Abstract
Aims
Dexmedetomidine (DEX), a highly selective and potent α2-adrenergic receptor agonist, has anti-apoptotic, anti-inflammatory, and anti-oxidative stress effects in diabetes mellitus (DM) rats. The underlying molecular mechanisms and signaling pathways of diabetic cardiomyopathy remain poorly understood. This study aimed to elucidate the effect of DEX on cardiac function in DM rats.
Methods
Eight-week-old male Sprague Dawley rats were divided into three groups: control (n = 5), diabetes (DM, n = 7), and diabetes + DEX (DM + DEX, n = 10). DM was induced via intraperitoneal injection of streptozotocin (70 mg/kg); at 3 days later, DEX (1 µg/kg/h) was administered for 4 weeks. Cardiac function was evaluated using pressure–volume loop analysis and echocardiography. Left ventricular (LV) histological sections were used to analyze the interstitial collagen fraction. Using the LV samples, we performed a western blot analysis to evaluate signaling pathways and autophagic markers.
Results
The DM group had lower body weight and higher blood glucose level and heart weight/body weight ratio than the control group. However, metabolic changes did not differ between the DM and DM + DEX groups. Pressure–volume loop analysis and echocardiography showed impaired cardiac function, evidenced by a decrease in systolic and diastolic function, in both DM groups. DEX treatment in DM rats was associated with increased LV end-systolic pressure, LV contractility, cardiac output, and relaxed LV function compared with that in non-treated DM rats. LC3B and autophagy-related gene (ATG) proteins increased in the hearts of DM rats compared with the hearts of control rats. However, DEX reduced the expression of LC3B and ATG proteins in the hearts of DM rats. Increased p-ERK and decreased p-AKT were reduced in the hearts of DEX-treated DM rats.
Conclusions
DEX reduces cardiac dysfunction and impaired autophagy in DM rats. This study reinforces our understanding of the potential anti-autophagic effect of DEX in patients with diabetic cardiomyopathy.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Diabetes mellitus (DM) is one of the most important risk factors for heart failure and is associated with increased morbidity and mortality. Diabetic cardiomyopathy (DCM) has become a major cause of DM-related mortality and occurs independently of coronary artery disease, hypertension, and other cardiovascular diseases [1, 2]. DM impairs cardiac structure and function, and is characterized by myocyte hypertrophy, myocardial interstitial fibrosis, increased apoptosis, and suppressed autophagy, eventually leading to left ventricular (LV) systolic and diastolic dysfunction [3].
Dexmedetomidine (DEX), a highly selective and potent α2 adrenergic receptor agonist, has sedative, analgesic, and anxiolytic properties, as well as anesthetic-sparing effects [4]. Moreover, recent animal and clinical studies showed that DEX provided protection against ischemia reperfusion injury in the kidney, heart, and brain through its anti-apoptotic, anti-inflammatory, and anti-oxidative stress effects in DM rats [5,6,7]. To date, there are few published data addressing the effects of DEX in animal models of DCM.
Autophagy is the physiologic process whereby cytoplasmic components, including long-lived proteins and organelles, are engulfed by a double-membrane structure and targeted for destruction in lysosomes [8]. It selectively removes damaged mitochondria as a cytoprotective mechanism to limit mitochondria-derived oxidative stress and prevent apoptosis [9]. A low level of constitutive autophagy is important for the maintenance of normal cellular function and quality of cardiac proteins and organelles. Defects in this process result in cardiac dysfunction and heart failure, particularly during increased cellular stress [10]. Although autophagy is implicated in various pathologic conditions, including cardiac hypertrophy, cardiomyopathy, and heart failure, little information is available about its pathophysiologic roles in the pathogenesis of DCM. Despite the importance of this complication, the underlying molecular mechanisms and signaling pathways of DCM remain poorly understood. In this study, we focused on the effects of DEX treatment on DCM, especially the molecular signaling pathways. We aimed to elucidate the potential effect of DEX on alterations in cardiac dysfunction in streptozotocin (STZ)-induced DM rats and the mechanisms involved in autophagy.
Materials and methods
Ethical approval
All animal procedures were approved by the Committee for the Care and Use of Laboratory Animals at Yonsei University College of Medicine (no. 2015-0129) and were performed in accordance with the Guide for the Care and Use of Laboratory Animals published by the US National Institutes of Health. All animals were housed in a temperature-controlled room (25 ± 2 °C) with a 12-h light/12-h dark cycle and humidity of 60–65% and were provided with free access to normal chow and tap water. Except for drinking water, rats were fasted for 8 h before surgery and were allowed free access to food and water after surgery.
STZ models and study groups
Sprague Dawley rats are widely used in animal studies on type 1 DM induced by STZ. Male Sprague Dawley rats aged 8–10 weeks received a single intraperitoneal injection of STZ (Sigma-Aldrich, Saint Louis, MO, USA) to induce severe DM. DM rats were intraperitoneally injected with STZ at a dose of 70 mg/kg (n = 20), whereas control animals received an equivalent volume of citric acid buffer (n = 5) [11, 12]. Glucose and insulin levels were determined on the third day following STZ injection. In this study, 80% of rats became diabetic. Rats with a fasting blood glucose level > 200 mg/dL were considered diabetic and randomized into two groups: non-treated DM group (n = 7) or DEX-treated DM group (1 µg/kg/h, n = 10) [13]. A group of randomly selected STZ-induced DM rats was treated with DEX (1 µg/kg/h), which was administered via an osmotic pump (ALZET model 2ML4 2002, DURECT Corp., Cupertino, CA, USA) for 28 days. After anesthesia induction with isoflurane, the pump was subcutaneously implanted between the scapulae of DM rats. The adequacy of anesthesia was monitored based on the lack of pedal withdrawal reflex, slow constant breathing, and absence of response to surgical manipulation.
Chemical reagents and antibodies
The following chemical agents were used: STZ and radioimmunoprecipitation assay with protease and phosphatase inhibitors were obtained from Sigma-Aldrich (St. Louis, MO, USA). Citric acid was purchased from the Shanghai Chemical Reagent Co., Ltd. (Shanghai, China), whereas DEX was obtained from Hospira (Rocky Mount, NC, USA). Moreover, 0.45% sodium chloride injections and a Bradford protein assay kit were obtained from Thermo Fisher Scientific (Rockford, IL, USA), and an Amersham enhanced chemiluminescence (ECL) western blotting detection reagent was acquired from GE Healthcare (Little Chalfont, UK). Anti-LC3 was obtained from Novus (San Diego, CA, USA); anti-Beclin1, anti-Atg3, anti-Atg5, anti-Atg7, anti-Atg12, anti-Atg16L1, anti-p-AKT and total-AKT, anti-p-ERK and total-ERK, and anti-p-p70S6 and anti-total-p70S6 were acquired from Cell Signaling Technology (Beverly, MA, USA); and anti-glyceraldehyde 3-phosphate dehydrogenase (GAPDH) was acquired from Santa Cruz Biotechnology (Santa Cruz, CA, USA).
Biochemical analysis
Blood samples were obtained from the femoral artery of rats after overnight fasting. Fasting blood glucose level was measured using a blood glucose monitoring system (CareSens II, Wonju, Korea). Insulin level was determined using a Mercodia rat insulin enzyme-linked immunosorbent assay kit [oxidase method, with a biochemical auto-analyzer (Beck-man CX-7 Biochemical Auto-analyzer, Brea, CA, USA)]. Data on heart weight and body weight were collected and measured. Heart tissues were snap-frozen in liquid nitrogen and stored at − 80 °C to obtain protein.
Pressure–volume (PV) loop analysis
Pressure and volume were calibrated using the MPVS-Ultra system (Millar Inc., Houston, TX, USA). Anesthetized rats were placed on a heating pad in the supine position, and an incision was created in the anterior midline of the neck to expose the trachea. The right carotid artery was dissected and exposed, and a Millar catheter was introduced into the artery and advanced into the LV via the aortic valve. After stabilization for 5–10 min, PV loop signals were continuously recorded at a sampling rate of 1000 Hz using an MPVS-Ultra Single Segment Pressure–volume unit (Millar Inc., Houston, TX, USA). Using a special PV loop analysis software (Millar Inc., Houston, TX, USA), we computed and calculated the heart rate (HR), LV end-systolic pressure (LVESP), LV end-diastolic pressure, pressure increment (dP/dtmax) and pressure decrement (dP/dtmin), stroke work (SW), and ejection fraction (EF). Stroke volume (SV) and cardiac output (CO) were calculated and corrected according to in vitro and in vivo volume calibrations using the PV loop analysis software. In addition, the relationship between LV and PV was evaluated based on the occlusion of the inferior cava. The slope of the LV end-systolic PV relationship (Emax), preload recruitable stroke work (PRSW), and slope of the end-diastolic PV relationship (EDPVR) were calculated. At the end of each experiment, 50 mL of 30% saline was intravenously injected to establish a parallel conductance volume from the shift of PV loop relations, and this was used to correct the volume of the cardiac mass. The volume was calibrated using a Millar volume calibration cuvette. All measurements were evaluated using LabChart8 (ADInstruments, Colorado Springs, CO, USA).
Echocardiography
Murine transthoracic echocardiography was performed using the GE Vivid 7 ultrasound system (General Electric, Atlanta, GA, USA) with a 10-MHz transducer. The rats were lightly anesthetized using 2% sevoflurane and restrained on a heated imaging table. Images were obtained using M-mode echocardiography in the parasternal short-axis view, pulse-wave Doppler imaging through the mitral valve in an apical four-chamber view, and tissue Doppler imaging at the level of the septal mitral annulus in an apical four-chamber view to calculate the cardiac diastolic and systolic functions.
Western blotting
Proteins were extracted from the heart using a radioimmunoprecipitation assay buffer with protease and phosphatase inhibitors for western blot analysis. We loaded 50 mg of protein in each well to perform sodium dodecyl sulfate-polyacrylamide gel electrophoresis. Membranes were blocked with 5% skim milk for 1 h and incubated overnight with the first antibodies. Signals were measured using the Amersham ECL western blotting detection reagent (GE Healthcare, Little Chalfont, UK), scanned using ImageQuant LAS 4000 Mini (GE Healthcare, Little Chalfont, UK), and quantified using the ImageJ software (National Institutes of Health, Bethesda, MD, USA).
Statistical analysis
All data are expressed as means ± standard deviations. Statistical analysis was performed using t test or one-way analysis of variance (Bonferroni test), and differences were determined to be statistically significant when p values were < 0.05. All experiments were performed at least three times.
Results
Metabolic changes in DM rats
Blood glucose levels (mg/dL), insulin levels (µg/L), body weight (g), heart weight (mg), and heart weight/body weight (mg/g) were measured to estimate the effects of DEX on metabolic changes in DM rats. We observed that DEX treatment failed to improve the metabolic changes in DM rats including the blood glucose level, insulin level, and heart weight. However, DEX significantly restored the body weight of DM rats (p < 0.05) (Fig. 1).
DEX attenuates metabolic changes in DM rats. a A schematic timeline of treatment, surgery, and evaluation. b Blood glucose levels (mg/dL). c and d insulin levels (µg/L). e Body weight (g). f Heart weight (mg). g Heart weight/body weight (mg/g). Results represent triplicate experiments. Data are expressed as mean ± standard deviation (n = 6). *p < 0.05 vs. the control group; #p < 0.05 vs. the DM group. DM diabetes mellitus, DEX dexmedetomidine, Con control
DEX treatment improved cardiac performance in DM rats
The baseline hemodynamic data of rats are summarized in Table 1. Most parameters in DM rats showed significant differences from those in control rats. Non-treated DM rats exhibited decreased HR, LVESP, SV, SW, CO, EF, dP/dtmax, and dP/dtmin and increased time constant of LV pressure decay (τ) compared with control rats. Similarly, DEX-treated DM rats showed a decrease in HR, LVESP, SV, SW, CO, EF, dP/dtmax, and dP/dtmin and an increase in tau compared with control rats. Further, SV, SW, CO, dP/dtmax, and dP/dtmin were higher and tau was lower in DEX-treated DM rats than in non-treated DM rats. Functional indices were derived using the PV loop analysis at different preloads during the transient occlusion of the inferior vena cava. Emax was steeper in control rats than in DM rats. In contrast, the EDPVR tended to be increased in DM rats, albeit without statistical significance. The PRSW values were significantly lower in DM rats than in control rats. DEX treatment in DM rats was associated with increased Emax and PRSW compared with that in non-treated DM rats.
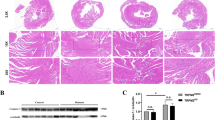
LV systolic function by echocardiography was lower in DM rats than in control rats; moreover, both LV end-diastolic dimension (LVEDD) and LV end-systolic dimension (LVESD) increased in DM rats compared with those in control rats. In DM rats treated with DEX, LV systolic function significantly improved, and both LVEDD and LVESD decreased compared with those in non-treated DM rats. Pulse-wave Doppler and tissue Doppler imaging indicated a significant increase in the E/E′ ratio and a significant decrease in the E/A ratio in DM rats, suggestive of diastolic dysfunction. Conversely, no significant changes in these diastolic dysfunction measures were observed in DM rats treated with DEX compared with those in control rats (Fig. 2).
DEX improves cardiac function in DM rats. a Representative M-mode images from rats in each group. b Representative pulsed-wave Doppler image of mitral valve inflow. c Representative tissue Doppler image of E/E′ ratio. d HR. e LVEDD. f LVESD. g FS (%). hE/A ratio. iE/E′ ratio. *p < 0.05 vs. the control group; #p < 0.05 vs. the DM group. HR heart rate, LVEDD LV end-diastolic dimension, LVESD LV end-systolic dimension, FS fractional shortening, DM diabetes mellitus, DEX dexmedetomidine, Con control
DEX regulates the expression of LC3 in the hearts of DM rats
To investigate the role of DEX in autophagic regulation in DM rats, we examined the expression of critical autophagic markers such as LC3 and beclin-1. The LC3-II protein level was significantly increased in DM rats compared with that in control rats (p < 0.05). However, DEX treatment in DM rats significantly suppressed the LC3-II protein level compared with that in non-treated DM rats (Fig. 3, p < 0.05).
DEX regulates the level of LC3 protein in the hearts of DM rats. Representative western blot data for LC3 and beclin-1 proteins. a Graph for the beclin-1 level. b Graph for the LC3-II level. c Graph for the LC3-II/LC-I ratio. GAPDH was used as the protein loading control. Results represent triplicate experiments. Data are expressed as mean ± standard deviation (n = 5). *p < 0.05 vs. the control group; #p < 0.05 vs. the DM group. GAPDH glyceraldehyde 3-phosphate dehydrogenase, DM diabetes mellitus, Con control, DEX dexmedetomidine
DEX treatment affected AKT and ERK signaling in DM rats
We confirmed the association of signaling pathway with representative western blot data for LC3 and beclin-1 proteins. AKT and p70S6 activation was significantly increased in DM rats (p < 0.05), and DEX treatment reversed the phosphorylation of AKT (Fig. 4a), but not of p70S6 (Fig. 4b). Moreover, ERK activation was significantly decreased in DM rats, and DEX treatment reversed the phosphorylation of ERK (Fig. 4c).
Effects of DEX on signaling pathway in the hearts of DM rats. Representative western blot data for AKT, p70S6, and ERK activation. a Data are expressed as the ratio of p-AKT to total AKT. b Ratio of p-p70S6 to total p70S6. c Ratio of p-ERK to total-ERK. GAPDH was used as the protein loading control. Results are representative of triplicate experiments. Data are expressed as mean ± standard deviation (n = 5). *p < 0.05 vs. the control group; #p < 0.05 vs. the DM group. GAPDH glyceraldehyde 3-phosphate dehydrogenase, STZ streptozotocin, DM diabetes mellitus, Con control
DEX reduced the autophagosomal markers that were increased in the hearts of DM rats
To further explore underlying autophagic molecular markers for the effects of DEX on STZ-induced autophagy, we performed western blotting for autophagy-related gene (ATG) proteins in the hearts of STZ-induced DM rats treated with or without DEX. The expressions of ATG5, ATG7, and ATG12 were significantly increased in DM rats, but were markedly decreased after DEX administration (Fig. 5, p < 0.05). Autophagy is a conserved, genetically controlled process that leads to the degradation of cytoplasmic components within lysosomes and has recently gained much attention because of its paradoxical relationship with apoptosis [14]. We observed an increase in the expression of autophagosomal markers such as LC3B and ATG proteins.
Effects of DEX on autophagic markers in the hearts of DM rats. Representative western blot data for ATG3, ATG5, ATG7, ATG12, and ATG16L1 proteins. GAPDH was used as the protein loading control. Results represent triplicate experiments. Data are expressed as mean ± standard deviation (n = 5). *p < 0.05 vs. the control group; #p < 0.05 vs. the DM group. GAPDH glyceraldehyde 3-phosphate dehydrogenase, DM diabetes mellitus, DEX dexmedetomidine, Con control
Discussion
To the best of our knowledge, this is the first study that evaluated the cardiac effects of DEX in STZ-induced DM rats with respect to autophagy. We showed that DEX restores the cardiac function in STZ-induced DM rats by maintaining the homeostasis of autophagic markers, including LC3B, ATG5, ATG7, and ATG12. Additionally, DEX regulates the impaired activation of ERK and AKT signaling pathways. Additionally, we showed that STZ-induced autophagy was suppressed by DEX treatment via the phosphorylation of AKT and dephosphorylation of ERK. AKT and ERK signaling pathways are important for the regulation of the effect of DEX on cardiac dysfunction in STZ-induced DM rats. Our findings suggest that DEX could be an effective therapeutic target to protect diabetic patients from experiencing cardiac dysfunction.
DEX is clinically well known to exert pharmacological effects such as sedation and analgesia [4, 15]. Recent studies on DEX, including clinical reports, have indicated its protective effects in the kidney, heart, lung, and brain of DM rats [5, 7, 16, 17]. In the present study, DEX treatment did not attenuate metabolic changes such as the blood glucose level, insulin level, and heart weight/body weight ratio in DM rats. Using PV loop analysis and echocardiography, we determined that DEX improved the cardiac function in STZ-induced DM rats. DEX treatment suppressed the cardiac autophagic activity enhanced in STZ-induced DM rats, as evidenced by the decrease in LC3-II/LC3-I ratio, which is an autophagic marker, and the expression of autophagy-related proteins.
DEX was reported to possess organ-protective properties in several injury models, including models of ischemia–reperfusion, inflammation, and trauma to multiple organs. The cardioprotective effects of DEX have been extensively shown in ischemic hearts, both regionally and globally [18,19,20]. Although the precise mechanisms of its protective effects in these organs are not well elucidated, several possible mechanisms have been reported, including modulation of cell death by apoptosis (inhibition of apoptosis and activation of the anti-apoptotic signaling pathway), activation of cell survival kinases, and modulation of inflammatory responses and oxidative stress [21,22,23]. In an incomplete cerebral ischemia–reperfusion model, DEX increased the concentration of anti-apoptotic proteins (B-cell lymphoma 2 and murine double minute 2) and inhibited the increase in the concentration of the proapoptotic protein (Bax) [24]. In a myocardial ischemia–reperfusion injury model, DEX preconditioning attenuated regional injury via the activation of ERK 1/2, AKT, and endothelial isoform of nitric oxide synthase [20]. DEX was reported to inhibit inflammation and autophagy in lipopolysaccharide-induced lung injury via the toll-like receptor 4-nuclear factor-κB pathway [25]. Furthermore, Shen et al. [26] reported that DEX inhibited neuronal autophagy mediated by the activation of the PI3K/AKT/mTOR pathway in traumatic brain injury. Moreover, it has been reported that DEX protected the mouse brain from ischemia–reperfusion injury via autophagy inhibition through the upregulation of hypoxia-inducible factor 1-α [27].
Autophagy plays an important role in maintaining normal cardiac function and morphology [28, 29]. Recent evidence showed that autophagy at baseline is an important homeostatic mechanism for the maintenance of normal cardiac function and morphology. In contrast, excessive induction of the autophagic process by environmental or intracellular stress plays an important role in several types of cardiomyopathy by functioning as a death pathway [28]. Moreover, autophagy is linked to cardiac dysfunction in diabetic patients, and myocardial fibrosis is related to PI3K/AKT signaling in rats with STZ-induced type 1 diabetes [2, 3, 30]. This suggests that autophagy carries out different functions depending on the cause of autophagy activation, which could be either an adaptor to maintain cardiac function or a contributor to the pathogenesis of heart disease. Interestingly, autophagic adaptations in DCM differ between type 1 and type 2 diabetes, with cardiac autophagic activity having been reported to be enhanced in type 1 diabetes, but suppressed in type 2 diabetes [31]. In this study, DEX treatment suppressed the cardiac autophagic activity enhanced in STZ-induced type 1 diabetes, which is consistent with the results of previous studies on autophagic activity [31, 32]. In contrast, some studies reported suppression of autophagy in type 1 DM models of STZ-induced mice and genetically engineered mice (diabetic OVE26 mice), proposing it as a response to limit or cause cardiac dysfunction [30, 33, 34]. Further investigation on assay tools or criteria for cardiac autophagic activity in diabetic models is warranted.
Cardiac hypertrophy usually occurs in the late stage of diabetes, which eventually leads to cardiac remodeling, dysfunction, and even heart failure. Consistent changes in hypertrophy and expression of ERK1/2 were also observed in the myocardium of STZ-induced DM rats. It is believed that ERK1/2 activation in response to hyperglycemia results in cardiac hypertrophy [12]. Many researchers have realized the involvement of mitogen-activated protein kinase (MAPK) during DCM development. MAPK signaling pathways (ERK1/2, JNK, and p38) are actively involved in myocardial dysfunction [35], hypertrophy [36, 37], fibrosis [38, 39], and heart failure [40,41,42]. ERK is a MAPK family member, and ERK signaling is important in cardiac hypertrophy [43, 44]. Recently, several reports have described the important role played by ERK signaling in accelerated DCM development [45, 46]. Additionally, MAPKs are involved in cardiac physiology and various cardiovascular diseases, resulting in further studies that explored pharmacological/genetic blockers/activators and downstream targets of the MAPK pathway in the heart [47, 48]. In relation to our results, these studies reported that DEX attenuated autophagy via the regulation of ERK and AKT signaling and improved cardiac malfunction. Although DEX improved cardiac function and inhibited autophagy in DM, it may exert an apoptosis-promoting effect at a higher concentration by protecting aberrant cells from metabolic stress and suppressing the cell death pathway. High doses of DEX (5 µg/kg or higher) have recently been reported to activate neuroapoptosis by stimulating the AKT/glycogen synthase kinase-3β signaling pathway [49]. Further studies should be performed to clarify the role of DEX in DM and are required to improve our understanding of the mechanism of DCM.
This study has several possible limitations. First, we did not perform behavioral tests during long-term DEX use; however, we did not observe any odd behavior during grooming. DEX may affect the behavior of rats via its sedative effect. Second, we did not evaluate cardiac function and measure autophagic markers after STZ administration and before DEX treatment owing to the insufficient time interval between them for DCM development. Even with the STZ model, we still do not exactly know the duration or magnitude of hyperglycemia that can lead to the occurrence of cardiac dysfunction and change in autophagic activity. Third, DEX is clinically administered via the intravenous, subcutaneous, or intranasal route. If chronically used in the treatment of DCM, oral or sublingual formulation would be more appropriate in the future.
In conclusion, this study reinforces our understanding of the potential anti-autophagic effect of DEX in patients with DCM. Our findings provide a greater understanding of the protective effects of DEX, with implications for the treatment of DCM.
Abbreviations
- DM:
-
Diabetes mellitus
- DCM:
-
Diabetic cardiomyopathy
- LV:
-
Left ventricular
- DEX:
-
Dexmedetomidine
- STZ:
-
Streptozotocin
- ECL:
-
Enhanced chemiluminescence
- ERK:
-
Extracellular signal-regulated kinase
- PV loop:
-
Pressure–volume loop
- HR:
-
Heart rate
- LVESP:
-
LV end-systolic pressure
- dP/dt max :
-
Maximal slope of the systolic pressure increment
- dP/dt min :
-
Maximal slope of the diastolic pressure decrement
- E max :
-
Slope of end-systolic pressure–volume relationship
- SW:
-
Stroke work
- EF:
-
Ejection fraction
- SV:
-
Stroke volume
- CO:
-
Cardiac output
- PRSW:
-
Preload recruitable stroke work
- EDPVR:
-
End-diastolic pressure–volume relationship
- LVEDD:
-
LV end-diastolic dimension
- LVESD:
-
LV end-systolic dimension
- ATG:
-
Autophagy-related gene
- TDI E/E′:
-
Ratio of transmitral Doppler early filling velocity to tissue Doppler early diastolic mitral annular velocity
- E/A :
-
Ratio between early (E)-to-late (A) diastolic mitral inflow
- MAPK:
-
Mitogen-activated protein kinase
References
Varga ZV, Giricz Z, Liaudet L, Hasko G, Ferdinandy P, Pacher P (2015) Interplay of oxidative, nitrosative/nitrative stress, inflammation, cell death and autophagy in diabetic cardiomyopathy. Biochim Biophys Acta 1852:232–242
Kobayashi S, Liang Q (2015) Autophagy and mitophagy in diabetic cardiomyopathy. Biochim Biophys Acta 1852:252–261
Ouyang C, You J, Xie Z (2014) The interplay between autophagy and apoptosis in the diabetic heart. J Mol Cell Cardiol 71:71–80
Carollo DS, Nossaman BD, Ramadhyani U (2008) Dexmedetomidine: a review of clinical applications. Curr Opin Anaesthesiol 21:457–461
Zeng X, Wang H, Xing X, Wang Q, Li W (2016) Dexmedetomidine protects against transient global cerebral ischemia/reperfusion induced oxidative stress and inflammation in diabetic rats. PLoS One 11:e0151620
Erbatur ME, Sezen SC, Bayraktar AC, Arslan M, Kavutcu M, Aydin ME (2017) Effects of dexmedetomidine on renal tissue after lower limb ischemia reperfusion injury in streptozotocin induced diabetic rats. Libyan J Med 12:1270021
Kip G, Celik A, Bilge M et al (2015) Dexmedetomidine protects from post-myocardial ischaemia reperfusion lung damage in diabetic rats. Libyan J Med 10:27828
Levine B, Klionsky DJ (2004) Development by self-digestion: molecular mechanisms and biological functions of autophagy. Dev Cell 6:463–477
Kim I, Rodriguez-Enriquez S, Lemasters JJ (2007) Selective degradation of mitochondria by mitophagy. Arch Biochem Biophys 462:245–253
Nakai A, Yamaguchi O, Takeda T et al (2007) The role of autophagy in cardiomyocytes in the basal state and in response to hemodynamic stress. Nat Med 13:619–624
Wang H, Wang J, Qu H et al (2016) In vitro and in vivo inhibition of mTOR by 1,25-dihydroxyvitamin D3 to improve early diabetic nephropathy via the DDIT4/TSC2/mTOR pathway. Endocrine 54:348–359
Xu Z, Sun J, Tong Q et al (2016) The role of ERK1/2 in the development of diabetic cardiomyopathy. Int J Mol Sci 17:2001. https://doi.org/10.3390/ijms17122001
Zhang Y, Wang Y, Yang K et al (2014) BMP4 increases the expression of TRPC and basal [Ca2+]i via the p38MAPK and ERK1/2 pathways independent of BMPRII in PASMCs. PLoS One 9:e112695
Gallagher LE, Williamson LE, Chan EY (2016) Advances in autophagy regulatory mechanisms. Cells 5:24. https://doi.org/10.3390/cells5020024
Sanders RD, Maze M (2007) Alpha2-adrenoceptor agonists. Curr Opin Investig Drugs 8:25–33
Venot M, Weis L, Clec’h C et al (2015) Acute kidney injury in severe sepsis and septic shock in patients with and without diabetes mellitus: a multicenter study. PLoS One 10:e0127411
Arslan M, Comu FM, Kip G et al (2014) Effect of dexmedetomidine on erythrocyte deformability during ischaemia–reperfusion injury of heart in diabetic rats. Bratisl Lek Listy 115:494–497
Riquelme JA, Westermeier F, Hall AR et al (2016) Dexmedetomidine protects the heart against ischemia–reperfusion injury by an endothelial eNOS/NO dependent mechanism. Pharmacol Res 103:318–327
Okada H, Kurita T, Mochizuki T, Morita K, Sato S (2007) The cardioprotective effect of dexmedetomidine on global ischaemia in isolated rat hearts. Resuscitation 74:538–545
Ibacache M, Sanchez G, Pedrozo Z et al (2012) Dexmedetomidine preconditioning activates pro-survival kinases and attenuates regional ischemia/reperfusion injury in rat heart. Biochim Biophys Acta 1822:537–545
Sun Y, Jiang C, Jiang J, Qiu L (2017) Dexmedetomidine protects mice against myocardium ischaemic/reperfusion injury by activating an AMPK/PI3K/Akt/eNOS pathway. Clin Exp Pharmacol Physiol 44:946–953
Behmenburg F, Pickert E, Mathes A et al (2017) The cardioprotective effect of dexmedetomidine in rats is dose-dependent and mediated by BKCa channels. J Cardiovasc Pharmacol 69:228–235
Cheng XY, Gu XY, Gao Q, Zong QF, Li XH, Zhang Y (2016) Effects of dexmedetomidine postconditioning on myocardial ischemia and the role of the PI3K/Akt-dependent signaling pathway in reperfusion injury. Mol Med Rep 14:797–803
Engelhard K, Werner C, Eberspacher E et al (2003) The effect of the alpha 2-agonist dexmedetomidine and the N-methyl-d-aspartate antagonist S(+)-ketamine on the expression of apoptosis-regulating proteins after incomplete cerebral ischemia and reperfusion in rats. Anesth Analg 96:524–531
Ding D, Xu S, Zhang H et al (2018) 3-Methyladenine and dexmedetomidine reverse lipopolysaccharide-induced acute lung injury through the inhibition of inflammation and autophagy. Exp Ther Med 15:3516–3522
Shen M, Wang S, Wen X et al (2017) Dexmedetomidine exerts neuroprotective effect via the activation of the PI3K/Akt/mTOR signaling pathway in rats with traumatic brain injury. Biomed Pharmacother 95:885–893
Luo C, Ouyang MW, Fang YY et al (2017) Dexmedetomidine protects mouse brain from ischemia–reperfusion injury via inhibiting neuronal autophagy through up-regulating HIF-1alpha. Front Cell Neurosci 11:197
Martinet W, Knaapen MW, Kockx MM, De Meyer GR (2007) Autophagy in cardiovascular disease. Trends Mol Med 13:482–491
Terman A, Brunk UT (2005) Autophagy in cardiac myocyte homeostasis, aging, and pathology. Cardiovasc Res 68:355–365
Xiao T, Luo J, Wu Z, Li F, Zeng O, Yang J (2016) Effects of hydrogen sulfide on myocardial fibrosis and PI3K/AKT1-regulated autophagy in diabetic rats. Mol Med Rep 13:1765–1773
Kanamori H, Takemura G, Goto K et al (2015) Autophagic adaptations in diabetic cardiomyopathy differ between type 1 and type 2 diabetes. Autophagy 11:1146–1160
Eguchi M, Kim YH, Kang KW et al (2012) Ischemia–reperfusion injury leads to distinct temporal cardiac remodeling in normal versus diabetic mice. PLoS One 7:e30450
Xie Z, Lau K, Eby B et al (2011) Improvement of cardiac functions by chronic metformin treatment is associated with enhanced cardiac autophagy in diabetic OVE26 mice. Diabetes 60:1770–1778
Xu X, Kobayashi S, Chen K et al (2013) Diminished autophagy limits cardiac injury in mouse models of type 1 diabetes. J Biol Chem 288:18077–18092
Javadov S, Jang S, Agostini B (2014) Crosstalk between mitogen-activated protein kinases and mitochondria in cardiac diseases: therapeutic perspectives. Pharmacol Ther 144:202–225
Wang S, Luo M, Zhang Z et al (2016) Zinc deficiency exacerbates while zinc supplement attenuates cardiac hypertrophy in high-fat diet-induced obese mice through modulating p38 MAPK-dependent signaling. Toxicol Lett 258:134–146
Zhang N, Yang Z, Yuan Y et al (2015) Naringenin attenuates pressure overload-induced cardiac hypertrophy. Exp Ther Med 10:2206–2212
Zhang L, Ding WY, Wang ZH et al (2016) Early administration of trimetazidine attenuates diabetic cardiomyopathy in rats by alleviating fibrosis, reducing apoptosis and enhancing autophagy. J Transl Med 14:109
Fei AH, Wang FC, Wu ZB, Pan SM (2016) Phosphocreatine attenuates angiotensin II-induced cardiac fibrosis in rat cardiomyocytes through modulation of MAPK and NF-kappaB pathway. Eur Rev Med Pharmacol Sci 20:2726–2733
Sager HB, Hulsmans M, Lavine KJ et al (2016) Proliferation and recruitment contribute to myocardial macrophage expansion in chronic heart failure. Circ Res 119:853–864
Martinez PF, Bonomo C, Guizoni DM et al (2016) Modulation of MAPK and NF-954;B signaling pathways by antioxidant therapy in skeletal muscle of heart failure rats. Cell Physiol Biochem 39:371–384
Thandavarayan RA, Giridharan VV, Arumugam S et al (2015) Schisandrin B prevents doxorubicin induced cardiac dysfunction by modulation of DNA damage, oxidative stress and inflammation through inhibition of MAPK/p53 signaling. PLoS One 10:e0119214
Kehat I, Molkentin JD (2010) Extracellular signal-regulated kinase 1/2 (ERK1/2) signaling in cardiac hypertrophy. Ann N Y Acad Sci 1188:96–102
Mutlak M, Kehat I (2015) Extracellular signal-regulated kinases 1/2 as regulators of cardiac hypertrophy. Front Pharmacol 6:149
Lakshmanan AP, Harima M, Sukumaran V et al (2012) Modulation of AT-1R/AMPK-MAPK cascade plays crucial role for the pathogenesis of diabetic cardiomyopathy in transgenic type 2 diabetic (Spontaneous Diabetic Torii) rats. Biochem Pharmacol 83:653–660
Zhang C, Huang Z, Gu J et al (2015) Fibroblast growth factor 21 protects the heart from apoptosis in a diabetic mouse model via extracellular signal-regulated kinase 1/2-dependent signalling pathway. Diabetologia 58:1937–1948
Ravingerova T, Barancik M, Strniskova M (2003) Mitogen-activated protein kinases: a new therapeutic target in cardiac pathology. Mol Cell Biochem 247:127–138
Gerits N, Kostenko S, Moens U (2007) In vivo functions of mitogen-activated protein kinases: conclusions from knock-in and knock-out mice. Transgenic Res 16:281–314
Perez-Zoghbi JF, Zhu W, Grafe MR, Brambrink AM (2017) Dexmedetomidine-mediated neuroprotection against sevoflurane-induced neurotoxicity extends to several brain regions in neonatal rats. Br J Anaesth 119:506–516
Acknowledgements
This study was supported by the following Grants: the Basic Science Research Program through the National Research Foundation of Korea, funded by the Ministry of Science, ICT & Future Planning (NRF2014R1A1A3053428).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
All animal procedures were approved by the Committee for the Care and Use of Laboratory Animals, Yonsei University College of Medicine (No. 2015–0129), and were performed in accordance with the Guide for the Care and Use of Laboratory Animals published by the US National Institutes of Health.
Human and animal rights
This article does not contain any studies with human participants performed by any of the authors. All procedures performed in studies involving animals were in accordance with the ethical standards of the institution.
Informed consent
For this type of study formal consent is not required.
Additional information
Managed by Massimo Porta.
Rights and permissions
About this article
Cite this article
Oh, J.E., Jun, J.H., Hwang, H.J. et al. Dexmedetomidine restores autophagy and cardiac dysfunction in rats with streptozotocin-induced diabetes mellitus. Acta Diabetol 56, 105–114 (2019). https://doi.org/10.1007/s00592-018-1225-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00592-018-1225-9