Abstract
Aims
Glucose-dependent insulinotropic polypeptide (GIP) is released primarily from the proximal small intestine and glucagon-like peptide-1 (GLP-1) from the more distal small intestine and colon. Their relative importance to the incretin effect in health has been contentious in the past, although it now appears that GIP has the dominant role. It is uncertain whether there is a relationship between GIP and GLP-1 secretion. We aimed to evaluate the relationship between plasma GIP and GLP-1 responses to a 75-g oral glucose load in individuals with normal (NGT) and impaired glucose tolerance (IGT).
Methods
One hundred healthy subjects had measurements of blood glucose, serum insulin, plasma GIP and GLP-1 concentrations for 240 min after a 300 mL drink containing 75 g glucose.
Results
Fifty had NGT and 41 IGT; 9 had type 2 diabetes and were excluded from analysis. In both groups, there were increases in plasma GIP and GLP-1 following the glucose drink, with no difference in the magnitude of the responses between t = 0–240 min. There was a weak relationship between the iAUC0–240 min for GIP and GLP-1 in the combined (r = 0.23, P = 0.015) and in the IGT (r = 0.34, P = 0.01), but not in the NGT (r = 0.15, P = 0.14) group.
Conclusions
There is a weak relationship between oral glucose-induced GIP and GLP-1 secretions in non-diabetic subjects.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Glucose-dependent insulinotropic polypeptide (GIP) and glucagon-like peptide-1 (GLP-1) are released from the small intestine in response to macronutrient exposure (carbohydrate, lipid or protein). GIP is released from enteroendocrine ‘K’ cells, located predominantly in the proximal small intestine, and GLP-1 from ‘L’ cells, located predominantly in the distal small intestine and colon [1]. It has been suggested that GIP and GLP-1 account, in approximately equal proportions, for the ‘incretin effect’ (the amplified insulin secretory response to oral compared with intravenous glucose) in health, although the outcome of a recent study is indicative of a dominant contribution of GIP [2]. The incretin effect is a major determinant of the postprandial insulin secretory response in health, although its magnitude is reduced in type 2 diabetes (T2D), at least in part because the insulinotropic effect of GIP is markedly diminished [3]. GLP-1, unlike GIP, largely retains its insulinotropic (and glucagonostatic) properties in T2D, which has stimulated the development, and current widespread use, of GLP-1-based therapy (GLP-1 receptor agonists and dipeptidyl peptidase 4 (DPP-4) inhibitors) for T2D. Augmenting endogenous GLP-1 has been shown to be therapeutically beneficial—both GLP-1 receptor agonists (> tenfold) and DPP-IV inhibitors (approx. twofold) enhance active GLP-1 concentrations [4]. Moreover, elevated GLP-1 concentrations post-bariatric surgery is considered to be a key mechanism underlying its impressive metabolic improvements [5].
There is renewed interest in GIP—in addition to the growing recognition of its dominant contribution to postprandial insulin release, a specific GIP antagonist is now available [6] and a recent trial of a novel dual GIP/GLP-1 agonist reported superior results for both glucose lowering and weight loss compared with a selective GLP-1 agonist (dulaglutide) at 26 weeks [7]. It is uncertain, however, whether GIP and GLP-1 secretion are related. A study by Nauck et al. in first-degree relatives of people with T2D and healthy controls reported a strong correlation between plasma GIP and GLP-1 responses following a 75-g oral glucose tolerance test (OGTT) [8, 9], but the number of subjects (n = 15 relatives, n = 10 controls) was small. In general, macronutrients will be exposed earlier to ‘K’ than ‘L’ cells, and unlike rodents, GIP probably does not influence GLP-1 secretion in humans [9]. Our study was conducted to determine whether there is a relationship between plasma GIP and GLP-1 levels in a larger cohort of individuals with normal (NGT), or impaired (IGT), glucose tolerance.
Materials and methods
Subjects
One hundred healthy Caucasian subjects were recruited through local advertisement. Subjects with a history of gastrointestinal disease, other significant medical illness or taking medication known to affect gastrointestinal motility were excluded.
The protocol was approved by the Human Research Ethics Committee of the Royal Adelaide Hospital, and each subject provided written informed consent. All experiments were carried out in accordance with the Declaration of Helsinki.
Protocol
Individuals attended the Royal Adelaide Hospital, at ∼ 08.30 h after an overnight fast (14 h for solids; 12 h for liquids) [10]. They were seated in an armchair, and a cannula was inserted into an antecubital vein for blood sampling. After a ‘rest period’ of 15–30 min, each subject consumed a drink containing 75 g glucose in 300 mL water within 2 min. Time zero (t = 0) was defined as the time of completion of the drink.
Venous blood samples (~ 12 mL) were obtained immediately prior to the commencement of the drink (t = −3 min) and at t = 30, 60, 90, 120, 180 and 240 min. The IV cannula was then removed, and the subject offered a light lunch before leaving the laboratory.
Measurements
Blood glucose
Blood glucose was determined using a portable glucometer (Medisense Companion 2 Meter, Medisense Inc., Waltham, MA, USA) [11, 12]. Subjects with fasting blood glucose ≥ 7.0 mmol/L and/or 2 h blood glucose ≥ 11.1 mmol/L were classified, according to WHO criteria, as having diabetes [13].
Serum insulin
Serum insulin was determined by ELISA (10–1113 Mercodia, Uppsala, Sweden), with assay sensitivity of 1.0 mU/L and coefficient of variation 2.5% within assays and 7.4% between assays [14].
Plasma GIP and GLP-1
Plasma GIP was measured by radioimmunoassay. The minimum detectable limit was 2 pmol/L, inter-assay CV was 9.4% and intra-assay CV was 4.4% [15]. Total GLP-1 was measured by radioimmunoassay (GLPIT-36HK, Millipore, Billerica, MA). The minimum detectable limit was 3 pmol/L, and intra- and inter-assay CVs were 8.0% and 10.0%, respectively [16].
Statistical analysis
Blood glucose, serum insulin, plasma GIP and GLP-1 were analyzed and presented as absolute values. Linear mixed-model analyses with group and time as factors and Bonferroni’s correction for post hoc comparisons were used to analyze blood glucose, insulin, GIP and GLP-1. Incremental areas under the curve (iAUC0–240 min) for GIP and GLP-1 were determined for the NGT, IGT and whole groups, and Pearson’s correlation was used to assess linear relationships between variables. A P value < 0.05 was considered significant in all analyses. Data are presented as mean values ± SEMs, unless stated otherwise.
Results
All subjects tolerated the study well, and there were no adverse events. Nine subjects had diabetes and were excluded from the analysis. Of the remaining 91 subjects (46 male and 45 female, mean age 68 ± 0.8 years, mean BMI 26 ± 0.3 kg/m2), 50 had NGT and 41 had IGT. A fixed sample size of 41 in the IGT group had the ability to obtain an unbiased estimate of the correlation coefficient of 0.2 to within ± 0.3.
Blood glucose
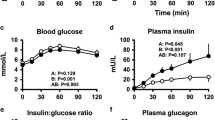
There was a substantial rise in blood glucose (time effect: P < 0.001) following the glucose load in both groups. Blood glucose concentrations were greater in IGT (group effect: P < 0.001 and group × time interaction: P < 0.001) with significant differences at t = 30, 60, 90 and 120 min (P < 0.05 for each, Fig. 1a).
Blood glucose (a), serum insulin (b), plasma GIP (c) and plasma GLP-1 (d) levels immediately before and following a 75-g oral glucose load in individuals with NGT (n = 50) and IGT (n = 41). Results of the linear mixed-model analysis are reported as P values for differences by group (group), differences over time (time) and differences due to the interaction of group and time (group × time). Post hoc comparisons, adjusted by Bonferroni’s correction, were made if mixed-model analysis values (group × time) were significant. *P < 0.05 and ***P < 0.01. Data are means ± SEMs.
Serum insulin
There was a substantial rise in serum insulin (time effect: P < 0.001) following the glucose load in both groups. The overall insulinemic response was slightly greater in IGT (group × time interaction: P < 0.001, Fig. 1b). Serum insulin concentration was lower at t = 30 min and greater at t = 120 min in IGT (P < 0.05 for both, Fig. 1b).
Plasma GIP and GLP-1
There was a substantial rise in plasma GIP (time effect: P < 0.001) following the glucose load in both groups. There was no difference in the overall GIP response between the two groups (group effect: P = 0.54, group × time interaction: P = 0.09, Fig. 1c). In both cohorts, plasma GIP levels were low at baseline, and rose promptly following the glucose drink with peak levels at ~ t = 30 min, maintained until ~ t = 120 min and followed by a decline to near baseline level at t = 240 min (Fig. 1c).
There was a substantial rise in plasma GLP-1 (time effect: P < 0.001) following the glucose load in both groups. There was no difference in the overall GLP-1 response between the two groups (group effect: P = 0.55, group × time interaction: P = 0.39, Fig. 1d). In both cohorts, plasma GLP-1 levels were low at baseline, and rose promptly following the glucose drink with peak levels at ~ t = 30 min, followed by a decline to the baseline level at t = 120 min, which was maintained until t = 240 min (Fig. 1d).
There was no relationship between baseline GIP and GLP-1 in the combined group (r = 0.14, P = 0.17), NGT (r = 0.13, P = 0.37) or IGT (r = 0.16, P = 0.33). The difference between the NGT and IGT group for the relationship between baseline GIP and GLP-1 was not statistically significant (z = −0.14, P = 0.89).
There was a significant relationship between the iAUC0–240 min for GIP and GLP-1 in the combined group (r = 0.23, P = 0.015), and in the IGT group (r = 0.34, P = 0.01), but not in the NGT group (r = 0.15, P = 0.14) (Fig. 2). The difference between the NGT and IGT group for the relationship between the iAUC0–240 min for GIP and GLP-1 was not statistically significant (z = −0.93, P = 0.35).
Discussion
We have evaluated, in subjects with NGT or IGT, whether there is a relationship between the GIP and GLP-1 responses to a 75-g OGTT. Our observations establish that there is a significant relationship albeit weakly positive. The lack of statistical significance in the NGT group alone is likely to represent a type II error. Factors potentially affecting GLP-1 and GIP secretion following macronutrient ingestion include the type of macronutrient stimulus (e.g., carbohydrate and lipid appear to be more potent stimuli of GLP-1 secretion than protein), caloric content, the rate of delivery of nutrients from the stomach to the small intestine (i.e., gastric emptying), integrity (or lack) of upper gastrointestinal anatomy (for example, GLP-1 secretion is markedly enhanced post-Roux-en-Y gastric bypass surgery) and the gastrointestinal autonomic system [9]. An association between GIP and GLP-1 levels (and presumably secretion) might reflect (i) a common stimulus or (ii) the influence of one on the other. While GIP and GLP-1 are known to be released from distinct intestinal cells, some enteroendocrine cells may co-secrete GIP and GLP-1 [17]. GIP and GLP-1 share some key commonalities (the same macronutrient stimuli induce the secretion of GIP and GLP-1 and the same ubiquitous enzyme, DPP-4, is responsible for the degradation of both [13]). Intuitively, as K cells are located more proximally, GIP may influence GLP-1 secretion, which is the case in some animal species [18]. However, intravenous infusion of supra-physiological doses of exogenous GIP does not stimulate GLP-1 secretion in humans [19].
Our analysis is indicative of only a modest correlation between the secretory responses of the two hormones, which was weaker than that observed by Nauck et al. [8] in a small cohort of first-degree relatives of people with T2D and healthy controls. We did not relate the incretin response to the rate of gastric emptying [20]. Gastric emptying exhibits a wide inter-individual variation (between 1 and 4 kcal/min in health) and our previous studies employing a naso-duodenal catheter to infuse glucose directly into the proximal duodenum have shown that the rate of intra-duodenal delivery of glucose has a major, and differential, impact on both GIP and GLP-1 secretion [20]. When the rate was increased from 1 to 4 kcal/min (i.e., within two extremes of the physiological range of gastric emptying), GIP secretion increased proportionately. In contrast, there was minimal, if any, elevation in GLP-1 at rates of 1 to 2 kcal/min, but a sustained and exaggerated response when the rate was increased to 3 kcal/min and beyond, indicative of a ‘threshold’ rate of delivery (or gastric emptying rate) for GLP-1 release of between 2 and 3 kcal/min [16]. It is, accordingly, possible that a stronger correlation between GIP and GLP-1 might be observed in individuals who have an intrinsically higher rate of gastric emptying (i.e., > 2 kcal/min).
In interpreting our observations, some limitations should be appreciated: (1) The correlation between GIP and GLP-1 secretion does not establish causality; (2) our cohort comprised predominantly of older men, and it is not known whether age affects the relationship; (3) we did not account for variations in gastric emptying or small intestinal absorption; (4) we characterized the response to glucose and not protein or fat; and (5) for convenience, we used a glucometer to determine glucose concentrations, which were taken from venous rather than capillary or arterial samples.
In conclusion, our study establishes that there is a weak, but significant, relationship between GIP and GLP-1 responses to oral glucose in individuals without diabetes.
Data availability
The datasets generated during and/or analyzed during the current study are not publicly available, but are available from the corresponding author on reasonable request.
References
Drucker DJ, Nauck MA (2006) The incretin system: glucagon-like peptide-1 receptor agonists and dipeptidyl peptidase-4 inhibitors in type 2 diabetes. Lancet 368(9548):1696–1705. https://doi.org/10.1016/S0140-6736(06)69705-5
Gasbjerg LS, Helsted MM, Hartmann B et al (2019) Separate and combined glucometabolic effects of endogenous glucose-dependent insulinotropic polypeptide and glucagon-like peptide 1 in healthy individuals. Diabetes 68(5):906–917. https://doi.org/10.2337/db18-1123
Nauck M, Stockmann F, Ebert R, Creutzfeldt W (1986) Reduced incretin effect in type 2 (non-insulin-dependent) diabetes. Diabetologia 29(1):46–52
Briere DA, Bueno AB, Gunn EJ, Michael MD, Sloop KW (2018) Mechanisms to elevate endogenous GLP-1 beyond injectable GLP-1 analogs and metabolic surgery. Diabetes 67(2):309–320. https://doi.org/10.2337/db17-0607
Madsbad S, Holst JJ (2014) GLP-1 as a mediator in the remission of type 2 diabetes after gastric bypass and sleeve gastrectomy surgery. Diabetes 63(10):3172–3174. https://doi.org/10.2337/db14-0935
Gasbjerg LS, Christensen MB, Hartmann B et al (2018) GIP(3–30)NH2 is an efficacious GIP receptor antagonist in humans: a randomised, double-blinded, placebo-controlled, crossover study. Diabetologia 61(2):413–423. https://doi.org/10.1007/s00125-017-4447-4
Frias JP, Nauck MA, Van J et al (2018) Efficacy and safety of LY3298176, a novel dual GIP and GLP-1 receptor agonist, in patients with type 2 diabetes: a randomised, placebo-controlled and active comparator-controlled phase 2 trial. Lancet 392(10160):2180–2193. https://doi.org/10.1016/s0140-6736(18)32260-8
Nauck MA, El-Ouaghlidi A, Gabrys B et al (2004) Secretion of incretin hormones (GIP and GLP-1) and incretin effect after oral glucose in first-degree relatives of patients with type 2 diabetes. Regul Pept 122(3):209–217. https://doi.org/10.1016/j.regpep.2004.06.020
Nauck MA, Meier JJ (2018) Incretin hormones: Their role in health and disease. Diabetes Obes Metab 20(Suppl 1):5–21. https://doi.org/10.1111/dom.13129
Gentilcore D, Doran S, Meyer JH, Horowitz M, Jones KL (2006) Effects of intraduodenal glucose concentration on blood pressure and heart rate in healthy older subjects. Dig Dis Sci 51(4):652–656
Chan JCN, Wong RYM, Cheung C-K et al (1997) Accuracy, precision and user-acceptability of self blood glucose monitoring machines. Diabetes Res Clin Pract 36(2):91–104. https://doi.org/10.1016/S0168-8227(97)00036-3
Weitgasser R, Straberger A, Schnoll F, Sailer S (1994) Clinical evaluation of the blood glucose monitors Accutrend, Companion 2, Glucometer 3 and One Touch II in comparison with the glucose oxidase reference method. Wien Klin Wochenschr 106(23):738–741
Alberti KG, Zimmet PZ (1998) Definition, diagnosis and classification of diabetes mellitus and its complications. Part 1: diagnosis and classification of diabetes mellitus provisional report of a WHO consultation. Diabet Med 15(7):539–553. https://doi.org/10.1002/(SICI)1096-9136(199807)15:7%3C539::AID-DIA668%3E3.0.CO;2-S
Trahair LG, Horowitz M, Marathe CS et al (2014) Impact of gastric emptying to the glycemic and insulinemic responses to a 75-g oral glucose load in older subjects with normal and impaired glucose tolerance. Physiol Rep 2(11):e12204. https://doi.org/10.14814/phy2.12204
Wishart J, Morris HA, Horowitz M (1992) Radioimmunoassay of gastric inhibitory polypeptide in plasma. Clin Chem 38(10):2156–2157
Trahair LG, Horowitz M, Rayner CK et al (2012) Comparative effects of variations in duodenal glucose load on glycemic, insulinemic, and incretin responses in healthy young and older subjects. J Clin Endocrinol Metabolism 97(3):844–851
Mortensen K, Petersen LL, Orskov C (2000) Colocalization of GLP-1 and GIP in human and porcine intestine. Ann N Y Acad Sci 921:469–472
Brubaker PL (1991) Regulation of intestinal proglucagon-derived peptide secretion by intestinal regulatory peptides. Endocrinology 128(6):3175–3182. https://doi.org/10.1210/endo-128-6-3175
Nauck MA, Heimesaat MM, Orskov C, Holst JJ, Ebert R, Creutzfeldt W (1993) Preserved incretin activity of glucagon-like peptide 1 [7-36 amide] but not of synthetic human gastric inhibitory polypeptide in patients with type-2 diabetes mellitus. J Clin Invest 91(1):301–307. https://doi.org/10.1172/jci116186
Marathe CS, Rayner CK, Bound M et al (2014) Small intestinal glucose exposure determines the magnitude of the incretin effect in health and type 2 diabetes. Diabetes 63(8):2668–2675. https://doi.org/10.2337/db13-1757
Acknowledgements
This research received no specific Grant from any funding agency.
Author information
Authors and Affiliations
Contributions
CSM conducted research, analyzed and interpreted data and reviewed paper; HP conducted research, analyzed and interpreted data and reviewed paper; JAM analyzed and interpreted data and reviewed paper; LGT conducted research and reviewed paper; LH analyzed data and reviewed paper; TW interpreted data and reviewed paper; LKP interpreted data and reviewed paper; CKR interpreted data and reviewed paper; MAN conceived and designed research and reviewed paper; MH conceived and designed research, interpreted data and reviewed paper; KLJ conceived and designed research, interpreted data, reviewed paper and approved the final version of paper.
Corresponding author
Ethics declarations
Conflict of interest
CSM is supported by a NHMRC Early Career Fellowship, TW by a Royal Adelaide Hospital Florey Fellowship and KLJ by the University of Adelaide William T Southcott Research Fellowship.
Ethical standard statement
All procedures involving human participants were in accordance with the ethical standards of the institutional research committee (Human Research Ethics Committee of the Royal Adelaide Hospital) and with the 1964 Helsinki Declaration and its later amendments.
Informed consent
Informed consent was obtained from all individual participants included in the study.
Additional information
Managed by Massimo Porta.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Marathe, C.S., Pham, H., Marathe, J.A. et al. The relationship between plasma GIP and GLP-1 levels in individuals with normal and impaired glucose tolerance. Acta Diabetol 57, 583–587 (2020). https://doi.org/10.1007/s00592-019-01461-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00592-019-01461-z