Abstract
Purposes
The objective of this study was to test the efficacy of an equine pericardial patch for repairing full-thickness defects of the stomach wall.
Methods
Circular defects, 1.5 cm in diameter, were created on the anterior wall of the stomach of 12 female New Zealand rabbits. The defects were repaired by an equine pericardial patch. After euthanasia at different time intervals (3 days to 8 weeks) a macroscopic evaluation of the abdominal cavity (including adhesion scoring), mechanical testing and a histological examination of the stomach were performed.
Results
The animals survived the surgical procedure and underwent an uneventful recovery until euthanasia. None of the patches failed. Adhesions were observed in all animals and were significant in 3/12 animals. Bursting pressure testing indicated that the repair was durable and that adequate strength to prevent patch failure was achieved by the second week. A histological examination showed gradual narrowing of the perforation site by mucosal and limited muscular regeneration.
Conclusions
The equine pericardial patch was successfully used to repair a gastric defect in our experimental model, and it seems that it could have potential as a material suitable for further research concerning the repair of upper gastrointestinal defects.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The repair of defects in the upper gastrointestinal tract constitutes an integral part of gastrointestinal surgery and is usually accomplished through primary suturing. However, the size of the defect is occasionally such that alternative approaches are required to ensure adequate repair.
Stomach defects are usually encountered as a result of ulcer perforation or iatrogenic perforation. Perforated peptic ulcers are usually repaired primarily with sutures or an omental patch (via either the Graham or Celan-Jones technique). The advances in laparoscopic surgery have made the laparoscopic approach increasingly popular due to its well-known advantages [1]. Although the technique has been well described by surgeons with extensive experience in laparoscopic operations [2–4], laparoscopic suturing can be a challenging task to perform, especially in the emergency setting. Closure using a patch could greatly facilitate the laparoscopic approach. Aside from its use in sealing a perforation, a patch could be used as a scaffold for tissue regeneration, facilitating the preservation of the stomach contour, such as in cases where a large gastrointestinal stromal tumor is excised.
Duodenal defects resulting from a perforated ulcer, injury or suture line dehiscence have been the subject of considerable debate as well as significant morbidity and mortality for the last 50 years [5]. The problem is especially acute in the presence of giant (>2 cm) defects, where approximation by primary repair is not always safe and can result in continued leaking of intestinal juices, dehiscence due to tension or gastric outlet obstruction [6]. The alternative techniques are difficult to perform and/or time consuming, while a patch repair could potentially minimize morbidity and mortality. The development of a patch repair technique could pave the way for the establishment of a minimally invasive approach.
The efficacy of different materials in closing upper gastrointestinal defects has been examined in a number of trials, mainly in animal models, but also in humans. Despite the encouraging results obtained so far, many surgeons have reservations about the use of synthetic materials in an infected field with the intention of sealing the lumen of the gastrointestinal tract. Biologically derived materials, which act as a scaffold that undergoes remodeling by host tissue, have been proposed as an alternative, but again, the durability in this setting is doubtful. It is therefore important to evaluate a wide range of available materials and examine the type and durability of their incorporation into the host tissue.
Glutaraldehyde-treated patches derived from pericardium have not been previously tested for their ability to bridge upper gastrointestinal defects. The cross-linking of collagen fibers in these patches enables them to resist degradation, incorporate into host tissue and thus enable a host tissue response adequate to permanently and robustly seal the defect. Based on this background, we hypothesized that a new equine pericardial patch would constitute an excellent bio-scaffold, allowing the healing of such a defect. We therefore decided to evaluate this material by studying its histological integration and mechanical strength in the repair of gastric and duodenal defects. We herein present the results of our first study of gastric defect repair in an animal model. Our aim was to evaluate the histological integration of equine pericardium and to assess whether it provided sufficient mechanical strength at different time points over a 2-month period.
Materials and methods
Study design and surgical technique
The research protocol was reviewed and approved by the veterinary department of the Prefecture of Athens (protocol number EL25 BIO 05) and the bioethics committee of the “Laiko” General Hospital of Athens.
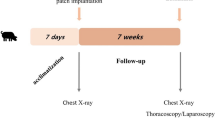
Twelve female white New Zealand rabbits, each weighing approximately 3.5 kg, were used in this study. The animals were housed under standard laboratory conditions (22 °C, 12 h light–dark cycle) and were left to acclimatize for 7 days before surgery, with free access to food until 12 h before surgery. The body weight (BW) was measured prior to surgery and weekly thereafter. The rabbits were anesthetized using ketamine at 35–50 mg/kg BW and xylazine at 5–10 mg/kg BW, and were intubated. The abdomen was shaved, cleaned and prepared with povidone iodine solution. All procedures were performed under aseptic conditions using sterile equipment. Surgery was performed via an upper midline laparotomy. The stomach was identified and mobilized with atraumatic forceps and a circular full-thickness defect of 1.5 cm in diameter was created on the anterior body of the stomach close to the greater curvature using scissors. The perforated stomach was left in the peritoneal cavity for 15 min before repair. A square patch of equine pericardium (Peripatch, PM Devices Inc.) was fashioned to measure 1.5 × 1.5 cm, and was secured with interrupted vicryl 3-0 sutures. After meticulous peritoneal lavage and drainage, the abdomen was closed with interrupted vicryl 2-0 sutures, and the skin was closed with a continuous intradermal 3-0 monocryl suture. A single dose of an antibiotic (fluoroquinolone 7.5 mg/kg BW) was administered. Postoperatively, the animals were checked continuously in order to evaluate their general condition and for signs of pain or distress. Pain medication was administered as needed. The rabbits were fed liquids in the immediate postoperative period and reverted to their normal diet on the first postoperative day.
Euthanasia and macroscopic examination
The rabbits were euthanized in pairs at different time intervals in order to observe the evolution of the healing process. The first group was euthanized after 3 days, the second group after 1 week, the third group after 2 weeks, the fourth after 4 weeks, the fifth after 6 weeks and the last group after 8 weeks. Euthanasia was performed by an intramuscular injection of ketamine, midazolam and atropine with dosing depending on each animal’s weight, followed by an intravenous injection of a concentrated solution of pentothal and potassium chloride. The abdomen was opened and a macroscopic examination was performed. The condition of the intestines was assessed, the presence of intraabdominal collections or abscess formation was documented, and the viability of the patched segment of the stomach, including the presence of local inflammation, was noted. The degree of adhesion formation and the type of adhesions was measured using a previously described scoring system [7] [(0) no adhesions, (1) avascular, easily lysed but fails to bleed, (2) vascular, is easily lysed but bleeds at the time of lysis, (3) thick, requires extensive sharp dissection]. The stomach was removed and placed in a container for mechanical testing, which was performed immediately after removal. Adhesions on the repair site, which could not be removed by gentle traction, were left in situ.
Mechanical testing
Bursting pressure testing was used to evaluate the integrity and mechanical strength of the repair. The esophagus was sealed and the pylorus was connected to insufflation tubing (Fig. 1a). The specimen was submerged in saline at a controlled temperature of 37–39 °C. The stomach was gradually filled with air and the intragastric pressure was monitored with a pressure transducer (RS pressure transducer, model 249-3864, range 0–1 bar), connected to a data logger (NI SCXI-1520 8-Channel Universal Strain Gage Input Module, National Instruments, Austin, TX, USA), using the LabVIEW 6.0 software program (National Instruments, Austin, TX, USA). In order to calibrate the system, five normal rabbit stomachs were tested first. The bursting pressure was measured and recorded for each specimen except for those excised from the first pair of animals (killed 3 days after surgery). After testing, the specimens were conserved in formalin for the histological examination.
a Preparation of the stomach for bursting pressure testing. b A post-mortem view of the abdomen. c Adhesion of the omentum to the patched site. d Adhesion of the spleen to the patched site. e Lysis of adhesions by blunt dissection. f Bursting pressure testing. Shown are air bubbles indicating perforation originating at a point distal to the repair site
Histological examination
Samples were placed in 10 % buffered formalin solution, and 4 μm paraffin-embedded sections were stained with hematoxylin/eosin. All specimens were evaluated by a pathologist blinded to the sequence of the biopsy specimens. Slides were evaluated with regard to the inflammatory reaction, type of inflammatory cells, development of granulation tissue, degree of granulation tissue maturation, possible degradation of the patch by multinuclear giant cells, re-epithelialization and closure of the defect. A microscopic evaluation was performed to quantify the number of foreign body giant cells, polymorphonuclear cells (PMNs) and mononuclear cells (MNs) at the patch area, as well as to evaluate the amount of collagen deposition and the number of blood vessels, as described previously [8] (Table 1). Five fields per section were counted by the same individual at 400× magnification (Nikon, eclipse 50i) in a blinded fashion. Moreover, immunohistochemistry was performed to detect the possible expression of inflammatory cytokines, like IL-6, and angiogenic factors, like VEGF. The following antibodies were used: anti-IL-6 (goat, polyclonal, Santa Cruz Biotechnology, Santa Cruz, CA) diluted 1:100 and anti-VEGF (clone G153-694, monoclonal, Pharmingen BD Company, San Diego, CA) diluted 1:75. The incubation time was 1 h at room temperature for VEGF and 18 h at 4 °C for IL-6. The buffers, blocking solutions, secondary antibodies, avidin–biotin complex reagents and chromogen were supplied in a detection kit (LSAB detection kit; Dako). To enhance antigen retrieval, sections were microwave-treated in 0.01 M citrate buffer (pH 6.0) at 750 W for 20 min.
Results
Surgical outcomes: macroscopic examination
The operation was concluded successfully on all animals. All animals survived the operation. The animals resumed a normal diet and activity with normal bowel function. There were no clinical signs of postoperative complications, and euthanasia was carried out according to the protocol. At relaparotomy, there was neither evidence of gross abdominal contamination nor any signs of intraabdominal sepsis or abscess formation in any animal (Fig. 1b). The repair was found to be intact. Adhesions were observed in all of the animals, and included the omentum, liver and spleen (Fig. 1c, d). It was possible to lyse adhesions with gentle blunt dissection in all but three cases (Fig. 1e), where the adhering organ was excised en bloc with the stomach so as not to disrupt the specimen before mechanical testing (Fig. 2a).
Mechanical testing
Calibration of the measurement technique and estimation of the normal bursting pressure of the experimental model was achieved using five normal rabbit stomachs. The mean pressure was 119 mmHg. Except for the first pair of animals, all other stomachs were subjected to the bursting pressure test. There were no air bubbles at the start of the insufflation, indicating a complete seal of the defect. The repair site failed in both animals in the second pair (1 week after surgery) at a pressure more than 40 % lower than normal (62 mmHg, 71 mmHg) and in one of the animals in the fourth pair (4 weeks after surgery) at a bursting pressure within the normal range (139 mmHg). All other stomachs failed at a location distant from the repair site (Fig. 1f) and exhibited a bursting pressure similar to that of the non-operated stomachs. The usual failure point was located at the lesser curvature. The results of the bursting pressure test are demonstrated in Fig. 2b.
Histological examination
On day 3 and during the first week, edema and infiltration of the area by granulocytes was observed. In this area, an increased number of IL-6-positive cells were observed compared to the adjacent normal gastric wall (Fig. 3a). This newly formed granulation tissue showed marked neovascularization as indicated by the VEGF immunohistochemistry findings (Fig. 3b). In two cases, polymorphonuclear collections organized into abscesses were noted. In week 2, a granulomatous reaction with multinuclear giant cells was observed, indicating that degradation of the patch had started (Fig. 3c). In addition, some degree of closure of the epithelioid layer of the mucosa was seen. At 4 weeks after the operation, a fibrotic layer started to form on the outside of the patch which developed into a well-organized, vascularized and structured layer during weeks 6 and 8. After 6 and 8 weeks, some newly formed muscle cells crossing the perforation site were found. During these weeks, the perforation site was slowly narrowing (Fig. 3d, e). The scoring of the histological parameters is shown in Fig. 4.
a IL-6 immunohistochemical staining. b VEFG immunohistochemical staining. c Granulation tissue beneath the patch at 2 weeks after the operation, showing a marked inflammatory reaction and multinuclear giant cells. d An overview of the defect site: a thick layer of fibrous tissue had formed beneath the patch. e The defect site at 6 weeks: there was narrowing of the defect, an increase in the thickness of the granulation tissue and the inflammation has subsided considerably
Discussion
The present study has demonstrated that, in our rabbit model, an equine pericardial patch constitutes an appropriate material for repairing a full-thickness gastric defect. The patch successfully sealed the repair site, and no evidence of leakage or abscess formation was observed even 3 days after implantation. The defect continued to shrink throughout the observational period, although it did not completely heal within 2 months, while the patch was being extruded into the bowel lumen. Moreover, a thick fibrous layer of tissue developed early on and further enhanced the sealing of the defect.
To facilitate laparoscopic repair of a gastric perforation, a lactide–glycolid–caprolactone (LGC) patch, or the “stamp” method, has been proposed and tested in rats, and compared favorably to omentoplasty [9, 10]. To investigate the feasibility of endoluminal gastrotomy repair, an absorbable plug was successfully tested in dogs [11]. The first report of using polytetrafluoroethylene (PTFE) for stomach repair was an experimental study using rats, which demonstrated that it could seal a gastric defect with abnormal epithelialization [12]. The novel small intestinal submucosa (SIS) biomaterial was used to repair a 1 cm gastric defect in rats [13], resulting in limited mucosal regeneration after 21 days. Functional evaluation in a similar experimental model showed muscle contraction in the regenerated part of the stomach, combined with complete mucosal, submucosal and some muscular regeneration at 6 months [14].
A number of experimental studies have been performed to investigate the patch repair of a large gastroduodenal perforation. PTFE has been used in five studies, and has been shown to enable mucosal regeneration, although the resulting neomucosa was thin and could not completely bridge large defects [15–19]. SIS has been used in two models of duodenal defect repair: in a rat model, in combination with a poly(lactic-co-glycolic acid) (PLGA) layer [20] and in a porcine model combined with a porcine elastin layer [21], while an acellular porcine dermal collagen matrix patch was tested in a model with a complex duodenal wall defect [22]. The use of both materials resulted in complete mucosal and incomplete muscular regeneration [20–22].
The clinical use of prosthetic materials on upper gastrointestinal tract defects has been limited, but some reports of this do exist. A sutureless method for the laparoscopic treatment of an acute perforated duodenal ulcer using a gelatin sponge plug and fibrin sealant was described in 1993 [23] and led to a randomized study, which showed that the prolonged laparoscopic operation could be reduced when repair with sutures was not employed, without increased morbidity [24]. Controversially, a report by Gómez et al. [25] described a case of a successful PTFE closure of a duodenal stump dehiscence. Similar cases have been reported [18].
In the last decade, there has been an increase in the number of studies on the use of prosthetic materials to repair gastroduodenal defects. There are several reasons for this, the first being the aforementioned need for an easy laparoscopic technique for gastric ulcer repair. This is perhaps the indication with the more promising results, as shown by the experimental and clinical data, reinforced by the progress accrued using endoscopic techniques, which have shown the feasibility of plug repair. The second reason involves an attempt to overcome surgeons’ reluctance to use prosthetic materials on hollow viscera. The successful (although preliminary) results of ePTFE use are encouraging, and this material has potential, especially if an alternate manufacturing process can further enhance its ability to integrate into host tissue [18]. However, the new generation of collagen-based biomaterials show great promise with regard to not only gastroduodenal repair, but also many other applications.
Our choice of material was based on a number of reasons. Pericardial allografts, such as our material, have been used for several years with proven safety, efficacy and adequate strength for indications such as cardiac valve repair, aortic arch reconstruction, abdominal wall defects, tracheal reconstruction, diaphragmatic repair and as pericardial and dural grafts [26–32]. Since we needed a material that could be used in the upper gastrointestinal tract, the fact that pericardial tissue has been shown to be resistant to bile and pancreatic juice in vitro, and to have superior qualities compared to porcine dermal matrix [33], suggested that it might be more suitable for gastroduodenal repair. Furthermore, use in the gastrointestinal lumen necessitates a material which provokes the creation of robust repair tissue and has the ability to support it for a sufficient amount of time, qualities which we expected from our patch, since it has been subjected to collagen cross-linking, which inhibits immunogenicity and reduces collagenase-dependent degradation [34]. Another potential advantage is the equine origin of our patch: although pericardial scaffolds from different sources are similar, with small differences in thickness and mechanical strength, the equine pericardium does not raise the same concerns about the potential spread of infectious disease (e.g., transmissible spongiform encephalopathies) as does the bovine pericardium [35].
Our study confirmed that the equine pericardial patch we selected possesses the benefits we expected. It showed that the material could effectively seal a gastric defect and provide adequate strength to the stomach wall. Due to cross-linking, our patch tended to not only swiftly induce a robust fibrous capsule, rapidly sealing the defect, but also to resist degradation, remaining mostly intact even after 8 weeks, attributes which make it suitable for bridging gastrointestinal defects. In summary, we herein showed that a glutaraldehyde-treated equine pericardial patch could act as an excellent bio-scaffold in the repair of gastric defects in our experimental rabbit model.
We acknowledge that there are a number of possible limitations associated with our experimental model. We felt that the inclusion of a comparison or control group in this study was not appropriate, as our main focus was to determine and evaluate the histological integration and mechanical strength of the patch on the gastric wall. Through the application of this simple experimental model, we were able to acquire valuable information, without unnecessary morbidity, while keeping the number of animal subjects to a minimum.
In conclusion, this study demonstrated that an equine pericardial patch could be a reliable alternative in the repair of a gastric defect in our experimental animal model. The patch proved adequate to seal the defect, enabled mucosal regeneration, while the repair site achieved adequate strength to resist rupture by 2 weeks after implantation. This study shows that the equine pericardial patch is a promising biomaterial for repairing gastric defects. However, further laboratory-based research is required to ascertain the feasibility and safety of the patch on moderate and large-sized gastric and duodenal defects in comparison to a simple “primary suture” control group before this can be considered for clinical evaluation in the form of randomized trials.
References
Sanabria AE, Morales CH, Villegas MI. Laparoscopic repair for perforated peptic ulcer disease. Cochrane Database Syst Rev. 2005;(4):CD004778.
Song KY, Kim TH, Kim SN, Park CH. Laparoscopic repair of perforated duodenal ulcers: the simple “one-stitch” suture with omental patch technique. Surg Endosc. 2008;22(7):1632–5.
Lam PW, Lam MC, Hui EK, Sun YW, Mok FP. Laparoscopic repair of perforated duodenal ulcers: the “three-stitch” Graham patch technique. Surg Endosc. 2005;19(12):1627–30.
Lee J, Sung K, Lee D, Lee W, Kim W. Single-port laparoscopic repair of a perforated duodenal ulcer: intracorporeal “cross and twine” knotting. Surg Endosc. 2010 Jun 12.
Tsuei BJ, Schwartz RW. Management of the difficult duodenum. Curr Surg. 2004;61(2):166–71.
Lal P, Vindal A, Hadke NS. Controlled tube duodenostomy in the management of giant duodenal ulcer perforation: a new technique for a surgically challenging condition. Am J Surg. 2009;198(3):319–23.
Chen MD, Teigen GA, Reynolds HT, Johnson PR, Fowler JM. Laparoscopy versus laparotomy: an evaluation of adhesion formation after pelvic and paraaortic lymphadenectomy in a porcine model. Am J Obstet Gynecol. 1998;178(3):499–503.
Liu Z, Tang R, Zhou Z, Song Z, Wang H, Gu Y. Comparison of two porcine-derived materials for repairing abdominal wall defects in rats. PLoS One. 2011;6(5):e20520.
Bertleff MJ, Liem RS, Bartels HL, Robinson PH, Van der Werff JF, Bonjer HJ, Lange JF. The “stamp method”: a new treatment for perforated peptic ulcer? Surg Endosc. 2006;20(5):791–3.
Bertleff MJ, Stegmann T, Liem RS, Kors G, Robinson PH, Nicolai JP, Lange JF. Comparison of closure of gastric perforation ulcers with biodegradable lactide–glycolide–caprolactone or omental patches. JSLS. 2009;13(4):550–4.
Cios TJ, Reavis KM, Renton DR, Hazey JW, Mikami DJ, Narula VK, Allemang MT, Davis SS, Melvin WS. Gastrotomy closure using bioabsorbable plugs in a canine model. Surg Endosc. 2008;22(4):961–6.
Cağa T, Gürer F. Polytetrafluoroethylene patch grafting for closure of stomach defects in the rat. Br J Surg. 1993;80(8):1013–4.
De la Fuente SG, Gottfried MR, Lawson DC, Harris MB, Mantyh CR, Pappas TN. Evaluation of porcine-derived small intestine submucosa as a biodegradable graft for gastrointestinal healing. J Gastrointest Surg. 2003;7(1):96–101.
Ueno T, de la Fuente SG, Abdel-Wahab OI, Takahashi T, Gottfried M, Harris MB, Tatewaki M, Uemura K, Lawson DC, Mantyh CR, Pappas TN. Functional evaluation of the grafted wall with porcine-derived small intestinal submucosa (SIS) to a stomach defect in rats. Surgery. 2007;142(3):376–83.
Kung SP. Teflon-felt grafting of giant gastroduodenal perforation in a canine model. Surg Today. 2004;34(2):145–9.
Ozlem N, Erdogan B, Gültekin S, Dedeoglu S, Aydin A. Repairing great duodenal defects in rabbits by ePTFE patch. Acta Chir Belg. 1999;99(1):17–21.
Nikeghbalian S, Atefi S, Kazemi K, Jalaeian H, Roshan N, Naderi N, Hajizadeh R, Tanideh N. Repairing large duodenal injuries in dogs by expanded polytetrafluoroethylene patch. J Surg Res. 2008;144(1):17–21.
Oh DS, Manning MM, Emmanuel J, Broyles SE, Stone HH. Repair of full-thickness defects in alimentary tract wall with patches of expanded polytetrafluoroethylene. Ann Surg. 2002;235(5):708–11; (discussion 711–2).
Astarcioğlu H, Koçdor MA, Sökmen S, Karademir S, Ozer E, Bora S. Comparison of different surgical repairs in the treatment of experimental duodenal injuries. Am J Surg. 2001;181(4):309–12.
De Ugarte DA, Choi E, Weitzbuch H, Wulur I, Caulkins C, Wu B, Fonkalsrud EW, Atkinson JB, Dunn JC. Mucosal regeneration of a duodenal defect using small intestine submucosa. Am Surg. 2004;70(1):49–51.
Kajitani M, Wadia Y, Xie H, Hinds MT, Shalaby SW, Swartz KR, Gregory KW. Use of a new elastin patch and glue for repair of a major duodenal injury. ASAIO J. 2000;46(4):409–14.
Eckert MJ, Perry JT, Sohn VY, Keylock JB, Munaretto JA, Beekley AC, Martin MJ. Bioprosthetic repair of complex duodenal injury in a porcine model. J Trauma. 2009;66(1):103–9.
Tate JJ, Dawson JW, Lau WY, Li AK. Sutureless laparoscopic treatment of perforated duodenal ulcer. Br J Surg. 1993;80(2):235.
Lau WY, Leung KL, Kwong KH, Davey IC, Robertson C, Dawson JJ, Chung SC, Li AK. A randomized study comparing laparoscopic versus open repair of perforated peptic ulcer using suture or sutureless technique. Ann Surg. 1996;224(2):131–8.
Gómez NA, Roura E, León CJ, Vargas P, Zapatier JE. Use of a polytetrafluoroethylene tube and patch in the repair of a difficult duodenal stump. Acta Gastroenterol Latinoam. 2003;33(1):9–12.
Mueller XM, von Segesser LK. A new equine pericardial stentless valve. J Thorac Cardiovasc Surg. 2003;125(6):1405–11.
Montinaro A, Gianfreda CD, Proto P. Equine pericardium for dural grafts: clinical results in 200 patients. J Neurosurg Sci. 2007;51(1):17–9.
Morell VO, Wearden PA. Experience with bovine pericardium for the reconstruction of the aortic arch in patients undergoing a Norwood procedure. Ann Thorac Surg. 2007;84(4):1312–5.
Van Tuil C, Saxena AK, Willital GH. Experience with management of anterior abdominal wall defects using bovine pericard. Hernia. 2006;10(1):41–7.
Knott PD, Lorenz RR, Eliachar I, Murthy SC. Reconstruction of a tracheobronchial tree disruption with bovine pericardium. Interact Cardiovasc Thorac Surg. 2004;3(4):554–6.
Von Segesser L, Jornod N, Faidutti B. Repeat sternotomy after reconstruction of the pericardial sac with glutaraldehyde-preserved equine pericardium. J Thorac Cardiovasc Surg. 1987;93(4):616–9.
Santillan-Doherty P, Jasso-Victoria R, Sotres-Vega A, Olmos R, Arreola JL, Garcia D, Vanda B, Gaxiola M, Santibañez A, Martin S, Cabello R. Thoracoabdominal wall repair with glutaraldehyde-preserved bovine pericardium. J Invest Surg. 1996;9(1):45–55.
Hoeppner J, Marjanovic G, Helwig P, Hopt UT, Keck T. Extracellular matrices for gastrointestinal surgery: ex vivo testing and current applications. World J Gastroenterol. 2010;16(32):4031–8.
Butler CE. The role of bioprosthetics in abdominal wall reconstruction. Clin Plast Surg. 2006;33:199–211.
WHO guidelines on transmissible spongiform encephalopathies 2003.
Acknowledgments
The authors would like to thank Prof. Despina Perrea Director of the “N.S. Christeas” Laboratory for Experimental Surgery and Surgical Research, at the University of Athens Medical School, where the experimental surgical procedures took place and Dr. Alkistis Pantopoulou who was the supervising veterinarian. We would also like to thank Prof. Stavros Kourkoulis and Mr. Athanasios Mitousoudis from the National Technical University of Athens for the bursting pressure measurements and Ms. Myrto Kogevina for the editing of the manuscript. This work was supported by a University of Athens research grant (Kapodistrias programme).
Conflict of interest
Doctors Kostantinos Spiliopoulos, Charalampos Markakis, Periklis Tomos, Hariklia Gakiopoulou, Ioannis Nikolopoulos, Eleftherios Spartalis, Kostantinos Kontzoglou and Michael Safioleas have no conflicts of interest or financial ties to disclose.
Author information
Authors and Affiliations
Corresponding author
Additional information
K. Spiliopoulos and C. Markakis made equal contribution to this study.
Rights and permissions
About this article
Cite this article
Spiliopoulos, K., Markakis, C., Tomos, P. et al. Repair of gastric defects with an equine pericardial patch. Surg Today 45, 83–90 (2015). https://doi.org/10.1007/s00595-014-1072-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00595-014-1072-4