Abstract
Background
Cardiac autonomic neuropathy (CAN) is a common complication of type 2 diabetes mellitus (T2DM). We sought to determine whether sodium-glucose co-transporter-2 (SGLT-2) inhibitors affect indices of CAN in patients with T2DM.
Methods
We searched for parallel group or cross-over randomized controlled trials (RCTs) enrolling adult subjects with T2DM, assigned to a SGLT-2 inhibitor versus placebo or active comparator and addressing their effect on CAN. PubMed, Cochrane Library and gray literature sources were searched. We set as primary efficacy outcome the change in the low-frequency-to-high-frequency (LF/HF) ratio. We set as secondary efficacy outcomes: first, the change in the standard deviation of all 5 min mean normal RR intervals and second, the change in the square root of the mean of the sum of the squares of differences between adjacent RR intervals (r-MSSD). Protocol has not been registered at a publicly available repository.
Results
We pooled data from four RCTs in a total of 247 subjects with T2DM. SGLT-2 inhibitor treatment did not have a significant effect on LF/HF ratio (MD = − 0.11, 95% CI − 0.35 to 0.12, I2 = 0%, p = 0.36). SGLT-2 inhibitor treatment did not have a significant impact either on SDNN (MD = − 2.83, 95% CI − 7.41 to 1.75, I2 = 31%, p = 0.23), or on r-MSSD (MD = − 0.14, 95% CI − 3.52 to 3.25, I2 = 46%, p = 0.94). Overall risk of bias was graded as low across the selected RCTs.
Conclusion
SGLT-2 inhibitor treatment in patients with T2DM does not seem to provide any significant beneficial effect on CAN indices.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Cardiac autonomic neuropathy (CAN) is a rather common complication of type 2 diabetes mellitus (T2DM), which is expected to rise, due to the growing epidemic of diabetes worldwide [1]. Recent studies have suggested that CAN is associated with coronary microvascular dysfunction [2] and progressive loss of kidney function in patients with T2DM [3]. In a recently published post hoc analysis of the Action to Control Cardiovascular Risk in Diabetes (ACCORD) study, it was demonstrated that among subjects with T2DM, but without heart failure (HF) at baseline, CAN was highly predictive of the future risk incident HF [4]. Overall, CAN represents a prognostic risk marker for cardiovascular disease among patients with T2DM, also associated with an increased risk for all-cause mortality [5].
Besides the prognostic role of CAN in cardiovascular disease among individuals with T2DM, it has also been associated with a higher recurrence rate of vaso-vagal syncope (VVS) among those patients compared to normoglycemic controls [6]. A recently published, prospective, multicenter study showed that patients with T2DM have higher heart rate variability (HRV) dysfunction compared to normoglycemic controls, and these alterations, associated with CAN, are highly predictive of VVS [6].
Sodium-glucose co-transporter-2 (SGLT-2) inhibitors have been shown to provide outstanding cardiovascular and renal benefits in patients with T2DM during recent years [7]. In addition, they are recommended for patients with HF with reduced left ventricular ejection fraction (HFrEF), regardless of T2DM status at baseline, while, recent data also recommend their use in patients with HF, regardless of left ventricular ejection fraction, as they decrease the risk for recurrent hospitalization for HF decompensation and cardiovascular mortality [8, 9]. It has been hypothesized that amelioration of CAN by SGLT-2 inhibitors might partially account for their beneficial effects in patients with HF [10]. However, high-level evidence is relatively limited. Therefore, we sought to determine whether SGLT-2 inhibitors affect CAN indices, in patients with T2DM, pooling data from relevant published randomized controlled trials (RCTs).
Methods
This systematic review and meta-analysis was conducted in accordance with the PRISMA (Preferred Reporting Items for Systematic reviews and Meta-Analyses) guidelines [11].
We searched for parallel group or cross-over RCTs enrolling adult subjects with T2DM, assigned to a SGLT-2 inhibitor versus placebo or active comparator, addressing their effect on CAN indices. We excluded trials enrolling subjects with type 1 diabetes mellitus (T1DM).
We used the following search strategy implemented on 24th May 2022 both in the PubMed and Cochrane Library databases: ((((((((((((SGLT2 inhibitor) OR (empagliflozin)) OR (dapagliflozin)) OR (canagliflozin)) OR (ertugliflozin)) OR (sotagliflozin)) OR (ipragliflozin)) OR (luseogliflozin)) OR (tofogliflozin)) OR (bexagliflozin)) OR (licogliflozin)) AND (((sympathetic nervous system activity) OR (heart rate variability)) OR (SDNN))). We searched the clinicaltrials.gov registry, as well. We did not impose any filter regarding sample size, study setting or publication language.
We set as primary efficacy outcome the change in the low-frequency-to-high-frequency (LF/HF) ratio, observed with SGLT-2 inhibitor treatment compared to control. We set as secondary efficacy outcomes: first, the change in the standard deviation of all 5-min mean normal RR intervals (SDANN) and second, the change in the square root of the mean of the sum of the squares of differences between adjacent RR intervals (r-MSSD).
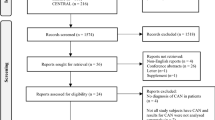
After deduplication, two independent reviewers (D.P., C.P.) screened all records at title and abstract level and then assessed the full text of eligible records. Any disagreements between reviewers were resolved by consultation of a third senior reviewer (M.D.).
Three independent reviewers (D.P., N.F. and C.P.) extracted the data from the eligible reports. Extracted information were first author, year of study conduction, study setting, study sample size, country of origin, type of administered SGLT-2 inhibitor, type of control (placebo or active comparator), methods for the assessment of cardiac autonomic function, follow-up duration, mean age of participants, male-to-female ratio, major co-morbidities, baseline pharmacotherapy of interest and method for the assessment of CAN.
Differences were calculated with the use of mean difference (MD), with standard error (SE), after implementation of the inverse variance (IV) random effects formula. In those studies not reporting SE, we calculated SE from sample size and standard deviation (SD).
Statistical heterogeneity among studies was assessed by using I2 statistics [2]. I2 ranging between 0 and 40% is considered as low, I2 ranging between 50 and 90% may represent substantial heterogeneity and I2 ranging between 75 and 100% may be indicative of considerable heterogeneity [12]. All analyses were performed at the 0.05 significance level, with the RevMan 5.3. software [13].
Two independent reviewers (D.P. and C.P.) assessed the quality of the included RCTs, by using the Revised Cochrane risk of bias tool for randomized trials (RoB 2.0) for the primary efficacy outcome [14]. Discrepancies between reviewers were solved by discussion, consensus or arbitration by a third senior reviewer (M.D.).
Results
We pooled data from four RCTs in a total of 247 subjects with T2DM [15,16,17,18]. Three trials were parallel-group [15,16,17], while the remaining one was cross-over [18]. Two trials assessed the effect of dapagliflozin on CAN [15, 18], while two trials evaluated the corresponding effect of empagliflozin [16, 17]. Study selection process is depicted in Fig. 1. A detailed description of participants’ baseline characteristics of interest is summarized in supplementary table 1.
Flow diagram depicting the study selection process. *Consider, if feasible to do so, reporting the number of records identified from each database or register searched (rather than the total number across all databases/registers). **If automation tools were used, indicate how many records were excluded by a human and how many were excluded by automation tools
Regarding the primary efficacy outcome, SGLT-2 inhibitor treatment did not have a significant effect on LF/HF ratio in patients with T2DM (MD = − 0.11, 95% CI − 0.35 to 0.12, I2 = 0%, p = 0.36), as shown in Fig. 2. Concerning the secondary efficacy outcomes, SGLT-2 inhibitor treatment did not have a significant impact either on SDNN (MD = − 2.83, 95% CI − 7.41 to 1.75, I2 = 31%, p = 0.23), or on r-MSSD (MD = − 0.14, 95% CI − 3.52 to 3.25, I2 = 46%, p = 0.94), as shown in Figs. 3 and 4, respectively. Low statistical heterogeneity was documented for the assessed comparisons. Overall risk of bias is considered as low across the selected RCTs (supplementary Table 2).
Restriction of the analyses to those RCTs enrolling patients with T2DM and concomitant coronary artery disease [16, 17] did not reveal any statistically significant effect on the assessed electrocardiographic indices of CAN.
A sensitivity analysis excluding the only cross-over RCT performed by Ang et al. [18] did not have a significant impact on the generated results: SGLT-2 inhibitors did not have a significant effect on LF/HF ratio (p = 0.23, I2 = 0%), on SDNN (p = 0.24, I2 = 52%) and on r-MSSD (p = 0.82, I2 = 57%).
Alternate pooling methods (random-effects versus fixed-effects) did not have a significant effect on the generated results, as shown in supplementary Table 3.
Lack of access to individual patients’ data did not permit us to perform subgroup analyses according to history of major co-morbidities, or background treatment of interest (such as beta-blockers).
Finally, visual assessment of the corresponding funnel plot did not document any asymmetry, indicative of absence of significant publication bias (Supplementary Fig. 1). According to Cochrane Guidelines, no formal statistical testing (for example, Egger’s test) was performed, due to the fact that there were less than 10 eligible RCTs.
Discussion
Herein, we present the first relevant meta-analysis in the literature so far, suggesting that SGLT-2 inhibitor treatment in patients with T2DM, with or without cardiovascular co-morbidities, does not exert any significant effect on CAN indices (Fig. 5).
A former meta-analysis of observational studies in a total of 2932 participants, with half of them having background T2DM, showed that T2DM is significantly associated with CAN, with lower SDNN, r-MSSD and LF/HF ratio values been observed in patients with T2DM compared to healthy controls [19]. Besides glycemic control, age, male gender and blood pressure were identified as significant determinants of impaired HRV among the enrolled participants with T2DM [19].
It has also been confirmed that cardiac autonomic dysfunction is observed in patients with T2DM regardless of level of glycemic control, although worse glycemic control was associated with more impaired CAN [20]. As shown in two recently published studies, young-onset T2DM in adolescents and young adults is also associated with significant cardiac autonomic dysfunction, closely related to poor glycemic control, while it is also indicative of a worse overall cardiovascular risk profile [21, 22]. Therefore, CAN might also be predictive of the presence of asymptomatic coronary artery lesions, and thus of coronary artery disease [23], besides its predictive role in cardiovascular disease in patients with T2DM [5]. Therefore, prevention or amelioration of CAN would be ideal for those patients. Finally, the presence of CAN is responsible for an increased risk for mortality with an estimated relative risk of 3.65, based on the results of a meta-analysis of 15 studies [24].
Of course, it has to be admitted that diagnosis of CAN is challenging and multi-disciplinary. Besides assessment of HRV, 123I-labeled metaiodobenzylguanidine (MIBG) cardiac scintigraphy has also been utilized for the assessment of CAN in clinical practice. Former studies, more than two decades ago, found that patients with insulin-dependent diabetes mellitus had a reduced global myocardial uptake of 123I-MIBG, even in the absence of electrocardiographic indications of CAN [25]. More recent studies have also documented the role of 123I-MIBG scintigraphy for the diagnosis of CAN in other settings [26, 27], however, highlighting the crucial and independent role of hyperglycemia on cardiac sympathetic denervation, which is associated with worse prognosis, strongly correlated with surrogate endpoints, such as heart failure incidence and all-cause death [26].
Current guidelines highlight the absence of any treatment capable of reversing established CAN, with treatment options being focused on ameliorating symptoms [28]. Therefore, early preclinical data suggesting that SGLT-2 inhibitors could modify the course of CAN were welcomed. For instance, SGLT-2 inhibitors improved dipping and the circadian rhythm of sympathetic nervous activity in rat models of obesity and metabolic syndrome, while in another study with standard chow- and high-fat-fed rats, dapagliflozin attenuated the sympathetic nervous system response to glucose load [29,30,31]. In addition, a recently published prospective study, after propensity score matching, assessing 324 patients with well-controlled T2DM and VVS, documented that SGLT-2 inhibitor users, compared to non-users, had significantly lower incidence rates of CAN, assessed by electrocardiographic Holter analysis, while they experienced a significantly lower recurrence rate of VVS after 1-year, with SGLT-2 inhibitor treatment being an independent predictor of reduced risk of VVS recurrence by 45% [32].
Several mechanisms have been suggested for the cardio-protective effects of SGLT-2 inhibitors in clinical practice [33]. Most of them might also be associated with an improvement in CAN, as demonstrated in previously published experimental studies [34, 35]. T2DM is associated with low-grade, chronic inflammation [36], while, some cytokines, such as the pro-inflammatory interleukin-1β, might also be associated with T2DM pathogenesis. Sympathetic nervous system over-activation is also documented in individuals with T2DM, which has been shown to be associated with age and insulin resistance, irrespective of co-morbidities, such as obesity, hypertension, or metabolic syndrome [37, 38]. Over-inflammation and over-activation of sympathetic nervous system are directly implicated into pathogenesis of CAN and are therefore promising treatment targets [39].
SGLT-2 inhibitors have been shown to significantly ameliorate inflammatory burden in patients with T2DM and coronary artery disease treated with coronary artery bypass grafting (CABG), an effect associated with significant improvement in surrogate clinical endpoints, including cardiovascular outcomes and all-cause death, underlining the prominent role of inflammation in cardiovascular disease among patients with T2DM and the value of SGLT-2 inhibitor treatment [40]. In another prospective, observational study enrolling subjects with T2DM and acute myocardial infarction, it was shown that previous SGLT-2 inhibitor users compared to non-users experienced a significantly decreased inflammatory response, associated with smaller infarct size, regardless of age and level of glycemic control at baseline [41]. These results provide novel insights into the anti-inflammatory effects of SGLT-2 inhibitors and the clinical implications of such effects, since they are associated with improved cardiovascular outcomes.
However, evidence retrieved from our meta-analysis does not support an otherwise reasonable hypothesis of a beneficial effect of SGLT-2 inhibitors on CAN indices, as we have shown that SGLT-2 inhibitors do not provide any improvement in electrocardiographic parameters of CAN. Of course, our results are preliminary and should be interpreted with caution since this is the first meta-analysis of the relevant RCTs. In addition, our results are in contrast with the results of a recently published observational study, which supported a beneficial role of SGLT-2 inhibitor treatment on CAN indices and their protective role against VVS recurrence among patients with T2DM [32].
The use of b-blockers was high in the two studies that included patients with established cardiovascular disease [16, 17]. This might have influenced the results of the interventions, as b-blockers are able to modify sympatho-vagal balance through vagal activation, possibly eliminating any benefits that could possibly otherwise be evident with the use of SGLT-2 inhibitors [42].
Of note, we should also clarify that cardiovascular autonomic reflex tests (CARTs), namely, heart rate variations during deep breathing, Valsalva maneuver, and lying-to-standing (heart rate tests) as indices of parasympathetic function, along with the orthostatic hypotension, the blood pressure response to a Valsalva maneuver and sustained isometric muscular strain as indices of sympathetic function, remain the mainstay for the assessment of CAN in clinical practice [43]. CARTs are safe, easy to perform, sensitive, specific, reproducible, while they allow CAN staging from early to advanced involvement, as documented by the CAN Subcommittee of the Toronto Consensus Panel on Diabetic Neuropathy [43]. The present meta-analysis pooled data from trials primarily assessing HRV, while only Ang and colleagues performed CARTs [18]. Thus, this can be considered as a main limitation of our meta-analysis.
We consider as additional limitations of our meta-analysis the limited number of relevant RCTs, the small sample size of the eligible trials, and the lack of access to individual participants’ information, which would permit us to perform subgroup analyses of interest. As shown in supplementary table 1, enrolled subjects across the eligible RCTs had different levels of glycemic control, were mostly overweight or obese, had differential usage rates of cardiac acting drug classes other than b-blockers, while, availability of data concerning prior cardiovascular disease, CAN, cardio-respiratory fitness, or other co-morbidities of interest, is scarce and limited, restricting the generation of definite conclusions and the applicability of generated results into clinical practice. All these factors could have influenced CAN variables and thus limit the robustness of our results, in the absence of relevant sub-group analyses.
Importantly, inadequate glycemic control, as stated previously, and central obesity have been shown to be independent predictors of CAN development among subjects with T2DM or metabolic syndrome [44,45,46]. Three out of four trials included in our meta-analysis enrolled adults with insufficient glycemic control, overweight or obese, while only one trial, that performed by Shimizu and colleagues [16], recruited adult subjects with T2DM and almost within normal range body mass index and glycated hemoglobin levels. This might partially explain the non-significant results of our meta-analysis, whereas a former observational study by Sardu et al. [32], which enrolled subjects with almost optimal glycemic control and low relative frequency rates of obesity, documented a significant effect of long-term administration of SGLT-2 inhibitors on the risk for VVS recurrence.
Moreover, the duration of electrocardiographic recordings differed among the included studies. Eligible RCTs utilized only empagliflozin or dapagliflozin, while, currently, there are no available trials utilizing other SGLT-2 inhibitors with established cardio-protective effects, such as canagliflozin, sotagliflozin or ertugliflozin. In addition, we did not register prospectively our protocol at a publicly available repository. Finally, there is a number of limitations in the assessment of CAN with the use of Holter monitoring that should not be overlooked, mainly the absence of standardized reference values [47]. It would be interesting if future trials also assessed the effect of SGLT-2 inhibitors on head-up tilt test, which may be positive in patients with CAN [48], and of course to assess the effect of SGLT-2 inhibitors on heart denervation, as assessed with 123I-MIBG scintigraphy.
Conclusion
SGLT-2 inhibitor treatment in patients with T2DM does not seem to provide any significant beneficial effect on CAN indices. Further, larger RCTs are required to provide definitive answers on this sound scientific question.
References
Spallone V (2019) Update on the impact, diagnosis and management of cardiovascular autonomic neuropathy in diabetes: what is defined, what is new, and what is unmet. Diab Metab J 43:3–30. https://doi.org/10.4093/dmj.2018.0259
von Scholten BJ, Hansen CS, Hasbak P et al (2016) Cardiac autonomic function is associated with the coronary microcirculatory function in patients with type 2 diabetes. Diabetes 65:3129–3138. https://doi.org/10.2337/db16-0437
Laursen JC, Rasmussen IKB, Zobel EH et al (2021) The association between cardiovascular autonomic function and changes in kidney and myocardial function in type 2 diabetes and healthy controls. Front Endocrinol 12:780679. https://doi.org/10.3389/fendo.2021.780679
Kaze AD, Yuyun MF, Erqou S et al (2022) Cardiac autonomic neuropathy and risk of incident heart failure among adults with type 2 diabetes. Eur J Heart Fail 24:634–641. https://doi.org/10.1002/ejhf.2432
Chowdhury M, Nevitt S, Eleftheriadou A et al (2021) Cardiac autonomic neuropathy and risk of cardiovascular disease and mortality in type 1 and type 2 diabetes: a meta-analysis. BMJ Open Diab Res Care 9:e002480. https://doi.org/10.1136/bmjdrc-2021-002480
Sardu C, Paolisso P, Santamaria M et al (2019) Cardiac syncope recurrence in type 2 diabetes mellitus patients vs. normoglycemics patients: the CARVAS study. Diab Res Clin Pract 151:152–162. https://doi.org/10.1016/j.diabres.2019.04.015
Zelniker TA, Wiviott SD, Raz I et al (2019) SGLT2 inhibitors for primary and secondary prevention of cardiovascular and renal outcomes in type 2 diabetes: a systematic review and meta-analysis of cardiovascular outcome trials. Lancet 393:31–39. https://doi.org/10.1016/S0140-6736(18)32590-X
Zannad F, Ferreira JP, Pocock SJ et al (2020) SGLT2 inhibitors in patients with heart failure with reduced ejection fraction: a meta-analysis of the EMPEROR-Reduced and DAPA-HF trials. Lancet 396:819–829. https://doi.org/10.1016/S0140-6736(20)31824-9
Pandey AK, Dhingra NK, Hibino M, Gupta V, Verma S (2022) Sodium-glucose cotransporter 2 inhibitors in heart failure with reduced or preserved ejection fraction: a meta-analysis. ESC Heart Fail 9:942–946. https://doi.org/10.1002/ehf2.13805
Sano M (2020) A paradigm shift in the treatment of type 2 diabetes and heart failure. J Atheroscler Thromb 27:727–731. https://doi.org/10.5551/jat.RV17042
Liberati A, Altman DG, Tetzlaff J et al (2009) The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate healthcare interventions: explanation and elaboration. BMJ 339:b2700. https://doi.org/10.1136/bmj.b2700
Deeks JJ, Higgins JPT, Altman DG, Higgins JPT, Green S (2011) Cochrane handbook for systematic reviews of interventions version 5.1.0 (updated March 2011). In: The cochrane collaboration; chapter 9: analysing data and undertaking meta-analyses
Review Manager (RevMan) [Computer program] Version [5.3] Copenhagen: The Nordic Cochrane Centre TCC; (2014)
Sterne JAC, Savović J, Page MJ et al (2019) RoB 2: a revised tool for assessing risk of bias in randomised trials. BMJ 366:l4898. https://doi.org/10.1136/bmj.l4898
van Bommel EJM, Smits MM, Ruiter D et al (2020) Effects of dapagliflozin and gliclazide on the cardiorenal axis in people with type 2 diabetes. J Hypertens 38:1811–1819. https://doi.org/10.1097/HJH.0000000000002480
Shimizu W, Kubota Y, Hoshika Y, et al EMBODY trial investigators (2020) Effects of empagliflozin versus placebo on cardiac sympathetic activity in acute myocardial infarction patients with type 2 diabetes mellitus: the EMBODY trial. Cardiovasc Diabetol 19:148. https://doi.org/10.1186/s12933-020-01127-z
Garg V, Verma S, Connelly KA et al (2020) Does empagliflozin modulate the autonomic nervous system among individuals with type 2 diabetes and coronary artery disease? The EMPA-HEART CardioLink-6 Holter analysis. Metabol Open 7:100039. https://doi.org/10.1016/j.metop.2020.100039
Ang L, Kidwell KM, Dillon B et al (2021) Dapagliflozin and measures of cardiovascular autonomic function in patients with type 2 diabetes (T2D). J Diab Complicat 35:107949. https://doi.org/10.1016/j.jdiacomp.2021.107949
Benichou T, Pereira B, Mermillod M et al (2018) Heart rate variability in type 2 diabetes mellitus: a systematic review and meta-analysis. PLoS ONE 13:e0195166. https://doi.org/10.1371/journal.pone.0195166
Yu Y, Hu L, Xu Y et al (2020) Impact of blood glucose control on sympathetic and vagus nerve functional status in patients with type 2 diabetes mellitus. Acta Diabetol 57:141–150. https://doi.org/10.1007/s00592-019-01393-8
Shah AS, Jaiswal M, Dabelea D et al (2020) Cardiovascular risk and heart rate variability in young adults with type 2 diabetes and arterial stiffness: the search for diabetes in youth study. J Diab Complicat 34:107676. https://doi.org/10.1016/j.jdiacomp.2020.107676
Shah AS, El Ghormli L, Vajravelu ME et al (2019) Heart rate variability and cardiac autonomic dysfunction: prevalence, risk factors, and relationship to arterial stiffness in the treatment options for type 2 diabetes in adolescents and youth (TODAY) study. Diab Care 42:2143–2150. https://doi.org/10.2337/dc19-0993
Liu L, Wu Q, Yan H et al (2020) Association between cardiac autonomic neuropathy and coronary artery lesions in patients with type 2 diabetes. Dis Markers 2020:6659166. https://doi.org/10.1155/2020/6659166
Vinik AI, Ziegler D (2007) Diabetic cardiovascular autonomic neuropathy. Circulation 115:387–397. https://doi.org/10.1161/CIRCULATIONAHA.106.634949
Schnell O, Muhr D, Weiss M et al (1996) Reduced myocardial 123I-metaiodobenzylguanidine uptake in newly diagnosed IDDM patients. Diabetes 45:801–805. https://doi.org/10.2337/diab.45.6.801
Paolisso P, Bergamaschi L, Rambaldi P et al (2021) Impact of admission hyperglycemia on heart failure events and mortality in patients with takotsubo syndrome at long-term follow-up: data from HIGH-GLUCOTAKO investigators. Diab Care 44:2158–2161. https://doi.org/10.2337/dc21-0433
Marfella R, Barbieri M, Sardu C et al (2016) Effects of α-lipoic acid therapy on sympathetic heart innervation in patients with previous experience of transient takotsubo cardiomyopathy. J Cardiol 67:153–161. https://doi.org/10.1016/j.jjcc.2015.07.012
Pop-Busui R, Boulton AJM, Feldman EL et al (2017) Diabetic neuropathy: a position statement by the American diabetes association. Diab Care 40:136–154. https://doi.org/10.2337/dc16-2042
Rahman A, Fujisawa Y, Nakano D, Hitomi H, Nishiyama A (2017) Effect of a selective SGLT2 inhibitor, luseogliflozin, on circadian rhythm of sympathetic nervous function and locomotor activities in metabolic syndrome rats. Clin Exp Pharmacol Physiol 44:522–525. https://doi.org/10.1111/1440-1681.12725
Wan N, Rahman A, Hitomi H, Nishiyama A (2018) The effects of sodium-glucose cotransporter 2 inhibitors on sympathetic nervous activity. Front Endocrinol 9:421
Sato D, Nakamura T, Amarume J et al (2022) Effects of dapagliflozin on peripheral sympathetic nerve activity in standard chow- and high-fat-fed rats after a glucose load. J Pharmacol Sci 148:86–92. https://doi.org/10.1016/j.jphs.2021.09.009
Sardu C, Massimo Massetti M, Rambaldi P et al (2022) SGLT2-inhibitors reduce the cardiac autonomic neuropathy dysfunction and vaso-vagal syncope recurrence in patients with type 2 diabetes mellitus: the SCAN study. Metabolism. https://doi.org/10.1016/j.metabol.2022.155243
Cowie MR, Fisher M (2020) SGLT2 inhibitors: mechanisms of cardiovascular benefit beyond glycaemic control. Nat Rev Cardiol 17:761–772. https://doi.org/10.1038/s41569-020-0406-8
Gueguen C, Burke SL, Barzel B et al (2020) Empagliflozin modulates renal sympathetic and heart rate baroreflexes in a rabbit model of diabetes. Diabetologia 63:1424–1434. https://doi.org/10.1007/s00125-020-05145-0
Zhang N, Feng B, Ma X et al (2019) Dapagliflozin improves left ventricular remodeling and aorta sympathetic tone in a pig model of heart failure with preserved ejection fraction. Cardiovasc Diabetol 18:107. https://doi.org/10.1186/s12933-019-0914-1
Esser N, Legrand-Poels S, Piette J et al (2014) Inflammation as a link between obesity, metabolic syndrome and type 2 diabetes. Diabetes Res Clin Pract 105:141–150. https://doi.org/10.1016/j.diabres.2014.04.006
Grassi G, Biffi A, Dell’Oro R et al (2020) Sympathetic neural abnormalities in type 1 and type 2 diabetes: a systematic review and meta-analysis. J Hypertens 38:1436–1442. https://doi.org/10.1097/HJH.0000000000002431
Coats AJ, Cruickshank JM (2014) Hypertensive subjects with type-2 diabetes, the sympathetic nervous system, and treatment implications. Int J Cardiol 174:702–709. https://doi.org/10.1016/j.ijcard.2014.04.204
Bakkar NZ, Mougharbil N, Mroueh A et al (2020) Worsening baroreflex sensitivity on progression to type 2 diabetes: localized vs. systemic inflammation and role of antidiabetic therapy. Am J Physiol Endocrinol Metab 319:E835–E851. https://doi.org/10.1152/ajpendo.00145.2020
Sardu C, Massetti M, Testa N et al (2021) Effects of sodium-glucose transporter 2 inhibitors (SGLT2-I) in patients with ischemic heart disease (IHD) treated by coronary artery bypass grafting via MiECC: inflammatory burden, and clinical outcomes at 5 years of follow-up. Front Pharmacol 12:777083. https://doi.org/10.3389/fphar.2021.777083
Paolisso P, Bergamaschi L, Santulli G et al (2022) Infarct size, inflammatory burden, and admission hyperglycemia in diabetic patients with acute myocardial infarction treated with SGLT2-inhibitors: a multicenter international registry. Cardiovasc Diabetol 21:77. https://doi.org/10.1186/s12933-022-01506-8
Spallone V, Bellavere F, Scionti L et al, On behalf of the Diabetic Neuropathy Study Group of the Italian Society of Diabetology (2011) Recommendations for the use of cardiovascular tests in diagnosing diabetic autonomic neuropathy. Nutr Metab Cardiovasc Dis 21:69–78. https://doi.org/10.1016/j.numecd.2010.07.005
Spallone V, Ziegler D, Freeman R et al, Toronto Consensus Panel on Diabetic Neuropathy (2011) Cardiovascular autonomic neuropathy in diabetes: clinical impact, assessment, diagnosis, and management. Diab Metab Res Rev 27:639–653. https://doi.org/10.1002/dmrr.1239
Carvalho LP, Di Thommazo-Luporini L, Mendes RG et al (2018) Metabolic syndrome impact on cardiac autonomic modulation and exercise capacity in obese adults. Auton Neurosci 213:43–50. https://doi.org/10.1016/j.autneu.2018.05.008
Verma S, Alam R, Ahmad I et al (2018) Effect of glycemic control and disease duration on cardiac autonomic function and oxidative stress in type 2 diabetes mellitus. J Diab Metab Disord 17:149–158. https://doi.org/10.1007/s40200-018-0354-6
Silva LRB, Gentil P, Seguro CS et al (2021) High fasting glycemia predicts impairment of cardiac autonomic control in adults with type 2 diabetes: a case-control study. Front Endocrinol 12:760292. https://doi.org/10.3389/fendo.2021.760292
De Maria B, Dalla Vecchia LA, Porta A, La Rovere MT (2021) Autonomic dysfunction and heart rate variability with Holter monitoring: a diagnostic look at autonomic regulation. Herzschrittmacherther Elektrophysiol 32:315–319
Shinohara T, Ebata Y, Ayabe R et al (2014) Cardiac autonomic dysfunction in patients with head-up tilt test-induced vasovagal syncope. Pacing Clin Electrophysiol 37:1694–1701. https://doi.org/10.1111/pace.12484
Acknowledgements
None.
Funding
None.
Author information
Authors and Affiliations
Contributions
MD and DP conceived and designed the study. DP and AK performed search of relevant studies, literature screening and data extraction. DP and AK made the statistical analysis. DP, AK, NF and CP wrote the first draft. MD critically revised the first draft. All authors agree to its submission.
Corresponding author
Ethics declarations
Conflict of interest
None declared.
Ethical standard statement
Not required for this type of manuscript.
Human and animal rights disclosure
No experiment involving humans or animals was performed.
Informed consent
Not required.
Additional information
Managed By Massimo Federici.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Patoulias, D., Katsimardou, A., Fragakis, N. et al. Effect of SGLT-2 inhibitors on cardiac autonomic function in type 2 diabetes mellitus: a meta-analysis of randomized controlled trials. Acta Diabetol 60, 1–8 (2023). https://doi.org/10.1007/s00592-022-01958-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00592-022-01958-0