Abstract
Currently, glycated hemoglobin A1c (HbA1c) has been widely used to assess the glycemic control of patients with diabetes. However, HbA1c has certain limitations in describing both short-term and long-term glycemic control. To more accurately evaluate the glycemic control of diabetes patients, the continuous glucose monitoring (CGM) technology has emerged. CGM technology can provide robust data on short-term glycemic control and introduce new monitoring parameters such as time in range, time above range, and time below range as indicators of glycemic fluctuation. These indicators are used to describe the changes in glycemic control after interventions in clinical research or treatment modifications in diabetes patient care. Recent studies both domestically and internationally have shown that these indicators are not only associated with microvascular complications of diabetes mellitus but also closely related to cardiovascular disease complications and prognosis. Therefore, this article aims to comprehensively review the association between CGM-based glycemic parameters and cardiovascular disease complications by analyzing a large number of domestic and international literature. The purpose is to provide scientific evidence and guidance for the standardized application of these indicators in clinical practice, in order to better evaluate the glycemic control of diabetes patients and prevent the occurrence of cardiovascular disease complications. This research will contribute to improving the quality of life for diabetes patients and provide important references for clinical decision-making.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Background
Diabetes mellitus is a metabolic disorder characterized by elevated blood glucose levels due to defects in insulin secretion and/or action [1]. According to estimates, the global number of individuals aged 20–79 years with diabetes was 536.6 million in 2021 and is projected to increase to 783.2 million by 2045 [2]. Among individuals with diabetes, cardiovascular disease (CVD) is the leading cause of death, with a twofold increased risk compared to those without diabetes [3, 4]. The annual direct treatment cost for diabetes exceeds $827 billion globally. Additionally, between 2011 and 2030, the estimated GDP loss due to diabetes worldwide will reach $1.7 trillion, with high-income countries accounting for $900 billion and middle- to low-income countries accounting for $800 billion [5]. Research has shown that the major contributors to diabetes treatment costs are the macrovascular and microvascular complications, including coronary artery disease, myocardial infarction, peripheral vascular disease, retinopathy, end-stage renal disease, and peripheral neuropathy [6, 7]. Large-scale studies such as the Diabetes Control and Complications Trial (DCCT) [8] and the UK Prospective Diabetes Study (UKPDS) [9] have demonstrated a significant correlation between the incidence of clinical complications and blood glucose levels, as indicated by HbA1c. Strict control of blood glucose levels can reduce the occurrence of long-term complications associated with diabetes. Furthermore, it has been found that for every 1% reduction in HbA1c, there is a significant decrease in the risk of diabetes-related endpoints, diabetes-related mortality, myocardial infarction, and microvascular complications. The results also indicate that there is no observed threshold for these risks, meaning that the risk decreases proportionally with any decrease in blood glucose levels. Although there is substantial evidence linking increased HbA1c levels to diabetes-related complications, patients with the same HbA1c value can have different risks of complications. For example, in the DCCT cohort [10, 11], only 11% of the variation in the risk of retinopathy could be explained by overall glycemic exposure (HbA1c and duration of diabetes). Another study [12] found a weak correlation between average HbA1c levels and the presence and severity of cardiovascular autonomic neuropathy. The measurement of HbA1c results can also be influenced by various factors unrelated to blood glucose levels, such as age, race, anemia, chronic kidney failure, pregnancy, and medication use [13]. Moreover, HbA1c can only reflect blood glucose exposure over the past 8–12 weeks and cannot describe daily or intraday blood glucose fluctuations. A study by Beck et al. [14] demonstrated that there can be diverse blood glucose fluctuations at the same HbA1c level. Therefore, these limitations of HbA1c highlight the necessity of using other supplementary methods to assess blood glucose levels in diabetic patients, taking into account individual patient characteristics, other blood glucose monitoring indicators, and potential risk factors for complications. In recent years, with the continuous development of continuous glucose monitoring (CGM) technology and the emergence of multiple novel blood glucose control indices, it is now possible to obtain comprehensive information on a patient's blood glucose control throughout the day and the actual situation of patients with significant blood glucose fluctuations. For example, CGM blood glucose parameters such as time in range (TIR), time above range (TAR), and time below range (TBR) not only provide a visual representation of blood glucose fluctuations and effectively identify the risk of hypoglycemia but have also been widely used in clinical practice to evaluate the blood glucose control status of diabetic patients, treatment efficacy, and predict the incidence of clinical complications [15]. However, numerous domestic and international studies have shown that these indices are not only associated with microvascular complications in diabetes but also closely linked to macrovascular complications such as CVD. Therefore, based on a comprehensive analysis of a large number of domestic and international literature, this study aims to provide a review of the relationship between CGM-based blood glucose indices and the occurrence of cardiovascular disease complications. The objective is to provide a scientific basis and guidance for the standardized application of these indices in clinical practice.
The progress of blood glucose monitoring technology and the emergence of time in range (TIR) as a key indicator for glycemic control
Previous studies have shown that blood glucose can be monitored using various methods. Traditional blood glucose monitoring methods mainly include biochemical analyzers and home blood glucose meters, which utilize the reactions of glucose oxidase and hexokinase. Biochemical analyzers provide high accuracy in measuring blood glucose but require professional operation and are time-consuming, while home blood glucose meters are small in size, provide rapid results, and can be operated by patients themselves. However, both of these techniques cannot continuously record the dynamic changes in blood glucose levels. On the other hand, CGM technology, which combines the advantages of biochemical analyzers and home blood glucose meters, can continuously record the dynamic changes in blood glucose levels, making it widely used in clinical practice [16]. CGM mainly consists of a disposable wired sensor inserted into subcutaneous tissue, a transmitter connected to the sensor, and a receiver (or application) that displays glucose data. The sensor is the core component and directly determines the accuracy of blood glucose monitoring. Subsequently, CGM technology has made revolutionary advancements, including improvements in accuracy, reduction in device size and invasiveness, extension of sensor lifespan, and the approval of insulin dosing decisions. Additionally, factory-calibrated CGM devices no longer require fingerstick blood glucose measurements. These improvements have reduced the burden on diabetes patients, increased patient satisfaction and compliance with device use and medication therapy, and enhanced clinical awareness of CGM technology. As a result, CGM is widely applied in patients with type 1 diabetes mellitus (T1DM) and those receiving intensified insulin therapy for type 2 diabetes mellitus (T2DM) [17]. The advancements in CGM technology have also led to significant progress in its integration with continuous subcutaneous insulin infusion (CSII) or insulin pumps. This integration enables more accurate insulin delivery, as CGM technology provides real-time blood glucose data and trends, allowing the insulin pump to make more precise adjustments based on blood glucose fluctuations. Furthermore, the development of closed-loop systems, also known as automated insulin delivery systems, has emerged. These systems continuously monitor blood glucose and automatically adjust insulin delivery to maintain stable blood glucose levels. CGM technology has also facilitated the development and validation of novel blood glucose indices, including TIR, TAR, TBR, and coefficient of variation (CV) as measures of glycemic fluctuation. Research has shown that TIR is more accurate than self-monitoring of blood glucose (SMBG) and HbA1c in assessing blood glucose control [18]. Currently, CGM is divided into two categories: intermittent CGM (iCGM) and real-time CGM (rtCGM). There is increasing evidence of the significant advantages of CGM technology in improving blood glucose control. Multiple studies have demonstrated the clinical efficacy, safety, and other benefits of using rtCGM and iCGM in patients with T1DM and T2DM. The DIAMOND trial showed that compared to traditional SMBG, the use of rtCGM in patients receiving multiple daily injections (MDI) for T1DM and T2DM can improve HbA1c levels, reduce time spent in hypoglycemia and hyperglycemia, and lower the occurrence of moderate to severe hypoglycemia [19, 20]. Researchers have also reported reduced diabetes-related distress and increased confidence in managing hypoglycemia among rtCGM users [21]. Additionally, a recent randomized trial found that the use of rtCGM significantly increased the time spent in normal blood glucose range for individuals with impaired hypoglycemia awareness and reduced the occurrence of severe hypoglycemia [22]. The IMPACT study and the REPLACE randomized controlled trial have also demonstrated the association of iCGM use with reduced hypoglycemia, increased TIR, decreased blood glucose variability, and improved patient satisfaction, although no significant reduction in HbA1c was observed [23, 24]. However, a recent prospective observational study showed that compared to SMBG, the use of iCGM significantly reduced HbA1c levels in a large population of patients with T1DM and T2DM [25]. Some of these studies have also shown that the use of iCGM can significantly reduce hospitalizations due to hypoglycemia [26, 27]. In 2017, an expert consensus on interpreting continuous glucose monitoring (CGM) data was established, which identified 14 key CGM metrics for assessing blood glucose status, including the recommendation of TIR as a key indicator for blood glucose control in clinical trials [28]. In 2019, the expert group reconvened to establish specific CGM targets related to these metrics, including TIR measurement methods, technical requirements, and individualized patient goal setting, to assist healthcare professionals and diabetes patients in interpreting and utilizing CGM data for routine clinical care [29]. The 2020 DM Prevention and Treatment Guidelines [1] introduced TIR as an indicator for evaluating blood glucose levels. From consensus to guidelines, TIR has gained increasing attention from both healthcare professionals and patients due to its simple and intuitive presentation of blood glucose levels. It is considered a "promising" indicator that has the potential to surpass HbA1c in evaluating treatment effectiveness and blood glucose control (Table 1, 2).
The blood glucose fluctuation indices of CGM and their definitions
-
1.
Average Glucose Profile (AGP): Divides the blood glucose data within a 24-h period into multiple time segments (usually 2 or 3 h per segment) and calculates the average blood glucose value for each segment. Reflects the daily synchronicity of blood glucose patterns and is typically represented by the interquartile range (IQR) of the AGP [30].
-
2.
Mean Blood Glucose (MBG): Represents the average blood glucose level over a specific period of time, typically measured in millimoles per liter (mmol/L) or milligrams per deciliter (mg/dL) [31].
-
3.
Time in Range (TIR): The percentage of time within the target blood glucose range (70–180 mg/dL or 3.9–10.0 mmol/L) within a 24-h period [31].
-
4.
Time Above Range (TAR): Part of the High Blood Glucose Index (HBGI), indicating the time when blood glucose levels are above the target range (181–250 mg/dL or 10.1–13.9 mmol/L).
-
5.
Time Below Range (TBR): Part of the Low Blood Glucose Index (LBGI), indicating the time when blood glucose levels are below the target range (54–69 mg/dL or 3.0–3.8 mmol/L) and (< 54 mg/dL or < 3.0 mmol/L).
-
6.
Coefficient of Variation (CV): Calculated as (standard deviation/mean) × 100%, CV is an independent parameter reflecting the degree of blood glucose variability.
-
7.
Standard Deviation (SD): The square root of variance, representing the distribution or variability of blood glucose readings around the mean. SD is greatly influenced by the mean blood glucose level.
-
8.
Variability Independent of Mean (VIM): Derived from logarithmic curve fitting, VIM is calculated as 100 × SD/meanβ, where β is the regression coefficient of the natural logarithm of the standard deviation to the natural logarithm of the mean [32]. A smaller VIM value indicates less blood glucose fluctuation and more stable blood glucose control.
-
9.
Mean Amplitude of Glycemic Excursions (MAGE): Calculated by summing the amplitudes of all valid glycemic fluctuations (excluding those below a certain threshold, typically 1 standard deviation) and dividing by the number of valid fluctuations.
The relationship between CGM blood glucose indicators and cardiovascular diseases (CVD)
The relationship between TIR, TAR, TBR, and CVD.
Arterial stiffness (AS)
Foreman et al. [33] observed in the Maastricht study population that an increase in daily glycemic variability (GV) and a decrease in TIR were associated with greater arterial stiffness. However, Helleputte et al. [34] found no association between arterial stiffness and any CGM parameters (TIR, TBR, TAR, or GV parameters) in patients with T1DM (all p-values > 0.26). It is important to note that the current research on the relationship between TIR and AS is still relatively limited and there are some differences in research methods and sample sizes. Furthermore, the causal relationship between TIR and arterial stiffness has not been fully elucidated and further research is needed to validate and better understand this relationship. In summary, maintaining a proportion of time within the target range for blood glucose levels may contribute to improving arterial elasticity and reducing stiffness, but the specific mechanisms influencing and factors require further investigation.
Cardiac function (CF)
In a study conducted by Wang et al. [35], a HFA-PEFF score was performed on 523 patients with T2DM. A HFA-PEFF score of ≥ 5 was defined as a positive result, and it was found that T2DM patients with lower TIR had an increased risk of heart failure with preserved ejection fraction (HFpEF). A lower TIR was associated with a higher likelihood of developing HFpEF. Further research and clinical practice are needed to better understand the relationship between TIR and cardiac function and to provide more effective management strategies for the cardiac health of patients with diabetes.
Arrhythmias
Guo et al. [36] found that in patients with diabetes and atrial fibrillation (AF), an increase in the quartiles of TIR was significantly associated with a reduced risk of stroke. Zhou et al. demonstrated that MAGE and TAR were positively correlated with the occurrence of cardiac arrhythmias in middle-aged patients with diabetes, while TIR was negatively correlated with the occurrence of cardiac arrhythmias in elderly patients with diabetes. MAGE and TBR were positively correlated with cardiac arrhythmias in elderly patients [37]. Therefore, actively controlling glycemic variability in patients with diabetes may help reduce the risk of severe cardiac arrhythmias and their complications.
Cardiac autonomic neuropathy (CAN)
CAN is a common and serious complication, and dysfunction in any part of the reflex arc can lead to disorders in orthostatic blood pressure response, including orthostatic hypotension (OH) and orthostatic hypertension (OHT). OH is a major risk factor for syncope and falls in the elderly population, while OHT is associated with the early stages of diabetic neuropathy. Yuan et al. [38] reported that low TIR and low body mass index (BMI) were independent risk factors for OH in patients with T2DM, while low TIR and high BMI were independent risk factors for OHT. Additionally, there was a negative correlation between absolute blood pressure changes and TIR. GUO et al. [39] conducted a CAN function test on 349 patients with type 2 diabetes to explore the correlation between TIR and CAN. The results showed a significant negative correlation between TIR and the total CAN score. After adjusting for age, duration of diabetes, gender, blood lipids, blood creatinine, BMI, blood pressure, HbA1c, and other glycemic variability indicators, TIR remained significantly correlated with CAN, independent of HbA1c, and other glycemic variability indicators. A study involving 284 outpatient patients with type 2 diabetes also confirmed a significant negative correlation between TIR and CAN. It was also found that TAR was independently associated with the presence and severity of CAN [40]. Jun et al., after adjusting for mean blood glucose and clinical risk factors for CAN, found that SD, CV, MAGE, percentage of time spent in level 1 (blood glucose 54–69 mg/dL) and level 2 (blood glucose < 54 mg/dL) hypoglycemia, area under the curve (AUC) during level 2 hypoglycemia, LBGI, HBGI, and percentage of time spent in the range of 70 to 180 mg/dL were all independently associated with CAN, with the highest independent association observed for the percentage of time spent in level 2 hypoglycemia [41]. Jaiswal et al. also indicated a negative correlation between LBGI, AUC, and CAN in patients with type 1 diabetes [42]. Racca et al. also confirmed a correlation between low blood glucose parameters such as TBR and LBGI and a lower prevalence of CAN [43].
The aforementioned studies indicate a close association between TIR, TBR, and CAN, suggesting that they serve as protective factors for CAN. Obtaining data from both TIR and TBR are beneficial for the clinical assessment of CAN risk. Therefore, maintaining good glycemic control can improve cardiovascular autonomic function. However, it is crucial to consider the influence of other factors when managing diabetes and preventing cardiac autonomic neuropathy. A comprehensive treatment strategy is of utmost importance in effectively managing diabetes and preventing the development of cardiovascular autonomic neuropathy.
Carotid artery stenosis (CAS)
Carotid intima-media thickness (CIMT) is an early clinical indicator of carotid atherosclerosis. A prospective cohort study conducted in 2019 demonstrated that carotid plaques and CIMT can enhance the identification of cardiovascular events (CVE) and serve as independent predictors of cardiovascular events [44]. Lu et al. analyzed the relationship between CIMT and TIR obtained from CGM in 2,215 patients with type 2 diabetes. The study found that patients with abnormal CIMT had significantly lower TIR levels compared to those with normal CIMT. After adjusting for traditional cardiovascular disease risk factors, every 10% increase in TIR was associated with a 6.4% decrease in the risk of abnormal CIMT [45]. SUN et al. also found a negative correlation between CIMT and TIR, as well as a positive correlation between CIMT and TAR. Decreased TIR and increased TAR were identified as risk factors for CIMT thickening in patients with type 2 diabetes [46]. Duan et al. further confirmed the negative correlation between CIMT and TIR [47]. However, a cross-sectional study conducted on patients with type 1 diabetes yielded different results. The study aimed to evaluate the relationship between TIR collected in the short-term and long-term and CIMT in patients with type 1 diabetes. It included 70 patients with type 1 diabetes and 35 healthy controls. The results showed a significant increase in CIMT in patients with type 1 diabetes compared to healthy controls, but no significant association was found between TIR and CIMT. The study suggested that young patients with type 1 diabetes may exhibit early vascular abnormalities, but TIR is not related to subclinical atherosclerosis [48]. The contradictory results may be attributed to the short interval between CGM measurements and vascular studies (2 weeks, 3 months, and 6 months), which may not influence the measurement of atherosclerosis markers. Additionally, the wide range of TIR standards may not accurately differentiate between young patients with some metabolic compensation and poorly controlled patients. This study emphasizes the complexity of the interaction between diabetes and atherosclerosis, and further prospective research is needed to confirm the relationship between the two. Furthermore, several studies have indicated a positive correlation between the occurrence of carotid atherosclerosis in patients with type 2 diabetes and TAR, as well as a negative correlation with TIR. Decreased TIR and increased TAR are independent risk factors for T2DM combined with CAS [49, 50]. However, a study conducted on patients with T1DM suggested that only TBR was significantly associated with carotid atherosclerosis after adjusting for the average HbA1c over 5 years, while TIR, TAR, and other indicators were only independently associated with microvascular complications [51].
Therefore, there is a certain relationship between TIR and CIMT, and controlling TIR may help reduce the risk of CAS. Both high and low blood glucose levels may exacerbate the development of carotid atherosclerosis. However, further research is still needed to clarify this relationship and provide more specific guidance for the treatment of patients with diabetes.
Coronary artery disease (CAD)
After controlling for confounding factors such as HbA1C, Wang et al. found a significant independent correlation between TIR and the severity of CAD as well as the risk of acute coronary syndrome (ACS) in patients with T2DM and concomitant CAD. A lower TIR level was associated with a higher SYNTAX score (indicating greater severity of coronary artery disease) and an increased risk of acute coronary syndrome [52]. In a study by Snell-Bergeon et al. [53], coronary artery calcification was found to be associated with TIR and TAR in male patients with T1DM, but no such association was observed in females. The potential gender differences in this relationship warrant further investigation. Currently, direct research on the relationship between TIR and coronary artery stenosis is lacking, and further original studies are needed to confirm these findings.
Lower extremity arterial disease (LEAD)
A cross-sectional observational study conducted by Li et al. [54] involving 336 patients with T2DM demonstrated an association between TIR and LEAD. Patients with lower TIR levels in the T2DM population showed a higher prevalence of LEAD (P < 0.01), while the incidence of LEAD decreased with increasing quartiles of TIR (P < 0.05). In a prospective study by Wang et al. [55] involving 1351 adult patients with T2DM, a negative correlation was also found between TIR and the risk of LEAD. After adjusting for multiple variables, a 10% decrease in TIR was associated with a 7% increased risk of progressive LEAD. Furthermore, the study by Wen et al. [56] indicated a correlation between lower extremity arterial disease and carotid artery disease. As the severity of lower extremity arterial disease increased, so did the severity of carotid artery disease, and both were associated with the occurrence of cardiovascular and cerebrovascular disease (CCVD). These findings provide guidance for using TIR as a predictor of cardiovascular and cerebrovascular disease.
Major adverse cardiovascular events (MACE)
Multiple studies have shown a close correlation between blood glucose fluctuations and the occurrence of MACE in patients with diabetes, highlighting the importance of clinical attention to this issue. The DEVOTE trial [57], which included 7637 patients diagnosed with CVD or at high risk of CVD with T2DM, calculated the TIR based on the 8-point glucose profile (8 pp) data of these patients within the target range. The results demonstrated a correlation between TIR and the risk of first MACE. When comparing patients with TIR > 70% to those with TIR ≤ 50% or TIR ≤ 70%, the former group showed a reduced risk of first MACE (HR 0.74, 95% CI 0.60–0.91, P < 0.01). Additionally, Bergenstal et al. conducted a post hoc analysis investigating the association between the within the target TIR (derived TIR,dTIR) obtained from the 8-point glucose profile of T2DM patients in the DEVOTE trial and cardiovascular events or severe hypoglycemic events. At 12 months, there was a significant negative correlation between dTIR and time to first MACE (P = 0.0087) as well as time to severe hypoglycemic episode (P < 0.0001). There was also a nonsignificant trend observed between HbA1c and these outcomes, but this association became nonsignificant after adding dTIR to the model. These findings support setting a TIR target of > 70% and suggest that dTIR can serve as a clinical biomarker, potentially replacing the use of HbA1c in certain situations [58]. Another prospective cohort study involving 6,225 Chinese adults with type 2 diabetes revealed that for every 10% decrease in TIR, the risk of CVD mortality increased by 5% (HR 1.05, 95% CI 1.00–1.11), and the risk of all-cause mortality increased by 8% (HR 1.08, 95% CI 1.05–1.12) [59]. This indicates that lower TIR is associated with an increased long-term risk of all-cause and cardiovascular mortality. In a study by Bezerra et al. [60], it was demonstrated that TIR is also associated with cardiovascular complications in patients with type 1 diabetes and may be a better predictive indicator than HbA1c.
However, the DIGAMI 2 study [61] reported no relationship between 48-h TIR and acute cardiovascular complications in 578 patients with type 2 diabetes and acute myocardial infarction (AMI). The study results also indicated that glycemic variability was not associated with a composite measure of death, non-fatal stroke, and reinfarction during the initial hospitalization and up to 1-year follow-up. The discrepancy in these findings may be attributed to the relatively short duration of CGM in the patients. Similarly, a study [62] by EI Malahi et al. also indicated no association between TIR, SD, CV, and macrovascular complications in patients with T1DM, possibly due to the significant impact of insulin use on blood glucose in these patients. Furthermore, Mita et al. [63] designed a prospective observational study in 2019 to investigate the relationship between blood glucose fluctuations and cardiovascular events in patients with type 2 diabetes. The results of this study are expected to be published in 2024.
The above-mentioned studies suggest that assessing TIR over an extended period of time in patients with diabetes can help identify individuals at higher risk of developing cardiovascular disease, enabling early prevention. However, further research is needed to validate these findings.
The relationship between other CGM blood glucose variability indices and cardiovascular disease.
Cardiac structure and function
Yang et al. conducted a study involving 48 elderly patients with T2DM to investigate the correlation between blood glucose variability and cardiac function using dynamic blood glucose monitoring and echocardiography. The results revealed a significant negative correlation between MAGE and cardiac output (CO) as well as left ventricular ejection fraction (LVEF) [64]. Additionally, a study involving 100 asymptomatic patients with type 2 diabetes and no coronary artery disease demonstrated that glycemic variability assessed by SD was associated with left ventricular diastolic function [65]. Dzhun et al. also found that increased SD was related to diastolic dysfunction and could potentially contribute to the progression and occurrence of heart failure in patients with type 2 diabetes and no coronary artery disease [66]. Wang et al. also discovered a positive correlation between HFA-PEFF score and CV, SD, and MAGE [35]. These findings suggest that reducing glycemic variability may serve as a potential novel therapeutic strategy for preventing the development of HFpEF in patients with type 2 diabetes.
Cardiac autonomic neuropathy (CAN)
Wang et al. pointed out that the duration of diabetes and MAGE are risk factors for diabetic CAN [67]. Xu et al. also found a significant correlation between MAGE and CAN, with higher MAGE values associated with a higher prevalence of CAN [68]. Naaman et al. discovered that increased CV, SD, and MAGE were all associated with a higher prevalence of CAN in patients with T1DM [69]. Racca et al. further revealed a significant positive correlation between CV and CAN after adjusting for confounding factors [43].
In summary, blood glucose variability indices such as MAGE are closely associated with the occurrence of CAN. Therefore, in addition to controlling blood glucose levels, it is important to pay attention to the management of blood glucose fluctuations to reduce the risk of CAN in patients with diabetes. These research findings provide important theoretical evidence for the prevention and treatment of CAN and offer guidance for the clinical management of patients with diabetes.
Carotid artery stenosis (CAS)
Several studies have reported a positive correlation between CIMT and blood glucose variability indices, including standard deviation of blood glucose (SDBG), MBG, MAGE, and maximum amplitude of glycemic excursions (MAXAGE). These indices are considered risk factors for CIMT thickening in patients with type 2 diabetes [46, 47]. A meta-analysis has indicated that controlling glycemic variability can improve insulin resistance, reduce the thickness of the carotid intima-media layer, and lower the risk of CVD [70]. However, a study by Cesana et al. [71] exploring the relationship between blood glucose variability and CIMT in patients with uncomplicated T1DM found that the thickness and stiffness of the carotid intima-media were significantly associated with age, blood pressure, heart rate, and daily insulin intake but, not significantly correlated with indices such as MBG, SD, MAGE, and postprandial hyperglycemic peaks. The discrepancy in these results may be attributed to the relatively short duration of continuous glucose monitoring, which only captured 24-h blood glucose levels. Additionally, the inclusion of a limited number of study subjects may have affected the strength of the results.
Coronary artery disease (CAD)
Some studies have shown an association between blood glucose variability and the progression of CAD. For instance, Tsuyoshi Ito et al. [72] found that patients with higher MAGE were more likely to experience coronary artery spasm, and MAGE was positively correlated with changes in coronary artery diameter under acetylcholine stimulation. These findings suggest that daily blood glucose variability may play a role in the mechanism of coronary artery spasm. Snell-Bergeon et al. [53] found a relationship between coronary artery calcification and MBG and SD in male patients with type 1 diabetes, but no such association was observed in females. Benalia et al. [73] discovered that T2DM patients admitted for AMI with poor glycemic control had significantly higher SYNTAX scores, and patients with higher GV represented by MAGE had increased severity of CAD, independent of HbA1c levels. Su et al. [74] conducted coronary angiography on 344 patients with T2DM and chest pain symptoms, revealing that 252 patients had coronary artery disease (luminal diameter stenosis ≥ 50%), while 92 patients did not. Furthermore, the study found that MAGE (p < 0.001) was significantly higher in patients with coronary artery disease compared to those without, and MAGE ≥ 3.4 mmol/L was an independent predictor for coronary artery disease. Zhang et al. [75] also confirmed that T2DM patients with higher MAGE had more severe coronary artery stenosis, and they highlighted a significant correlation between blood glucose fluctuations and intima-media thickness of the carotid artery in T2DM patients.
These studies indicate a correlation between blood glucose fluctuations and the presence and severity of CAD in diabetic patients. The impact of blood glucose variability on vascular complications should not be overlooked in diabetic patients. Therefore, early assessment of glycemic variability may help identify high-risk patients for coronary heart disease and serve as a therapeutic target for primary and secondary prevention.
Major adverse cardiovascular events (MACE)
Su et al. also found a significant correlation between admission MAGE levels and the incidence of MACE in AMI patients, indicating that increased GV during admission may be more important than admission blood glucose and previous long-term abnormal glycemic status in predicting MACE within one year in AMI patients [76]. Zhang et al. [77] also demonstrated a relationship between higher MAGE and MACE during in-hospital and 30-day follow-up periods in patients with first-time percutaneous coronary intervention for acute myocardial infarction. A study conducted abroad included 417 patients with reperfusion in ACS to explore the association between GV and ACS prognosis. The results showed that major adverse cardiovascular events were more common in the high MAGE group (p = 0.002). In multivariate analysis, GV assessed by MAGE was an independent predictor of adverse prognosis in patients with cerebrovascular disease and ACS (p = 0.045) [78]. A meta-analysis [79] also indicated that regardless of diabetes status and subtypes of coronary heart disease, MAGE at admission may be associated with the incidence of MACE during follow-up in coronary heart disease patients. Gerbaud et al. [80] proposed that during the initial hospitalization period, GV assessed by SD (critical value > 2.70 mmol/L) was an independent predictor of cardiovascular complications in DM patients with ACS, including recurrent AMI, acute heart failure, and cardiac death. Besch et al. [81] calculated the average daily δ blood glucose in the first two days after transcatheter aortic valve implantation (TAVI) in 160 patients and used it as a measure of postoperative glycemic variability to analyze major complications within 30 days, including death, stroke, myocardial infarction, acute heart failure, and life-threatening arrhythmias. The rate of major complications within 30 days was 12% in patients in the lowest quartile of glycemic variability, while it increased to 26% and 39% in the third and fourth quartiles, respectively. Multivariate analysis showed that glycemic variability was independently associated with the risk of major complications within 30 days (p = 0.006).
Although most studies have reported an association between GV and CAD and its prognosis, some literature has reported a lack of association between GV and CAD. The HEART2D study [82] reported that daily glycemic variability assessed by MAGE, mean absolute glucose (MAG), and SD was not associated with cardiovascular events in 1,115 T2DM patients after AMI, and it was suggested that treatment strategies targeting GV did not have significant benefits in reducing subsequent cardiovascular events. In addition, the FLAT-SUGAR trial [83] compared the effect of short-acting insulin with glucagon-like peptide-1 receptor agonists (GLP-1RA) on glycemic variability in patients receiving basal insulin therapy. They reported that GLP-1RA significantly reduced glycemic variability determined by CGM compared to prandial insulin. Although glycemic variability improved, there were no statistically significant differences in cardiac arrhythmias, cardiovascular risk biomarkers, or severe hypoglycemic events.
Nusca et al. [84] found a significant correlation between glycemic status and high platelet reactivity (HPR) in T2DM patients undergoing percutaneous coronary intervention (PCI) and receiving clopidogrel treatment. HbA1c could identify patients at higher risk of thrombosis. However, the combined use of GV represented by MAGE and CV with HbA1c could achieve higher diagnostic accuracy. Therefore, future research should focus on evaluating whether individualized anti-thrombotic and hypoglycemic treatments based on these parameters can reduce the incidence of thrombotic events in patients undergoing PCI.
Summary and outlook
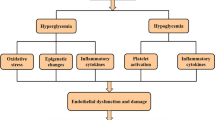
This review provides a comprehensive summary of the research progress on the association between glycemic fluctuation indices measured by CGM and CVD complications. Through a comprehensive analysis of numerous relevant studies, the following conclusions have been drawn. Firstly, there is a clear association between glycemic fluctuation indices and CVD complications. Studies have indicated that both high and low glycemic fluctuations are closely related to the occurrence and development of CVD. Increased glycemic fluctuations may lead to endothelial dysfunction, enhanced inflammatory response, and intensified oxidative stress, thereby increasing the risk of CVD [85]. Secondly, the application of CGM technology provides more accurate and comprehensive data support for the study of glycemic fluctuation indices and CVD complications. Compared to traditional blood glucose monitoring methods, CGM technology can provide continuous and real-time glucose data, which better reflects glycemic fluctuations. This offers researchers a more reliable basis for exploring the relationship between glycemic fluctuation and CVD. However, there are still some controversies and uncertainties regarding the underlying mechanisms of the association between glycemic fluctuation indices and CVD complications. On one hand, different research results show some heterogeneity, possibly influenced by factors such as sample size, study design, and statistical methods. On the other hand, there are differences in the definition and assessment methods of glycemic fluctuation indices, leading to inconsistent research findings. Future studies can be conducted in the following aspects: firstly, further elucidating the underlying biological and molecular mechanisms of the association between glycemic fluctuation indices and CVD complications. Secondly, strengthening research on different populations and different types of diabetes to better understand whether there are differences in the relationship between glycemic fluctuation and CVD. Additionally, standardizing the definition and assessment methods of glycemic fluctuation indices can improve the comparability and reliability of research results. In summary, research on the association between glycemic fluctuation indices measured by CGM and CVD complications has made certain progress, but there are still issues that require further investigation and exploration. Through further in-depth research, we can better understand the relationship between glycemic fluctuation and CVD and provide more effective strategies and methods for the prevention and treatment of CVD.
References
Advani A (2020) Positioning time in range in diabetes management. Diabetologia 63(2):242–252. https://doi.org/10.1007/s00125-019-05027-0
Sun H, Saeedi P, Karuranga S et al (2022) IDF diabetes atlas: global, regional and country-level diabetes prevalence estimates for 2021 and projections for 2045. Diabetes Res Clin Pract 183:109119. https://doi.org/10.1016/j.diabres.2021.109119
Fox CS, Coady S, Sorlie PD et al (2007) Increasing cardiovascular disease burden due to diabetes mellitus: the Framingham Heart Study. Circulation 115(12):1544–1550. https://doi.org/10.1161/CIRCULATIONAHA.106.658948
Preis SR, Hwang SJ, Coady S et al (2009) Trends in all-cause and cardiovascular disease mortality among women and men with and without diabetes mellitus in the Framingham Heart Study, 1950 to 2005. Circulation 119(13):1728–1735. https://doi.org/10.1161/CIRCULATIONAHA.108.829176
Henning RJ (2018) Type-2 diabetes mellitus and cardiovascular disease. Future Cardiol 14(6):491–509. https://doi.org/10.2217/fca-2018-0045
Andersson E, Persson S, Hallén N et al (2020) Costs of diabetes complications: hospital-based care and absence from work for 392,200 people with type 2 diabetes and matched control participants in Sweden. Diabetologia 63(12):2582–2594. https://doi.org/10.1007/s00125-020-05277-3
Jeyam A, McGurnaghan SJ, Blackbourn LAK et al (2020) Diabetic neuropathy is a substantial burden in people with type 1 diabetes and is strongly associated with socioeconomic disadvantage: a population-representative study from Scotland. Diabetes Care 43(4):734–742. https://doi.org/10.2337/dc19-1582
The DCCT Research Group (1987) Diabetes Control and Complications Trial (DCCT): results of feasibility study. Diabetes Care 10(1):1–19. https://doi.org/10.2337/diacare.10.1.1
Stratton IM, Adler AI, Neil HA et al (2000) Association of glycaemia with macrovascular and microvascular complications of type 2 diabetes (UKPDS 35): prospective observational study. BMJ 321(7258):405–412. https://doi.org/10.1136/bmj.321.7258.405
Lachin JM, Genuth S, Nathan DM, Zinman B, Rutledge BN (2008) Effect of glycemic exposure on the risk of microvascular complications in the diabetes control and complications trial—revisited. Diabetes 57(4):995–1001. https://doi.org/10.2337/db07-1618
The relationship of glycemic exposure (HbA1c) to the risk of development and progression of retinopathy in the diabetes control and complications trial (1995). Diabetes 44(8):968–83
Lai YR, Huang CC, Chiu WC et al (2019) HbA1C variability is strongly associated with the severity of cardiovascular autonomic neuropathy in patients with type 2 diabetes after longer diabetes duration. Front Neurosci 13:458. https://doi.org/10.3389/fnins.2019.00458
Little RR, Rohlfing C, Sacks DB (2019) The National Glycohemoglobin standardization program: over 20 years of improving Hemoglobin A measurement. Clin Chem 65(7):839–848. https://doi.org/10.1373/clinchem.2018.296962
Beck RW, Connor CG, Mullen DM, Wesley DM, Bergenstal RM (2017) The fallacy of average: how using HbA1c alone to assess glycemic control can be misleading. Diabetes Care 40(8):994–999. https://doi.org/10.2337/dc17-0636
Vigersky RA (2019) Going beyond HbA1c to understand the benefits of advanced diabetes therapies. J Diabetes 11(1):23–31. https://doi.org/10.1111/1753-0407.12846
Tang YL, Li GX, Tan ZW, Lei P, Yao LP (2022) Review of continuous blood glucose monitoring techniques. China Med Dev 37(06):149–152. https://doi.org/10.3969/j.issn.1674-1633.2022.06.035
Galindo RJ, Aleppo G (2020) Continuous glucose monitoring: The achievement of 100 years of innovation in diabetes technology. Diabetes Res Clin Pract 170:108502. https://doi.org/10.1016/j.diabres.2020.108502
Beyond A1C Writing Group (2018) Need for regulatory change to incorporate beyond A1C glycemic metrics. Diabetes Care 41(6):e92–e94. https://doi.org/10.2337/dci18-0010
Beck RW, Riddlesworth T, Ruedy K et al (2017) Effect of continuous glucose monitoring on glycemic control in adults with type 1 diabetes using insulin injections: the DIAMOND randomized clinical trial. JAMA 317(4):371–378. https://doi.org/10.1001/jama.2016.19975
Beck RW, Riddlesworth TD, Ruedy K et al (2017) Continuous glucose monitoring versus usual care in patients with type 2 diabetes receiving multiple daily insulin injections: a randomized trial. Ann Intern Med 167(6):365–374. https://doi.org/10.7326/M16-2855
Polonsky WH, Hessler D, Ruedy KJ, Beck RW, DIAMOND Study Group (2017) The impact of continuous glucose monitoring on markers of quality of life in adults with type 1 diabetes: further findings from the DIAMOND randomized clinical trial. Diabetes Care 40(6):736–741. https://doi.org/10.2337/dc17-0133
van Beers CA, DeVries JH, Kleijer SJ et al (2016) Continuous glucose monitoring for patients with type 1 diabetes and impaired awareness of hypoglycaemia (IN CONTROL): a randomised, open-label, crossover trial. Lancet Diabetes Endocrinol 4(11):893–902. https://doi.org/10.1016/S2213-8587(16)30193-0
Bolinder J, Antuna R, Geelhoed-Duijvestijn P, Kröger J, Weitgasser R (2016) Novel glucose-sensing technology and hypoglycaemia in type 1 diabetes: a multicentre, non-masked, randomised controlled trial. Lancet 388(10057):2254–2263. https://doi.org/10.1016/S0140-6736(16)31535-5
Haak T, Hanaire H, Ajjan R, Hermanns N, Riveline JP, Rayman G (2017) Flash glucose-sensing technology as a replacement for blood glucose monitoring for the management of insulin-treated type 2 diabetes: a multicentre, open-label randomized controlled trial. Diabetes Ther 8(1):55–73. https://doi.org/10.1007/s13300-016-0223-6
Yaron M, Roitman E, Aharon-Hananel G et al (2019) Effect of flash glucose monitoring technology on glycemic control and treatment satisfaction in patients with type 2 diabetes. Diabetes Care 42(7):1178–1184. https://doi.org/10.2337/dc18-0166
Quality of Life and Glucose Control After 1 Year of Nationwide Reimbursement of Intermittently Scanned Continuous Glucose Monitoring in Adults Living With Type 1 Diabetes (FUTURE): a prospective observational real-world cohort study. Diabetes Care. 2020;43(2):389–397. https://doi.org/10.2337/dc19-1610
Fokkert M, van Dijk P, Edens M et al (2019) Improved well-being and decreased disease burden after 1-year use of flash glucose monitoring (FLARE-NL4). BMJ Open Diabetes Res Care 7(1):e000809. https://doi.org/10.1136/bmjdrc-2019-000809
Danne T, Nimri R, Battelino T et al (2017) International consensus on use of continuous glucose monitoring. Diabetes Care 40(12):1631–1640. https://doi.org/10.2337/dc17-1600
Battelino T, Danne T, Bergenstal RM et al (2019) Clinical targets for continuous glucose monitoring data interpretation: recommendations from the international consensus on time in range. Diabetes Care 42(8):1593–1603. https://doi.org/10.2337/dci19-0028
Tokutsu A, Okada Y, Mita T et al (2022) Relationship between blood glucose variability in ambulatory glucose profile and standardized continuous glucose monitoring metrics: subanalysis of a prospective cohort study. Diabetes Obes Metab 24(1):82–93. https://doi.org/10.1111/dom.14550
Sun B, Luo Z, Zhou J (2021) Comprehensive elaboration of glycemic variability in diabetic macrovascular and microvascular complications. Cardiovasc Diabetol 20(1):9. https://doi.org/10.1186/s12933-020-01200-7
Kim JA, Lee JS, Chung HS et al (2018) Impact of visit-to-visit fasting plasma glucose variability on the development of type 2 diabetes: a nationwide population-based cohort study. Diabetes Care 41(12):2610–2616. https://doi.org/10.2337/dc18-0802
Foreman YD, van Doorn WPTM, Schaper NC et al (2021) Greater daily glucose variability and lower time in range assessed with continuous glucose monitoring are associated with greater aortic stiffness: the Maastricht Study. Diabetologia 64(8):1880–1892. https://doi.org/10.1007/s00125-021-05474-8. (Epub 2021 May 15)
Helleputte S, Calders P, Rodenbach A et al (2022) Time-varying parameters of glycemic control and glycation in relation to arterial stiffness in patients with type 1 diabetes. Cardiovasc Diabetol 21(1):277. https://doi.org/10.1186/s12933-022-01717-z
Wang DY, Deng XR et al (2023) Association between time in range of glucoseand heart failure with preserved ejection fraction in patients with type 2 diabetes. J Chin Pract Diagn Ther 37(6):570–575. https://doi.org/10.13507/j.issn.1674-3474.2023.06.006
Guo J, Wang J, Zhao Z, Yu L (2021) Association between glycemic control assessed by continuous glucose monitoring and stroke in patients with atrial fibrillation and diabetes mellitus. Ann Palliat Med 10(8):9157–9164. https://doi.org/10.21037/apm-21-2198
Zhou ZP, Liu JF (2023) Relationship between blood glucose variability and arrhythmia in diabetes mellitus complicated with chronic cardiovascular and cerebrovascular diseases. China Mod Doctor 61(11):47–51. https://doi.org/10.3969/j.issn.1673-9701.2023.11.011
Yuan Y, Lu B, Guo Q et al (2022) Time in range, as an emerging metric of glycemic control, is associated with orthostatic blood pressure changes in type 2 diabetes. Diabetes Res Clin Pract 183:109179. https://doi.org/10.1016/j.diabres.2021.109179
Guo Q, Zang P, Xu S et al (2020) Time in range, as a novel metric of glycemic control, is reversely associated with presence of diabetic cardiovascular autonomic neuropathy independent of HbA1c in Chinese type 2 diabetes. J Diabetes Res 2020:5817074. https://doi.org/10.1155/2020/5817074
Kim MY, Kim G, Park JY et al (2021) The association between continuous glucose monitoring-derived metrics and cardiovascular autonomic neuropathy in outpatients with type 2 diabetes. Diabetes Technol Ther 23(6):434–442. https://doi.org/10.1089/dia.2020.0599
Jun JE, Lee SE, Lee YB et al (2019) Continuous glucose monitoring defined glucose variability is associated with cardiovascular autonomic neuropathy in type 1 diabetes. Diabetes Metab Res Rev 35(2):e3092. https://doi.org/10.1002/dmrr.3092
Jaiswal M, McKeon K, Comment N et al (2014) Association between impaired cardiovascular autonomic function and hypoglycemia in patients with type 1 diabetes. Diabetes Care 37(9):2616–2621. https://doi.org/10.2337/dc14-0445
Racca C, Bouman EJ, Van Beers CAJ et al (2022) Association between hypoglycaemic glucose variability and autonomic function in type1 diabetes with impaired hypoglycaemia awareness. Diabetes Res Clin Pract 189:109964. https://doi.org/10.1016/j.diabres.2022.109964
Cardoso CRL, Salles GC, Leite NC, Salles GF (2019) Prognostic impact of carotid intima-media thickness and carotid plaques on the development of micro- and macrovascular complications in individuals with type 2 diabetes: the Rio de Janeiro type 2 diabetes cohort study. Cardiovasc Diabetol 18(1):2. https://doi.org/10.1186/s12933-019-0809-1
Lu J, Ma X, Shen Y et al (2020) Time in range is associated with carotid intima-media thickness in type 2 diabetes. Diabetes Technol Ther 22(2):72–78. https://doi.org/10.1089/dia.2019.0251
Sun F, Huang SY, Liu S, Fu JY (2023) Association between glucose time in range and internal carotid artery intima-media thickness in type-2 diabetes mellitus. J Kunming Med Univ 44(4):48–52. https://doi.org/10.12259/j.issn.2095-610X.S20230427
Duan RX, Liu SW, Shang HF, Wu YR, Li YB, Li X (2021) Relationship betw een blood glucose fluctuation and carotid intim a-m edia thickness in newly diagnosed type 2 diabetic p atients and the predictive value of betatrophin. Chin J Health Manag 15(4):350–355. https://doi.org/10.3760/cma.j.cn115624-20210118-00031
Cutruzzolà A, Parise M, Scavelli FB, Barone M, Gnasso A, Irace C (2022) Time in range does not associate with carotid artery wall thickness and endothelial function in type 1 diabetes. J Diabetes Sci Technol 16(4):904–911
Yan XJ, Sun H, Du X, Chen C, Li HJ, Shi BM (2020) The relationship between continuous glucose monitoring metrics and carotid atherosclerosis in patients with type 2 diabetes. Shandong Med J 60(32):47–50. https://doi.org/10.3969/j.issn.1002-266X.2020.32.012
Wang T (2022) The clinical relationship between continuous glucose monitoring metrics and arterial atherosclerosis in elderly patients with type 2 diabetes. Fujian Med J 44(3):78–80. https://doi.org/10.3969/j.issn.1002-2600.2022.03.026
Mesa A, Giménez M, Pueyo I et al (2022) Hyperglycemia and hypoglycemia exposure are differentially associated with micro- and macrovascular complications in adults with Type 1 Diabetes. Diabetes Res Clin Pract 189:109938. https://doi.org/10.1016/j.diabres.2022.109938
Wang DY, Liu KK, Zhang Y et al (2021) Association of time in range with the severity of coronary artery disease in type 2 diabetic patien. Chin J Endocrinol Metab 37(11):973–978. https://doi.org/10.3760/cma.j.cn311282-20201203-00807
Snell-Bergeon JK, Roman R, Rodbard D et al (2010) Glycaemic variability is associated with coronary artery calcium in men with Type 1 diabetes: the Coronary Artery Calcification in Type 1 Diabetes study. Diabet Med 27(12):1436–1442. https://doi.org/10.1111/j.1464-5491.2010.03127.x
Li J, Li Y, Ma W et al (2020) Association of time in range levels with lower extremity arterial disease in patients with type 2 diabetes. Diabetes Metab Syndr 14(6):2081–2085. https://doi.org/10.1016/j.dsx.2020.09.028
Wang Y, Lu J, Shen Y et al (2022) Association of time in range with lower extremity atherosclerotic disease in type 2 diabetes mellitus: a prospective cohort study. Endocrine 76(3):593–600. https://doi.org/10.1007/s12020-022-03038-3
Wen ZG, Zhao C, He XC, Chen LJ, Zhang H, Lv LZ (2019) Correlation between lower-extremity and carotid arterial diseases and cerebrovascular and cardio-vascular diseases in patients with type 2 diabetes mellitus. J New Med 50(4):298–302. https://doi.org/10.3969/j.issn.0253-9802.2019.04.013
Bergenstal RM, Hachmann-Nielsen E, Kvist K, Buse JB (2020) 21-LB: derived time-in-range is associated with MACE in T2D: data from the DEVOTE Trial. Diabetes 69(21-LB). https://doi.org/10.2337/db20-21-LB
Bergenstal RM, Hachmann-Nielsen E, Kvist K, Peters AL, Tarp JM, Buse JB (2023) Increased derived time in range is associated with reduced risk of major adverse cardiovascular events, severe hypoglycemia, and microvascular events in type 2 diabetes: a post hoc analysis of DEVOTE. Diabetes Technol Ther 25(6):378–383. https://doi.org/10.1089/dia.2022.0447
Lu J, Wang C, Shen Y et al (2021) Time in range in relation to all-cause and cardiovascular mortality in patients with type 2 diabetes: a prospective cohort study. Diabetes Care 44(2):549–555. https://doi.org/10.2337/dc20-1862
Bezerra MF, Neves C, Neves JS, Carvalho D (2023) Time in range and complications of diabetes: a cross-sectional analysis of patients with type 1 diabetes. Diabetol Metab Syndr 15(1):244. https://doi.org/10.1186/s13098-023-01219-2
Mellbin LG, Malmberg K, Rydén L, Wedel H, Vestberg D, Lind M (2013) The relationship between glycaemic variability and cardiovascular complications in patients with acute myocardial infarction and type 2 diabetes: a report from the DIGAMI 2 trial. Eur Heart J 34(5):374–379. https://doi.org/10.1093/eurheartj/ehs384
El Malahi A, Van Elsen M, Charleer S et al (2022) Relationship between time in range, glycemic variability, HbA1c, and complications in adults with type 1 diabetes mellitus. J Clin Endocrinol Metab 107(2):e570–e581. https://doi.org/10.1210/clinem/dgab688
Mita T, Katakami N, Okada Y et al (2019) Protocol of a prospective observational study on the relationship between glucose fluctuation and cardiovascular events in patients with type 2 diabetes. Diabetes Ther 10(5):1565–1575. https://doi.org/10.1007/s13300-019-0665-8
Si SC, Yang W, Wang JY, Luo HY, Ma YX, Zhao H (2023) Study on glycemic variability and the relationship between mean amplitude of glycemic excursion and cardiac function in elderly patients with type 2 diabetes mellitus. Pract Geriatr 37(4):361–364. https://doi.org/10.3969/j.issn.1003-9198.2023.04.010
Yokota S, Tanaka H, Mochizuki Y et al (2019) Association of glycemic variability with left ventricular diastolic function in type 2 diabetes mellitus. Cardiovasc Diabetol 18(1):166. https://doi.org/10.1186/s12933-019-0971-5
Dzhun Y, Mankovsky G, Rudenko N, Marushko Y, Saienko Y, Mankovsky B (2023) Glycemic variability is associated with diastolic dysfunction in patients with type 2 diabetes. J Diabetes Comp 37(11):108519. https://doi.org/10.1016/j.jdiacomp.2023.108519
Wang ZZ, Cao YH, Dai W (2022) Relationship between time in range and diabetic cardiac autonomic neuropathy in patients with type 2 diabetes. Anhui Med J 43(4):386–390. https://doi.org/10.3969/j.issn.1000-0399.2022.04.004
Xu W, Zhu Y, Yang X et al (2016) Glycemic variability is an important risk factor for cardiovascular autonomic neuropathy in newly diagnosed type 2 diabetic patients. Int J Cardiol 215:263–268. https://doi.org/10.1016/j.ijcard.2016.04.078
Naaman SH, Mizrak HI, Lind N et al (2022) Cardiovascular autonomic neuropathy is associated with increased glucose variability in people with type 1 diabetes. Diabetes Care 45(10):2461–2465. https://doi.org/10.2337/dc22-0117
Liang S, Yin H, Wei C, Xie L, He H, Liu X (2017) Glucose variability for cardiovascular risk factors in type 2 diabetes: a meta-analysis. J Diabetes Metab Disord 16:45. https://doi.org/10.1186/s40200-017-0323-5
Cesana F, Giannattasio C, Nava S et al (2013) Impact of blood glucose variability on carotid artery intima media thickness and distensibility in type 1 diabetes mellitus. Blood Press 22(6):355–361. https://doi.org/10.3109/08037051.2013.791413
Ito T, Ichihashi T, Fujita H et al (2019) The impact of intraday glucose variability on coronary artery spasm in patients with dysglycemia. Heart Vessels 34(8):1250–1257. https://doi.org/10.1007/s00380-019-01353-w
Benalia M, Zeller M, Mouhat B et al (2019) Glycaemic variability is associated with severity of coronary artery disease in patients with poorly controlled type 2 diabetes and acute myocardial infarction. Diabetes Metab 45(5):446–452. https://doi.org/10.1016/j.diabet.2019.01.012
Su G, Mi S, Tao H et al (2011) Association of glycemic variability and the presence and severity of coronary artery disease in patients with type 2 diabetes. Cardiovasc Diabetol 10:19. https://doi.org/10.1186/1475-2840-10-19
Zhang X, Xu X, Jiao X, Wu J, Zhou S, Lv X (2013) The effects of glucose fluctuation on the severity of coronary artery disease in type 2 diabetes mellitus. J Diabetes Res 2013:576916. https://doi.org/10.1155/2013/576916
Su G, Mi SH, Tao H et al (2013) Impact of admission glycemic variability, glucose, and glycosylated hemoglobin on major adverse cardiac events after acute myocardial infarction. Diabetes Care 36(4):1026–1032. https://doi.org/10.2337/dc12-0925
Zhang JW, He LJ, Cao SJ, Yang Q, Yang SW, Zhou YJ (2014) Effect of glycemic variability on short term prognosis in acute myocardial infarction subjects undergoing primary percutaneous coronary interventions. Diabetol Metab Syndr 6:76. https://doi.org/10.1186/1758-5996-6-76
Takahashi H, Iwahashi N, Kirigaya J et al (2018) Glycemic variability determined with a continuous glucose monitoring system can predict prognosis after acute coronary syndrome. Cardiovasc Diabetol 17(1):116. https://doi.org/10.1186/s12933-018-0761-5
Pu Z, Lai L, Yang X et al (2020) Acute glycemic variability on admission predicts the prognosis in hospitalized patients with coronary artery disease: a meta-analysis. Endocrine 67(3):526–534. https://doi.org/10.1007/s12020-019-02150-1
Gerbaud E, Darier R, Montaudon M et al (2019) Glycemic variability is a powerful independent predictive factor of midterm major adverse cardiac events in patients with diabetes with acute coronary syndrome. Diabetes Care 42(4):674–681. https://doi.org/10.2337/dc18-2047
Besch G, Pili-Floury S, Morel C et al (2019) Impact of post-procedural glycemic variability on cardiovascular morbidity and mortality after transcatheter aortic valve implantation: a post hoc cohort analysis. Cardiovasc Diabetol 18(1):27. https://doi.org/10.1186/s12933-019-0831-3
Siegelaar SE, Kerr L, Jacober SJ, Devries JH (2011) A decrease in glucose variability does not reduce cardiovascular event rates in type 2 diabetic patients after acute myocardial infarction: a reanalysis of the HEART2D study. Diabetes Care 34(4):855–857. https://doi.org/10.2337/dc10-1684
FLAT-SUGAR Trial Investigators (2016) Glucose variability in a 26-week randomized comparison of mealtime treatment with rapid-acting insulin versus GLP-1 agonist in participants with type 2 diabetes at high cardiovascular risk. Diabetes Care 39(6):973–981. https://doi.org/10.2337/dc15-2782
Nusca A, Tuccinardi D, Proscia C et al (2019) Incremental role of glycaemic variability over HbA1c in identifying type 2 diabetic patients with high platelet reactivity undergoing percutaneous coronary intervention. Cardiovasc Diabetol 18(1):147. https://doi.org/10.1186/s12933-019-0952-8
Hirakawa Y, Arima H, Zoungas S et al (2014) Impact of visit-to-visit glycemic variability on the risks of macrovascular and microvascular events and all-cause mortality in type 2 diabetes: the ADVANCE trial. Diabetes Care 37(8):2359–2365. https://doi.org/10.2337/dc14-0199
Funding
None.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
This study is a review article that does not involve experiments, data collection, or human experiments, and therefore does not require informed consent. We have ensured that the study maintains a high level of transparency and credibility. All authors are fully aware and have consented to the modifications and publication of the article. Furthermore, there are no conflicts of interest associated with this study.
Ethical approval
Not applicable.
Informed consent
Not applicable.
Consent to participate
Not applicable.
Consent for publication
Not applicable.
Additional information
Managed By Antonio Secchi.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Zhang, L., Sun, XX. & Tian, Qs. Research progress on the association between glycemic variability index derived from CGM and cardiovascular disease complications. Acta Diabetol 61, 679–692 (2024). https://doi.org/10.1007/s00592-024-02241-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00592-024-02241-0