Abstract
Aims
To perform an automated functional assessment of retinal and choroidal microvasculature in eyes with low-grade diabetic retinopathy (DR) using optical coherence tomography angiography (OCT-A) and to identify potential perfusion changes in case of early vascular damage.
Methods
This is an observational, case–control study of consecutive diabetic patients with level 20 DR severity scale score and age-matched healthy subjects. A prototypal OCT-angiography was used to obtain the OCT-angiograms of the superficial capillary plexus (SCP), deep capillary plexus (DCP) and choriocapillaris (CC) layer. A validated automated microstructural analysis provided data on SCP, DCP and CC vascular perfusion density (VPD). A comparative assessment between different vascular layers and different groups was performed.
Results
Twenty-nine diabetic patients (7 females, 24%) and 20 healthy controls were enrolled. VPD values were significantly lower in the DCP (25.1% vs. 26.5%; p = 0.04) and CC (71.2% vs. 86.6%; p = 0.0001) of diabetic patients compared with controls. A statistically significant negative linear correlation was reported between CC VPD and DCP VPD in diabetic patients; at the reverse, a positive linear correlation between the same parameters was noticed in controls.
Conclusion
Retinal and choroidal vascular networks, although distinct entities, seem functionally interconnected: varying the degree of perfusion may be a mutual compensatory mechanism in response to an ischemic injury.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Diabetic retinopathy (DR) is one of the most relevant complications of diabetes mellitus (DM). It is characterized by a chronic microvascular damage, with increased vascular permeability causing macular edema, and late onset of retinal ischemia leading to retinal neovascularizations [1, 2]. Pericyte loss, basement membrane thickening and smooth muscle cell loss represent some of the earliest changes of retinal capillaries in DR [3]. Although most of the attention has been focused on retinal microangiopathy, choriocapillaris degeneration in diabetic patients may also occur. Hidayat and Fine firstly introduced the concept of diabetic choroidopathy, describing capillary dropout, basement membrane thickening and choroidal neovascularization in eyes from subjects with advanced diabetes [4]. Choriocapillaris (CC) loss, Bruch’s membrane degenerative changes, large and intermediate blood vessel tortuosity, microaneurysms and vascular hypercellularity have also been documented with scanning electron microscopy in human diabetic choroidal specimens [5]. A healthy and functional choroid is essential for the outer retina activity, and a potential choroidal flow impairment can result in photoreceptor dysfunction and death. Diabetic choroidopathy might be the cause of visual acuity loss described in diabetic patients who do not exhibit any signs of DR [6].
Fluorescein angiography (FA) and indocyanine green angiography (ICGA) were the first imaging modalities used to detect changes in the diabetic choroid [7]. Several authors reported the presence of a “salt and pepper” pattern, with both hyperfluorescent and hypofluorescent spots, in patients with clinical signs of DR which correlated with elevated serum levels of HbA1c [8]. Late choroidal non-perfusion regions and inverted flow phenomena have also been described on conventional dye-angiograms of diabetic patients [9].
The CC evaluation using conventional labeled angiography is not feasible due to the bidimensional nature of this imaging modality. Recently, with the introduction of optical coherence tomography angiography (OCT-A), a depth-resolved assessment of different retinal and choroidal vascular layers was made possible [10]. OCT-A has been largely applied in detecting and characterizing retinal microvascular perfusion abnormalities occurring in DR, but only few studies investigated its capability of analyzing pathological changes of choroidal perfusion in diabetes [10,11,12,13,14,15].
Retinal findings in DR are defined by discrete steps of disease worsening using the Early Treatment Diabetic Retinopathy Study DR severity score (DRSS), which is commonly used in clinical trials using color fundus stereo-photographs [16]. In clinical practice, simplified versions of this severity scale are routinely applied to describe patient status, to define intervals for follow-up and to decide for interventions. With the introduction of OCT-A, the capability of identifying the early perfusion changes both in retinal and choroidal microvasculature substantially increased. Therefore, a rigorous selection of the patient sample, using a definite scoring system, appears mandatory to understand the clinical meaning of the variable DR stage-related blood flow impairments.
In the current study, we investigated by OCT-A the potential perfusion abnormalities at the level of the CC and retinal circulations in diabetic patients with low-grade DR (DRSS score 20). By comparing these findings with those of healthy, control eyes, we attempted to understand the functional consequences of the diabetes-induced early choroidal damage.
Methods
Study design
This retrospective, case–control study was conducted at the Eye Clinic of the S. Maria della Misericordia Hospital (University of Perugia, Perugia, Italy) in accordance with the Declaration of Helsinki after approval by the Perugia Institutional Ethics Committee (IEC). Written informed consent was obtained from all individual participants included in the study.
Study sample
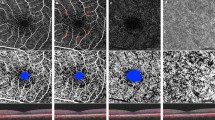
Diabetic patients with low-grade DR and age-matched healthy subjects were enrolled between June and December 2018. All subjects underwent a complete clinical evaluation including a detailed best-corrected visual acuity determination, applanation tonometry, anterior segment and fundus examination, FA and OCT-A by an expert medical retina specialist (M.L.). ICGA was also performed in order to exclude potential comorbidities involving the choroid that could negatively influence the evaluation of the CC on OCT-angiograms. According to the Diabetic Retinopathy Severity Scale (DRSS) score, patients with type 2 diabetes showing signs of level 20 DR (microaneurysms only) on FA and non-relevant vascular abnormalities of choroidal pertinence on ICGA were included in the study [16] (Fig. 1). Exclusion criteria were the presence of significant media opacities, refractive errors higher or equal than 4 diopters (spherical equivalent), previous retinal laser treatments, previous intravitreal injections and vitreo-retinal surgery, diagnosis of other retinal, choroidal or systemic diseases that might interfere with the purpose of the study. Patients showing any signs of cystoid macular edema as well as hard exudates in macular area [17], that may compromise a proper segmentation of OCT-angiograms or masking the flow signal coming from the CC, were also excluded. After image acquisition, all OCT-A examinations with bulk-motion artifacts, shadowgraphic artifacts or poor-quality images (quality index lower than 30) were as well, excluded.
Indocyanine green angiography (ICGA) during the venous phases (left) and 10 × 10° OCT-Angiography (OCT-A) image of the choriocapillaris (right) in a diabetic patient. The two imaging modalities show the correspondence between focal hypofluorescent areas on ICGA (arrowhead) and hypoperfused areas (yellow arrowhead) on OCT-A in the same area (white dashed line); microaneurysms can also be visualized on the ICGA image (arrows) (color figure online)
OCT-A image acquisition
The Spectralis OCT2 (Heidelberg Engineering, Heidelberg, Germany), based on a probabilistic amplitude decorrelation algorithm, was used to acquire OCT-A images. For additional details regarding the device or the OCT-A algorithm, we refer interested readers to our previously published studies [10, 13]. En-face OCT-angiograms were obtained by segmenting a 15 × 10° (4.5 × 3 mm approx.) volume scan at the level of the ganglion cell layer for the superficial capillary plexus (SCP) and at the inner nuclear layer for the deep capillary plexus (DCP). A 20-µm-thick slab shaped on the retinal pigmented epithelium (RPE) profile and placed underneath the Bruch’s membrane was used for the CC (Fig. 2). The in-built software (SP-X1701 Update 3, based on Heyex software version 1.9.215.0H, Heidelberg Engineering, Heidelberg, Germany) is equipped with a projection artifact removal (PAR) tool, which automatically removed any shadowgraphic artifact from the selected C-scan. The segmentation strategies, aimed to ensure a proper positioning of the slab edges, have been described in a reference study [13].
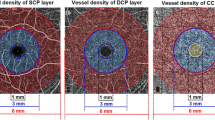
OCT-Angiography of different vascular layers in a diabetic patient and healthy control. a–c, OCT-angiograms of the superficial capillary plexus (a), deep capillary plexus (b) and choriocapillaris (c) of a healthy subject. d–f, OCT-angiograms of the superficial capillary plexus (a), deep capillary plexus (b) and choriocapillaris (c) of a diabetic patient with level 20 diabetic retinopathy severity scale score. A substantial rarefaction of the flow signals is shown in each vascular layer of the diabetic patient compared to the healthy control
Within a single visit, an experienced operator (M.L.) performed two OCT-A imaging sessions per patient, and all scans were then independently reviewed by two senior retinal specialists (M.L. and A.C.), in order to select the most accurate examination, to validate the segmentation and standardize the image quality as per study protocol. In case of potential issues related to the segmentation strategies, a manual correction of the alignment was allowed.
Image analysis
Three “en-face” OCT-angiograms per subject were evaluated in the quantitative assessment: the SCP, DCP and CC vascular layers. To limit the effects of artifacts at the lateral borders of the “en-face” OCT-angiogram, images were cropped to a 10 × 10° area corresponding to 3 × 3 mm (original image 15 × 10° area corresponding to 4.5 × 3 mm) [13]. This is an automated function provided by the AngiOCTool™ (version 4.0 Lupidi M, Coscas G, Centre de l’Odéon, Paris, France) which aligns different OCT-angiograms on the base of a reference infrared background. The software then identifies the center of the foveal avascular zone (FAZ), corrects for potential radial shifts and then computes 5° (1.5 mm) on the horizontal axis from both sides of the FAZ center. The software is able to rectify the color spectrum of all angiograms taking into account both brightness and contrast variations. The AngiOCTool™ also allows a quantification of SCP and DCP. Vessel detection was based both on the exact identification of each decorrelated pixel and on the trajectory taken by contiguous decorrelated pixels. With this process, the vessel thickness could be visualized and the shape of perfused structures obtained, with a vector representation [13].
Different parameters were automatically computed in the OCT-A analysis: The total vascular and avascular surfaces were assessed in order to calculate the vascular perfusion density (VPD) values. The magnification variation induced by the axial length variance was considered when computing the quantitative data [12].
The quantitative analysis of each “en-face” scan angiogram segmented at the level of the CC was obtained by FIJI imaging software (based on ImageJ, version 1.51 h, Wayne Rasband, National Institute of Health, USA). The CC VPD was computed as the ratio (expressed in percentage) between the number of “flow” pixels and the total number of pixels composing the OCT-angiogram after the proper application of an automated local thresholding algorithm to the image. The accurate description and validation of this approach have been previously reported [18].
Statistical analysis
Friedman test was used to compare the concordance of qualitative variables between the SCP, DCP and CC. Quantitative variables were described by their mean and standard deviation (SD). The Shapiro–Wilk test was applied to evaluate the normal distribution of the data. The Wilcoxon–Mann–Whitney test was used to compare paired data of both diabetic and healthy subjects. The Spearman rank correlation coefficient was applied to measure the statistical dependence of quantitative variables. A P value of < 0.05 was considered as statistically significant. All statistical analyses were performed using the SAS System for Windows (release 9.4, SAS Institute Inc., Cary, NC, USA).
Results
Demographic data
Twenty-nine eyes of 29 diabetic patients (7 females, mean age 69.2 years) and 20 eyes of 20 healthy controls (5 females, mean age 68.7 years) were enrolled. Mean duration of the disease was 11 ± 4 years (range 7–15 years), and mean HbA1c level was 7.6 ± 0.5% (range 6.1–8.3%). On the basis of the DRSS score, the whole DR sample was classified as level 20.
Quantitative data
VPD mean values at the level of the SCP were 26% ± 2% in the DR group and 27% ± 2% in the healthy group; the DCP VPD mean values were 25% ± 2% and 27% ± 2%, for diabetic eyes and healthy controls, respectively (Table 1).
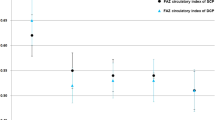
No statistically significant differences between the SCP and DCP VPDs were reported both in healthy and diabetic eyes (p > 0.05). When comparing DR and healthy eyes, the DCP VPD values were significantly lower in the diabetic group (p = 0.04). The CC VPD was 71% ± 5% in the diabetic group and 87% ± 2% in the control one, showing a statistically significant difference between the two samples (p = 0.001). The Spearman’s coefficient of rank correlation analysis revealed a significant negative linear correlation between DCP VPD and CC VPD in the diabetic group (− 0.448 p = 0.01). This negative linear correlation was not statistically significant when considering the SCP and CC VPDs (− 0.251 p = 0.06) (Fig. 3). Conversely, a positive linear correlation was found in the control group between VPD values of both retinal vascular plexuses and CC (SCP 0.454, p = 0.06; DCP 0.395, p = 0.08) (Fig. 3). In the DR group, four eyes did not have microaneurysms in macular area. When comparing these cases with those showing macular microaneurysms (25), no statistically significant differences were shown in terms of SCP VPD (p = 0.84), DCP VPD (p = 0.87), CC VPD (p = 0.79).
Images a and b show the correlation between vascular perfusion density (VPD) of the superficial and deep (DCP) capillary plexuses and the VPD of the choriocapillaris (CC). The negative linear correlation between CC and DCP in terms of VPD could be expression of a potential compensatory mechanism. In case of CC insufficiency, blood flow at the level of DCP might increase in order to supply outer retinal layers. Conversely, in case of retinal capillary damage, the CC might increase its perfusion to compensate the oxygen demand. Such a mechanism is not shown in healthy subjects, since no abnormalities of retinal or choroidal perfusion are present
Discussion
Aim of the current study was to investigate diabetes-related choroidal vascular perfusion changes in diabetic patients with low-grade DR and comparing them with age-matched healthy subjects. We previously evaluated through the use of OCT-A, the retinal vasculature impairment occurring in patients affected by type 2 DM and variable degrees of DR, ranging from mild nonproliferative DR (DRSS score 35) to severe nonproliferative DR (DRSS score 53). We observed that both the SCP and DCP seem to be diffusely affected by progressive non-perfusion, in a simultaneous but not symmetrical way [13]. This evidence was confirmed in the current study, since the quantitative evaluation of the DCP revealed significantly lower VPD values in diabetic patients with low-grade DR (DRSS score 20) compared to healthy controls (p = 0.04).
It has been shown that SCP and DCP are organized in an “in-series” vascular system, and a potentially increased arteriovenous transit due to diabetic microangiopathy might lead to an increased extraction of oxygen in the SCP causing a hypoxic environment at the level of the DCP [19]. The subsequent DCP damage could cause an impaired venous outflow and might be at the base of the reduced VPD that we observed in our corresponding OCT-angiograms. This finding confirms the presence of an evident microvascular perfusion impairment from the very early stages of the DR and highlights the importance of a timely DCP assessment in order to estimate the risk of developing severe macular complications (i.e., edema or hemorrhages).
The damage at the level of the CC occurring in patients affected by DR was already described in histological assessments [5, 6] and in vivo based on multimodal imaging modalities [7,8,9, 12, 14, 15]. Several studies focused on choroidal thickness evaluation and showed some degree of choroidal thinning in DR [20, 21].
Choi et al. demonstrated, through the use of the OCT-A, abnormalities of retinal and choroidal blood flow in eyes with both proliferative and nonproliferative DR, although these alterations were more severe and frequent in advanced stages of the disease [14]. Nesper et al. affirmed that retinal and CC vascular non-perfusion detected on OCT-angiograms was positively correlated (except for SCP in diabetic patients without DR) with the increasing severity of DR [22].
Although we included in the study only patients with DRSS score of 20, we observed a substantial vascular impairment at the level of the CC, since a statistically significant difference between healthy controls and diabetic patients in terms of VPD was noticed (p = 0.0001). These data highlight how the CC might show a significant functional impairment from the early stages of the disease, even before the retinal vascular layers, and confirm the sensitivity of OCT-A in detecting ocular microvascular changes occurring in DR.
The analysis of our results interestingly revealed the presence of a significant negative linear correlation between DCP VPD and CC VPD in diabetic subjects, whereas the correlation between SCP VPD and CC VPD was not significant (Fig. 3). Conversely, a positive linear correlation was found in healthy subjects between SCP or DCP VPD and CC VPD. These findings seem to suggest that retinal and choroidal vascular networks could be functionally interconnected. We hypothesize that the negative linear correlation between CC and DCP in terms of VPD could be expression of a potential compensatory mechanism. In case of CC insufficiency, blood flow at the level of DCP might slightly increase in order to supply outer retinal layers. At the reverse, in case of retinal capillary impairment, which more frequently involves the DCP in case of DR [15, 23], the CC might increase its perfusion to compensate the oxygen and metabolic demand (Fig. 4).
Explicative diagram showing the functional connection between retinal and choroidal circulations in normal (a) and diseased conditions (b–c). In case of choriocapillaris insufficiency, causing hypoxia of the outer retina, the blood flow at the level of the deep capillary plexus might slightly increase in order to supply outer retinal layers. At the reverse, in case of retinal capillary impairment, the choriocapillaris might increase its perfusion to compensate the oxygen and metabolic demand
It has been reported that the DCP seems to play a key role during the course of DR. In patients with type 1 DM without any sign of DR, Carnevali et al. observed a significantly decreased vessel density at the level of the DCP, suggesting that earliest vascular changes could occur at this level [15]. Scarinci et al. by evaluating patients with variable degrees of DR, including proliferative DR, reported how areas of DCP non-perfusion, detected on OCT-angiograms, co-localized with outer retinal changes on SD-OCT as thinning of outer nuclear layers, disruption of the ellipsoid zone and loss of the RPE integrity, suggesting that chronic ischemia at the level of the DCP is the main cause of focal photoreceptor damage [23]. Outer retina is mostly dependent on diffusion from CC for its oxygen and nutrients demand; nevertheless, previous studies have illustrated that photoreceptors rely on the retinal circulation for a certain part of their oxygen needs and that this percentage is even increased in eyes with DR [24,25,26].
Birol et al. reported that retinal circulation physiologically provides about 15% of the oxygen needs of the inner segments. [24]. Photoreceptors and OPL localized in the watershed zones, where both the choroidal and retinal circulations provide oxygen support, might be more susceptible to ischemic insults. We therefore argued that, in low-grade DR, a compensatory retinal vascular mechanism could be still effective. In patients with early damage of the CC, a moderate increase in blood flow at the level of DCP (or at the level of the CC in case of DCP insufficiency) could be supposed in order to maintain adequate metabolic supply to photoreceptors and outer retinal layers. In patients with more severe stages of DR and/or other concomitant cardiovascular diseases, these potential compensatory mechanisms might fail, causing severe outer retinal suffering.
To the authors’ knowledge, this is the first study that provides new insights on the functional correlation between retinal capillary plexuses and choroidal vasculature that could be present in early DR. We acknowledge as limitations of this study its retrospective nature and the relatively small sample size. Moreover, hemodynamic data at the moment of the OCT-A acquisition, that may influence the blood flow visualization [18], were not available for the enrolled subjects.
In conclusion, a fully automated quantitative OCT-A assessment is a useful approach for detecting diabetic-induced retinal and choroidal vascular impairment also in low-grade DR. Retinal and choroidal vascular networks although distinct entities seem functionally interconnected: varying the degree of perfusion might be a mutual compensatory mechanism in case of chronic retinal or choroidal insufficiency. Future studies will be necessary to further delineate the functional and anatomic consequences of retinal and choroidal plexuses alterations occurring in DR.
References
Klein R, Klein BE, Moss SE et al (1994) The Wisconsin epidemiologic study of diabetic retinopathy. XIV. Ten-year incidence and progression of diabetic retinopathy. Arch Ophthalmol 112:1217–1228
Engerman RL, Kern TS (1995) Retinopathy in animal models of diabetes. Diabetes Metab Rev 11:109–120
Hammes HP, Lin JH, Renner O et al (2002) Pericytes and the pathogenesis of diabetic retinopathy. Diabetes 51:3107–3112
Hidayat A, Fine B (1985) Diabetic choroidopathy: light and electron microscopic observations of seven cases. Ophthalmology 67:512–522
Lutty GA (2017) Diabetic choroidopathy. Vision Res 139:161–167
Cao J, McLeod DS, Merges CA, Lutty GA (1998) Choriocapillaris degeneration and related pathologic changes in human diabetic eyes. Arch Ophthalmol 116:589–597
Weinberger D, Kramer M, Priel E et al (1998) Indocyanine green angiographic findings in nonproliferative diabetic retinopathy. Am J Ophthalmol 126:238–247
Shiragami C, Shiraga F, Matsuo T et al (2002) Risk factors for diabetic choroidopathy in patients with diabetic retinopathy. Graefes Arch Clin Exp Ophthalmol 240(6):436–442
Hua R, Liu L, Wang X, Chen L (2013) Imaging evidence of diabetic choroidopathy in vivo: angiographic pathoanatomy and choroidal-enhanced depth imaging. PLoS ONE 8(12):e83494
Coscas G, Lupidi M, Coscas F (2017) Optical coherence tomography angiography in diabetic maculopathy. Dev Ophthalmol 60:38–49
Ishibazawa A, Nagaoka T, Takahashi A et al (2015) Optical coherence tomography angiography in diabetic retinopathy: a prospective pilot study. Am J Ophthalmol 160:35–44
Coscas G, Lupidi M, Coscas F, Chhablani J, Cagini C (2018) Optical coherence tomography angiography in healthy subjects and diabetic patients. Ophthalmologica 239(2–3):61–73
Lupidi M, Coscas G, Coscas F et al (2017) Retinal microvasculature in non-proliferative diabetic retinopathy: automated quantitative optical coherence tomography angiography assessment. Ophthalmic Res 58(3):131–141
Choi W, Waheed NK, Moult EM et al (2017) Ultrahigh speed swept source optical coherence tomography angiography of retinal and choriocapillaris alterations in diabetic patients with and without retinopathy. Retina 37(1):11–21
Carnevali A, Sacconi R, Corbelli E et al (2017) Optical coherence tomography angiography analysis of retinal vascular plexuses and choriocapillaris in patients with type 1 diabetes without diabetic retinopathy. Acta Diabetol 54(7):695–702
Early Treatment Diabetic Retinopathy Study (ETDRS) Research Group (1991) Grading diabetic retinopathy from stereoscopic color fundus photographs and extension of the modified Airlie house classification. ETDRS report number 10. Ophthalmology 98(5 suppl):786–806
Aiello LP, Beck RW, Bressler NM et al (2011) Rationale for the diabetic retinopathy clinical research network treatment protocol for center-involved diabetic macular edema. Ophthalmology 118(12):e5–e14
Cardillo Piccolino F, Lupidi M, Cagini C et al (2018) Choroidal vascular reactivity in central serous chorioretinopathy. Invest Ophthalmol Vis Sci 59:3897–3905
Freund KB, Sarraf D, Leong BCS et al (2018) Association of optical coherence tomography angiography of collaterals in retinal vein occlusion with major venous outflow through the deep vascular complex. JAMA Ophthalmol 136(11):1262–1270
Vujosevic S, Martini F, Cavarzeran F, Pilotto E, Midena E (2012) Macular and peripapillary choroidal thickness in diabetic patients. Retina 32(9):1781–1790
Querques G, Lattanzio R, Querques L et al (2012) Enhanced depth imaging optical coherence tomography in type 2 diabetes. Invest Ophthalmol Vis Sci 53(10):6017
Nesper PL, Roberts PK, Onishi AC et al (2017) Quantifying microvascular abnormalities with increasing severity of diabetic retinopathy using optical coherence tomography angiography. Invest Ophthalmol Vis Sci 58:BIO307–BIO315
Scarinci F, Nesper PL, Fawzi AA (2016) Deep retinal capillary nonperfusion is associated with photoreceptor disruption in diabetic macular ischemia. Am J Ophthalmol 168:129–138
Birol G, Wang S, Budzynski E et al (2007) Oxygen distribution and consumption in the macaque retina. Am J Physiol Heart Circ Physiol 293(3):H1696–H1704
Borrelli E, Palmieri M, Viggiano P et al (2019) Photoreceptor damage in diabetic choroidopathy. Retina. https://doi.org/10.1097/IAE.0000000000002538
Yi J, Liu W, Chen S et al (2015) Visible light optical coherence tomography measures retinal oxygen metabolic response to systemic oxygenation. Light Sci Appl 4:e334
Funding
This study was supported by Fondazione per la Macula Onlus, Genova, Italy, and Fondazione Ferrero, Alba, Italy.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical standards
This study was conducted in accordance with the Declaration of Helsinki after approval by the Perugia Institutional Ethics Committee (IEC).
Informed consent
Written informed consent was obtained from all individual participants included in the study.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This article belongs to the topical collection Eye Complications of Diabetes, managed by Giuseppe Querques.
Rights and permissions
About this article
Cite this article
Lupidi, M., Cerquaglia, A., Gujar, R. et al. Functional correlation between choroidal and retinal vascularity in low-grade diabetic retinopathy. Acta Diabetol 57, 983–990 (2020). https://doi.org/10.1007/s00592-020-01507-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00592-020-01507-7