Abstract
Aims
Several studies have revealed inconsistencies about the predictive properties of visceral adiposity index (VAI) in identifying chronic kidney disease (CKD). To date, it is unclear whether the VAI is a valuable diagnostic tool for CKD. This study intended to evaluate the predictive properties of the VAI in identifying CKD.
Methods
The PubMed, Embase, Web of Science, and Cochrane databases were searched for all studies that met our criteria from the earliest available article until November 2022. Articles were assessed for quality using the Quality Assessment of Diagnostic Accuracy Studies-2 (QUADAS-2). The heterogeneity was explored with the Cochran Q test and I2 test. Publication bias was detected using Deek’s Funnel plot. Review Manager 5.3, Meta-disc 1.4, and STATA 15.0 were used for our study.
Results
Seven studies involving 65,504 participants met our selection criteria and were therefore included in the analysis. Pooled sensitivity (Sen), specificity (Spe), positive likelihood ratio (PLR), negative likelihood ratio (NLR), diagnostic odds ratio (DOR) and area under the curve (AUC) were 0.67 (95%CI: 0.54–0.77), 0.75 (95%CI: 0.65–0.83), 2.7 (95%CI: 1.7–4.2), 0.44 (95%CI: 0.29–0.66), 6 (95%CI:3.00–14.00) and 0.77 (95%CI: 0.74–0.81), respectively. Subgroup analysis indicated that mean age of subjects was the potential source of heterogeneity. The Fagan diagram found that the predictive properties of CKD were 73% when the pretest probability was set to 50%.
Conclusions
The VAI is a valuable agent in predicting CKD and may be helpful in the detection of CKD. More studies are needed for further validation.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Chronic kidney disease (CKD) is an increasingly prominent health concern, with a prevalence of 11.6% in adults aged ≥ 45 years in the United States, and 10.8% in China [1, 2]. The early identifier of CKD is critical for its prognosis, and the clinical diagnosis of CKD mainly based on glomerular filtration rate (GFR) or albuminuria, but GFR or albuminuria needs to be measured repeatedly for more than three months [3]. CKD is relatively difficult for diagnosis, particularly in early stages [4]. Some renal functions have been damaged before symptoms appear, so it is a research issue to rely on biomedical laboratory indicators to diagnose CKD [4].
Obesity is closely related to CKD, and obesity-related subclinical inflammation and oxidative stress might directly contribute to renal damage [5, 6]. In addition, obesity-induced insulin resistance can lead to podocyte damage [7]. Several studies have confirmed that fat accumulation products are independent risk indicators for the prediction and diagnosis of CKD [8,9,10]. Data from human body constitutes these indicators, which reflect the level of fat metabolism. The visceral adiposity index (VAI), first developed by Amato and colleagues in 2009, is a mathematical method that consists of body mass index, waist circumference, triglycerides and high-density lipoprotein [11]. The VAI is a reliable agent of interior fat accumulation and dysfunction. The VAI has been linked with several metabolic diseases, including hypertension, prehypertension, type-2 diabetes, hyperuricemia, cardiovascular disease, and dementia [12,13,14,15,16,17].
The diagnostic value of the VAI is very attractive because laboratory indicators are cheap and routine, and the calculation is simple. A great many of studies have evaluated the diagnostic ability of the VAI in identifying CKD [8,9,10, 18,19,20], with several showing that the VAI is superior in comparison with other laboratory indicators[8, 19, 20]. Despite the benefit shown by these studies, the diagnostic properties of the VAI remain controversial. Besides, some limitations, such as insufficient sample size, subject variations, may affect the diagnostic value of different study. Therefore, the main purpose of current study is to perform a meta-analysis of diagnostic tests for predicting the accuracy of the VAI in identifying CKD.
Methods
This study was carried out following the PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses) guidelines [21].
Search strategy
A comprehensive bibliographic search was conducted using the PubMed, Embase, Cochrane, and Web of Science databases. All literatures published until November 7, 2022 were included in this review. The search strategy involved following key words: “visceral adiposity index,” “visceral fat indexes,” “visceral adipose index,” “VAI,” “VFI,” “chronic kidney diseases,” “chronic renal insufficiencies,” “chronic renal diseases,” “CKD,” and “diagnosis.”
Selection criteria
The inclusion criteria were as follows: (1) studies evaluated the relationship between VAI and CKD, and provided available data (the true-positive value (TP), false-positive value (FP), false-negative value (FN), and true-negative value (TN)) or studies have enough information to produce these data, (2) studies whose subjects were diagnosed with CKD based on a urinary albumin/creatinine ratio (UACR) of ≥ 30 mg/g or an estimated GFR (eGFR) of ≤ 60 ml/min/1.73 m2, and (3) studies published or translated into English.
The exclusion criteria were as follows: (1) Repetitive literature, animal research, review or case report, (2) studies without definite diagnostic criteria for CKD, and (3) studies without enough information to generate diagnosis related data.
Data extraction
The following data were extracted from each article: first author’s last name, year of publication, country, sample size, average age, diagnostic criteria for CKD, diagnostic data (TP, FP, FN, TN).
Quality assessment
Two independent reviewers (TT F and QL Z) assessed the articles. The disagreements were resolved by a third investigator (H Z). The Quality Assessment of Diagnostic Accuracy Studies-2 (QUADAS-2) criteria was used to assess these studies’ quality [22]. QUADAS-2 included nine questions, each question was answered as low risk, high risk, or unclear risk of bias. Review Manager 5.3 software was used to visualize the risk of bias in the included studies.
Statistical analysis
Meta-Disc 1.4 and STAT 15.0 software were used for analysis. The diagnostic value of VAI in patients with CKD was assessed by the pooled sensitivity (Sen), specificity (Spe), positive likelihood ratio (PLR), negative likelihood ratio (NLR), diagnostic odds ratio (DOR) and 95% confidence interval (CI), based on bivariate generalized linear mixed modelling. The area under the comprehensive receiver operating characteristic (SROC) curve was estimated. Threshold effect was tested using Spearman correlation analysis. Statistical heterogeneity was evaluated using the Cochran-Q test and I2 test. If there was significant heterogeneity (I2 > 50%, P < 0.05), the data were pooled by random effect model, otherwise, fixed effect model was used. We also used meta-regression and subgroup analysis to explore potential variability among groups, and subgroup was grouped according to country, gender, average age, and diagnostic criteria for CKD. Publication bias was assessed using Deek’s funnel plot. Diagnostic ability of the VAI was evaluated by a Fagan plot. P < 0.05 was considered significant.
Results
Search results
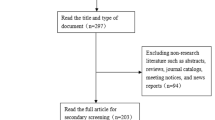
A total of 3,894 articles were collected, of which 3,113 were found to be duplicate records and were thus excluded. In addition, 699 articles were eliminated due to inclusion and exclusion criteria. Finally, 82 full-text articles were assessed for eligibility, among which, 64 records irrelevant to diagnostic test were excluded, 11 studies were removed due to insufficient data. Finally, 7 articles that met all of our selection criteria were included in this meta-analysis [8,9,10, 18,19,20, 23] (Table 1). The full search strategy is provided in Fig. 1.
Study characteristics and quality assessment
The characteristics of the screened studies are listed in Table 1. These studies were published between 2018 and 2022 and involved 65,504 participants. Six articles were from China (four were from Chinese mainland [9, 10, 18, 23], two from Taiwan [8, 19]), and one from South Korea [20]. Four articles adopted eGFR to diagnose CKD [8, 9, 20, 23], three articles diagnosed CKD by eGFR or UACR [10, 18, 19]. The subjects of three articles were younger than 60 years old [8, 9, 23], four articles were 60 years old or older [10, 18,19,20]. One article produced four groups diagnostic data (TP, FP, FN, TN) based on type of chronic kidney disease and gender. This article was divided into four studies, and pooled with other articles [23]. Four articles produced two groups diagnostic data (TP, FP, FN, TN), respectively, based on gender, and each article was divided into two studies, and pooled with other articles [9, 10, 18, 19]. All articles were evaluated by QUADAS-2, and the quality evaluation is shown in Fig. 2. The included studies were of moderate to high quality. Ultimately, 14 studies from 7 articles were included in the meta-analysis (Table 1).
Test of heterogeneity
Spearman rank correlation analysis between the logarithm of sensitivity and 1-specificity was − 0.064 (P = 0.829), implied no diagnostic threshold effects. There was no "shoulder-arm" distribution by drawing the SROC curve, showing that there was no threshold effect. The I2 heterogeneity of Sen, Spe, PLR, NLR, DOR was 97.4%, 99.7%, 98.7%, 98.6% and 98.5%, respectively, (P = 0.0001 each). So random effect model was used in this meta-analysis.
Diagnostic values of VAI for predicting CKD
The pooled Sen and Spe were 0.67 (95%CI: 0.54–0.77) and 0.75 (95%CI: 0.65–0.83), respectively. The pooled PLR and NLR were 2.7 (95%CI: 1.7–4.2) and 0.44 (95%CI: 0.29–0.66), respectively. The pooled DOR was 6 (95%CI:3.00–14.00). The corresponding AUC was 0.77 (95%CI: 0.74–0.81) (Table 2, Fig. 3, 4, 5, 6).
Subgroup analysis and meta-regression analysis
In this study, subgroup analyses and meta-regression analysis were conducted to explore the heterogeneity. Subgroup analyses were based on country, average age of subjects, diagnostic for CKD. The Sen, Spe, PLR, NLR, DOR of VAI for predicting CKD in subjects less than 60 years old were better than those 60 years old or more. Meta-regression analysis showed that mean age of subjects and diagnostic methods of CKD were a potential source of heterogeneity (P < 0.05) (Table 3, Fig. 7).
Sensitivity analysis and publication bias
The results did not significant alter after sensitivity analyses by eliminating studies one by one, indicating that the results were stable. Deek’s funnel plot was drawn to test publication bias. The results showed that P-value was 0.000 (< 0.05), suggesting that there was an obvious publication bias in our meta-analysis (Fig. 8).
Clinical application value
We could draw Fagan plot for clinical application analysis. The prior probability was 50%, and the post-test probability of VAI for predicting CKD was 73%, and 31% of LR-negative, suggesting that VAI was a valuable diagnostic tool for CKD (Fig. 9).
Discussion
CKD is one of the increasingly severe global public health concerns. As the current diagnostic indicators of CKD, proteinuria and serum creatinine are easily disturbed by patients’ physiological conditions. In addition, biopsy of CKD is not suitable for patients with contraindications, is also traumatic, and is insensitive to early progression [24]. Therefore, effective and convenient diagnostic methods have become important. In recent years, various new tests for CKD have emerged rapidly. Among a variety of examination methods, VAI, as a new method to detect visceral fat, has attracted extensive attention because of its role in patients with CKD [8], but there were inconsistencies in its diagnostic value.
In our meta-analysis, we found that VAI had medium diagnostic values for predicting CKD, the pooled Sen was 0.67 (95%CI: 0.54–0.77) and Spe was 0.75 (95%CI: 0.65–0.83), and AUC was 0.77 (95%CI: 0.74–0.81). A higher DOR value indicates a better diagnostic ability. The pooled DOR was 6 (95%CI:3.00–14.00), indicating diagnostic ability was not high. The pooled PLR was 2.7, suggesting that the probability of CKD was increased by 2.7-fold with the positive VAI. The pooled NLR was 0.44, indicating that probability could be 44% if VAI was negative. According to the criteria, PLR > 10 and NLR < 0.1 meant high accuracy, but our result did not up to par, suggesting the clinical value of VAI was limited.
The potential mechanisms linking VAI to CKD are still unclear. Visceral adipose tissue triggers an inflammatory response through free fatty acids (FFA) [25, 26]. Mitochondria plays a highly significant role in the metabolism of FFA and is critical factors in lipotoxicity [27]. Adipokines may cause kidney damage by mediating endothelial dysfunction, guiding oxidative stress and inflammation [27, 28]. In addition, altered adipokine levels may spoil the glomerular filtration barrier, resulting in decreased GFRs [29]. In conclusion, these findings show that obesity may cause CKD by various mechanisms.
The diagnostic values of VAI for predicting metabolic-associated fatty liver disease (MAFLD) have already been proven. Yi et al. [30] found that the combined Sen, Spe, PLR, NLR, DOR, AUC were 0.70, 0.67, 2.08, 0.39, 5.81, 0.79, respectively, and the VAI might be a valuable tool in the diagnosis of MAFLD. Bijari et al. [31]. considered that VAI had a moderate-to-high diagnostic value for metabolic syndrome (MetS), with Sen, Spe, AUC were 78%, 79%, 0.847, respectively. Chen et al. [8]. examined the role of VAI in CKD diagnosis and the Sen, Spe, AUC were 67.7%, 65.1%, 0.694, respectively, suggesting VAI might be a convenient tool for early detection of CKD in Taiwan. However, Dong et al. [9] showed that percentage body fat (PBF) was a more sensitive predictor for detecting CKD than other adiposity indices the VAI. PBF had a significantly higher AUC in both male and female groups (AUC for males: 0.593; AUC for females: 0.617) than VAI (AUC for males: 0.548; AUC for females: 0.577).
Subgroup analyses and meta-regression analysis were conducted to explore the heterogeneity, and found that mean age of subjects was a potential source of heterogeneity (P < 0.05). Subjects younger than 60 years old had a significantly higher Sen, Spe, PLR, NLR, DOR (0.75, 0.76, 3.1, 0.33, 10) than those 60 years old or more (Sen: 0.63; Spe: 0.72; PLR: 2.3; NLR: 0.51; DOR: 4). There were inconsistencies in diagnostic value of VAI for different age. Hu et al. [32] recognized the relationship between abdominal obesity and increasing age. His research showed that the prevalence of obesity was highest in men aged 45–54 years, while it was highest in women aged 55–64 years. Different from Hu et al.’ s study, Ahn et al. [33] found the VAI had better diagnostic ability in subjects younger than 65 years. Ageing is related to an increase in abdominal white adipose tissue (AT) and fat deposition in skeletal muscle, which significantly affect insulin sensitivity [34]. As an important component of MetS, insulin resistance is common in older adults [1]. The common cause of insulin resistance and MetS is abdominal obesity [35].
Limitations of the study
This study has several limitations that must be considered when interpreting its results. First, our studies may have potential heterogeneity and publication bias, so the results should be interpreted with caution. Second, all participants originated from Asian population, while most studies were from Chinese population, our study may have a population selection bias. Third, subjects in our studies may have comorbidities, which may affect the diagnostic power of VAI for CKD. Finally, inconsistent the VAI thresholds for CKD diagnosis may have influenced the final results.
Conclusion
In conclusions, our analysis shows that the VAI is a valuable predictor in diagnosing CKD and is feasible for clinical applications. This study will contribute to sifting patients with CKD with simple anthropometric index and provide basis for early diagnosis of CKD. Significant heterogeneity in the pooled estimates may have limited the reliability of our conclusions. Therefore, further large-scale studies are needed to confirm our findings.
Data availability
The original contributions presented in the study are included in the article/supplementary material.
References
McClellan WM, Abramson J, Newsome B et al (2010) Physical and psychological burden of chronic kidney disease among older adults. Am J Nephrol 31(4):309–317. https://doi.org/10.1159/000285113
Zhang L, Wang F, Wang L et al (2012) Prevalence of chronic kidney disease in China: a cross-sectional survey. Lancet 379(9818):815–822. https://doi.org/10.1016/s0140-6736(12)60033-60036
KDOQI (2007) KDOQI clinical practice guidelines and clinical practice recommendations for diabetes and chronic kidney disease. Am J Kidney Dis 49(2 Suppl 2):S12-154. https://doi.org/10.1053/j.ajkd.2006.12.005
Sherwood M, McCullough PA (2016) Chronic kidney disease from screening, detection, and awareness, to prevention. Lancet Glob Health 4(5):e288-289. https://doi.org/10.1016/s2214-109x(16)30049-3
Sharma K (2014) Obesity, oxidative stress, and fibrosis in chronic kidney disease. Kidney Int Suppl 4(1):113–117. https://doi.org/10.1038/kisup.2014.21
Stefan N, Artunc F, Heyne N et al (2016) Obesity and renal disease: not all fat is created equal and not all obesity is harmful to the kidneys. Nephrol Dial Transplant 31(5):726–730. https://doi.org/10.1093/ndt/gfu081
Sieber J, Lindenmeyer MT, Kampe K et al (2010) Regulation of podocyte survival and endoplasmic reticulum stress by fatty acids. Am J Physiol Renal Physiol 299(4):F821-829. https://doi.org/10.1152/ajprenal.00196.2010
Chen YC, Lai SH, Tsai YW et al (2018) Visceral adiposity index as a predictor of chronic kidney disease in a relatively healthy population in Taiwan. J Ren Nutr 28(2):91–100. https://doi.org/10.1053/j.jrn.2017.07.006
Dong Y, Wang Z, Chen Z et al (2018) Comparison of visceral, body fat indices and anthropometric measures in relation to chronic kidney disease among Chinese adults from a large scale cross-sectional study. BMC Nephrol 19(1):40. https://doi.org/10.1186/s12882-018-0837-1
Chen T, Wang X, Wang X et al (2020) Comparison of novel metabolic indices in estimation of chronic kidney diseases in a southern chinese population. Diabetes Metab Syndr Obes 13:4919–4927. https://doi.org/10.2147/dmso.S286565
Amato MC, Giordano C, Galia M et al (2010) Visceral Adiposity Index: a reliable indicator of visceral fat function associated with cardiometabolic risk. Diabetes Care 33(4):920–922. https://doi.org/10.2337/dc09-1825
Amato MC, Giordano C, Pitrone M et al (2011) Cut-off points of the visceral adiposity index (VAI) identifying a visceral adipose dysfunction associated with cardiometabolic risk in a Caucasian Sicilian population. Lipids Health Dis 10:183. https://doi.org/10.1186/1476-511x-10-183
Yang Y, Feng Y, Ma X et al (2015) Visceral adiposity index and insulin secretion and action in first-degree relatives of subjects with type 2 diabetes. Diabetes Metab Res Rev 31(3):315–321. https://doi.org/10.1002/dmrr.2615
Zhang M, Zheng L, Li P et al (2016) 4-year trajectory of visceral adiposity index in the development of type 2 diabetes: a prospective cohort study. Ann Nutr Metab 69(2):142–149. https://doi.org/10.1159/000450657
Fiorentino TV (2018) Visceral adiposity index (VAI), a powerful predictor of incident hypertension in prehypertensives. Intern Emerg Med 13(4):471–473. https://doi.org/10.1007/s11739-018-1849-3
Gu D, Ding Y, Zhao Y et al (2018) Positively increased visceral adiposity index in hyperuricemia free of metabolic syndrome. Lipids Health Dis 17(1):101. https://doi.org/10.1186/s12944-018-0761-1
Singh-Manoux A, Dugravot A, Shipley M et al (2018) Obesity trajectories and risk of dementia: 28 years of follow-up in the Whitehall II Study. Alzheimers Dement 14(2):178–186. https://doi.org/10.1016/j.jalz.2017.06.2637
Wan H, Wang Y, Xiang Q et al (2020) Associations between abdominal obesity indices and diabetic complications: Chinese visceral adiposity index and neck circumference. Cardiovasc Diabetol 19(1):118. https://doi.org/10.1186/s12933-020-01095-4
Chen IJ, Hsu LT, Lu MC et al (2021) Gender differences in the association between obesity indices and chronic kidney disease among middle-aged and elderly taiwanese population: a community-based cross-sectional study. Front Endocrinol (Lausanne) 12:737586. https://doi.org/10.3389/fendo.2021.737586
Seong JM, Lee JH, Gi MY et al (2021) Gender difference in the association of chronic kidney disease with visceral adiposity index and lipid accumulation product index in Korean adults: Korean national health and nutrition examination survey. Int Urol Nephrol 53(7):1417–1425. https://doi.org/10.1007/s11255-020-02735-0
Page MJ, McKenzie JE, Bossuyt PM et al (2021) The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ 372:n71. https://doi.org/10.1136/bmj.n71
Whiting PF, Rutjes AW, Westwood ME et al (2011) QUADAS-2: a revised tool for the quality assessment of diagnostic accuracy studies. Ann Intern Med 155(8):529–536. https://doi.org/10.7326/0003-4819-155-8-201110180-00009
Li H, Wang Q, Ke J et al (2022) Optimal obesity- and lipid-related indices for predicting metabolic syndrome in chronic kidney disease patients with and without type 2 diabetes mellitus in China. Nutrients 14(7):1334. https://doi.org/10.3390/nu14071334
Romagnani P, Remuzzi G, Glassock R et al (2017) Chronic kidney disease. Nat Rev Dis Primers 3:17088. https://doi.org/10.1038/nrdp.2017.88
Shi H, Kokoeva MV, Inouye K et al (2006) TLR4 links innate immunity and fatty acid-induced insulin resistance. J Clin Invest 116(11):3015–3025. https://doi.org/10.1172/jci28898
Schilling JD, Machkovech HM, He L et al (2013) Palmitate and lipopolysaccharide trigger synergistic ceramide production in primary macrophages. J Biol Chem 288(5):2923–2932. https://doi.org/10.1074/jbc.M112.419978
Zhu Y, Chen YL, Li C et al (2014) The effect of inhibition of endoplasmic reticulum stress on lipolysis in white adipose tissue in a rat model of chronic kidney disease. Acta Pharmacol Sin 35(3):356–362. https://doi.org/10.1038/aps.2013.177
Kershaw EE, Flier JS (2004) Adipose tissue as an endocrine organ. J Clin Endocrinol Metab 89(6):2548–2556. https://doi.org/10.1210/jc.2004-0395
Martínez-García C, Izquierdo-Lahuerta A, Vivas Y et al (2015) Renal lipotoxicity-associated inflammation and insulin resistance affects actin cytoskeleton organization in podocytes. PLoS ONE 10(11):e0142291. https://doi.org/10.1371/journal.pone.0142291
Yi X, Zhu S, Zhu L (2022) Diagnostic accuracy of the visceral adiposity index in patients with metabolic-associated fatty liver disease: a meta-analysis. Lipids Health Dis 21(1):28. https://doi.org/10.1186/s12944-022-01636-8
Bijari M, Jangjoo S, Emami N et al (2021) The Accuracy of Visceral Adiposity Index for the Screening of Metabolic Syndrome: A Systematic Review and Meta-Analysis. Int J Endocrinol 2021:6684627. https://doi.org/10.1155/2021/6684627
Hu L, Huang X, You C et al (2017) Prevalence of overweight, obesity, abdominal obesity and obesity-related risk factors in southern China. PLoS ONE 12(9):e0183934. https://doi.org/10.1371/journal.pone.0183934
Folsom AR, Kaye SA, Sellers TA et al (1993) Body fat distribution and 5-year risk of death in older women. JAMA 269(4):483–487
Arum O, Boparai RK, Saleh JK et al (2014) Specific suppression of insulin sensitivity in growth hormone receptor gene-disrupted (GHR-KO) mice attenuates phenotypic features of slow aging. Aging Cell 13(6):981–1000. https://doi.org/10.1111/acel.12262
Barzilai N, Huffman DM, Muzumdar RH et al (2012) The critical role of metabolic pathways in aging. Diabetes 61(6):1315–1322. https://doi.org/10.2337/db11-1300
Funding
This work was funded by Outstanding Leaders Training Program of Pudong Health Bureau of Shanghai (Grant No. PWRl2020-04).
Author information
Authors and Affiliations
Contributions
All authors contributed to data analysis, drafting or revising the article, gave final approval of the version to be published, and agree to be accountable for all aspects of the work.
Corresponding author
Ethics declarations
Conflict of interests
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Ethical approval
All author: this manuscript has not been published in whole or in part elsewhere; the manuscript is not currently being considered for publication in another journal; all authors have been personally and actively involved in substantive work leading to the manuscript, and will hold themselves jointly and individually responsible for its content.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This article belongs to the topical collection Diabetic Nephropathy, managed by Giuseppe Pugliese.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Fang, T., Zhang, Q., Wang, Y. et al. Diagnostic value of visceral adiposity index in chronic kidney disease: a meta-analysis. Acta Diabetol 60, 739–748 (2023). https://doi.org/10.1007/s00592-023-02048-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00592-023-02048-5