this number was small. As a result, only 1 patient with cN2–pN2 disease underwent pneumonectomy. Accordingly, we believe that hilar lymph node metastasis is a poor prognostic factor for patients with pN2 disease, and among these patients, those with cN2 disease showing bulky N1 should not undergo initial surgery. Using these 4 criteria, patients with cN2–pN2 are, thus, considered to have a favorable prognosis.
Previous reports have indicated that endobronchial ultrasonography (EBUS) is useful for the diagnosis of pN2 [17, 23]. Of 29 patients with c-N2 disease, 5 had false-positive N2. If the patients with false-positive N2 had received EBUS of the mediastinal lymph nodes, they might have been identified with pN0 or pN1 before surgery. However, they underwent initial surgery. In this study, 6 patients (25 %) with cN2–pN2 disease had multiple N2 stations, although this number is small. However, if these patients had undergone EBUS, they might have been identified as having multiple N2 station disease. Subsequently, their therapy plan should include chemo-radiation or induction therapy after surgery. Furthermore, we performed initial surgery for a more highly selected subset of patients with clinical N2 disease who fulfilled our criteria.
Recently, an improved prognosis has been reported in patients who are treated with adjuvant chemotherapy [24, 25]. A meta-analysis showed that cisplatin-based chemotherapy significantly improved the survival rates of patients with NSCLC [25]. In our study, no patient had only local disease at first recurrence, and we think that these patients thus require adjuvant chemotherapy after initial surgery. According to the findings of the aforementioned meta-analysis, 11 patients with cN2–pN2 disease were not able to receive adjuvant chemotherapy (5 had severe complications and a poor performance status, 3 were more than 75 years old, 3 refused treatment, and 2 did not receive this treatment for other reasons); the 5-year DFS was 60 % for these patients.
Patients with cN2 disease constitute a heterogeneous group, and many of these patients may not require induction therapy prior to surgical treatment or chemoradiotherapy. We believe that an initial resection should, therefore, be considered as a treatment option for this specific subset of patients. However, given the relative rarity of these patients, a multicenter trial is, therefore, required to fully validate the effect of initial surgery on the survival outcome of these patients.
Clinical N2 disease cases that fulfilled our specific criteria represent a highly distinct group associated with a favorable prognosis. We believe that an initial resection should, therefore, be considered as a treatment option for these patients.
References
Martini N, Flehinger BJ, Zaman MB, Bettie EJ Jr. Results of surgical treatment in N2 lung cancer. World J Surg. 1981;5:663–6.
Pearson FG, DeLarue NC, Ilves R, Todd TR, Cooper JD. Significance of positive superior mediastinal nodes identified at mediastinoscopy in patients with resectable cancer of the lung. J Thorac Cardiovasc Surg. 1982;83:1–11.
Rosell R, Gómez-Codina J, Camps C, Maestre J, Padille J, Cantó A, et al. A randomized trial comparing preoperative chemotherapy plus surgery with surgery alone in patients with non-small-cell lung cancer. N Engl J Med. 1994;330:153–8.
Albain KS, Rusch VW, Crowley JJ, Rice TW, Turrisi AT 3rd, Weick JK, et al. Concurrent cisplatin/etoposide plus chest radiotherapy followed by surgery for stages IIIA (N2) and IIIB non-small-cell lung cancer: mature results of Southwest Oncology Group phase II study 8805. J Clin Oncol. 1995;13:1880–92.
Albain KS, Swann RS, Rusch VW, Turrisi AT 3rd, Shepherd FA, Smith C, et al. Radiotherapy plus chemotherapy with or without surgical resection for stage III non-small-cell lung cancer: a phase III randomised controlled trial. Lancet. 2009;374:379–86.
van Meerbeeck JP, Kramer GW, Van Schil PE, Legrand C, Smit EF, Schramel F, et al. Randomized controlled trial of resection versus radiotherapy after induction chemotherapy in stage IIIA-N2 non-small-cell lung cancer. J Natl Cancer Inst. 2007;99:442–50.
Yoshino I, Yoshida S, Miyaoka E, Asamura H, Nomori H, Fujii Y, et al. Surgical outcome of stage IIIA-cN2/pN2 non-small-cell lung cancer patients in Japanese lung cancer registry study in 2004. J Thorac Oncol. 2012;7:850–5.
Suzuki K, Nagai K, Yoshida J, Nishimura M, Takahashi K, Nishiwaki Y. The prognosis of surgically resected N2 non-small cell lung cancer: the importance of clinical N status. J Thorac Cardiovasc Surg. 1999;118:145–53.
Andre F, Grunenwald D, Pignon JP, Dujon A, Pujol JL, Brichon PY, et al. Survival of patients with resected N2 non-small-cell lung cancer: evidence for a subclassification and implications. J Clin Oncol. 2000;18:2981–9.
Ichinose Y, Kato H, Koike T, Tsuchiya R, Fujisawa T, Shimizu N, et al. Overall survival and local recurrence of 406 completely resected stage IIIa-N2 non-small cell lung cancer patients: questionnaire survey of the Japan Clinical Oncology Group to plan for clinical trials. Lung Cancer. 2001;34:29–36.
Maniwa T, Okumura T, Isaka M, Nakagawa K, Ohde Y, Kondo H. Recurrence of mediastinal node cancer after lobe-specific systematic nodal dissection for non-small-cell lung cancer. Eur J Cardiothorac Surg. 2013;44:e59–64.
Robinson LA, Ruckdeschel JC, Wagner H Jr, Stevens CW, American College of Chest Physicians. Treatment of non-small cell lung cancer-stage IIIA: ACCP evidence-based clinical practice guidelines (2nd edition). Chest. 2007;132:243–65.
Yamamoto N, Nakagawa K, Nishimura Y, Tsujino K, Satouchi M, Kudo S, et al. Phase III study comparing second- and third-generation regimens with concurrent thoracic radiotherapy in patients with unresectable stage III non-small-cell lung cancer: West Japan Thoracic Oncology Group WJTOG0105. J Clin Oncol. 2010;28:3739–45.
Kubota K, Tamura T, Fukuoka M, Furuse K, Ikegami H, Ariyoshi Y, et al. Phase II study of concurrent chemotherapy and radiotherapy for unresectable stage III non-small-cell lung cancer: long-term follow-up results. Japan Clinical Oncology Group Protocol 8902. Ann Oncol. 2000;11:445–50.
NSCLC Meta-analysis Collaborative Group. Preoperative chemotherapy for non-small-cell lung cancer: a systematic review and meta-analysis of individual participant data. Lancet. 2014;383:1561–71.
Cerfolio RJ, Ojha B, Bryant AS, Raghuveer V, Mountz JM, Bartolucci AA. The accuracy of integrated PET–CT compared with dedicated PET alone for the staging of patients with nonsmall cell lung cancer. Ann Thorac Surg. 2004;78:1017–23.
Imai K, Minamiya Y, Saito H, Motoyama S, Sato Y, Ito A, et al. Diagnostic imaging in the preoperative management of lung cancer. Surg Today. 2014;44:1197–206.
Asamura H, Nakayama H, Kondo H, Tsuchiya R, Naruke T. Lobe-specific extent of systematic lymph node dissection for non-small cell lung carcinomas according to a retrospective study of metastasis and prognosis. J Thorac Cardiovasc Surg. 1999;117:1102–11.
Okada M, Tsubota N, Yoshimura M, Miyamoto Y. Proposal for reasonable mediastinal lymphadenectomy in bronchogenic carcinomas: role of subcarinal nodes in selective dissection. J Thorac Cardiovasc Surg. 1998;116:949–53.
Aokage K, Yoshida J, Ishii G, Hishida T, Nishimura M, Nagai K. Subcarinal lymph node in upper lobe non-small cell lung cancer patients: is selective lymph node dissection valid? Lung Cancer. 2010;70:163–7.
Prenzel KL, Mönig SP, Sinning JM, Baldus SE, Gutschow CA, Grass G, et al. Role of skip metastasis to mediastinal lymph nodes in non-small cell lung cancer. J Surg Oncol. 2003;82:256–60.
Misthos P, Sepsas E, Athanassiadi K, Kakaris S, Skottis I. Skip metastases: analysis of their clinical significance and prognosis in the IIIA stage of non-small cell lung cancer. Eur J Cardiothorac Surg. 2004;25:502–8.
Yasufuku K, Pierre A, Darling G, de Perrot M, Waddell T, Johnston M, et al. Prospective controlled trial of endobronchial ultrasound-guided transbronchial needle aspiration compared with mediastinoscopy for mediastinal lymph node staging of lung cancer. J Thorac Cardiovasc Surg. 2011;142:1393–400.
Winton T, Livingston R, Johnson D, Rigas J, Johnston M, Butts C, et al. Vinorelbine plus cisplatin vs. observation in resected non-small-cell lung cancer. N Engl J Med. 2005;352:2589–97.
Pignon JP, Tribodet H, Scagliotti GV, Douillard JY, Shepherd FA, Stephens RJ, et al. Lung adjuvant cisplatin evaluation: a pooled analysis by the LACE Collaborative Group. J Clin Oncol. 2008;26:3552–9.
Acknowledgments
None.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
Tomohiro Maniwa and the other co-authors have no conflicts of interest to declare in association with this study.
Rights and permissions
About this article
Cite this article
Maniwa, T., Takahashi, S., Isaka, M. et al. Outcomes of initial surgery in patients with clinical N2 non-small cell lung cancer who met 4 specific criteria. Surg Today 46, 699–704 (2016). https://doi.org/10.1007/s00595-015-1268-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00595-015-1268-2