Abstract
Aims
We evaluate prevalence of new abnormal glucose tolerance (AGT) in post-MI survivors without known diabetes (DM) if guidelines are followed and compare the ability of admission (APG), fasting (FPG) and 2-h post-load plasma glucose (2h-PG) to predict prognosis.
Methods
A total of 674 patients were followed up for 4 years for incidence of major adverse cardiovascular events (MACE) of cardiovascular death, non-fatal re-infarction or non-haemorrhagic stroke. Ability of models including APG, FPG and 2h-PG to predict MACE was compared.
Results
Of the total, 93–96% of impaired glucose tolerance and 64–75% of DM would be missed with current guidelines. MACE was higher in the upper quartiles of 2h-PG. When 2h-PG and FPG were included simultaneously in models, only 2h-PG predicted MACE (HR 1.12, CI 1.04–1.20, p = 0.0012), all cause mortality (HR 1.17, CI 1.05–1.30, p = 0.0039), cardiovascular mortality (HR 1.17, CI 1.02–1.33, p = 0.0205) and non-fatal MI (HR 1.10, CI 1.01–1.20, p = 0.0291). Adding 2h-PG significantly improved ability of models including FPG (χ2 = 16.01, df = 1, p = 0.0001) or FPG and APG (χ2 = 17.36, df = 1, p = 0.000) to predict MACE. Model including 2h-PG only had the lowest Akaike’s information criteria and highest Akaike weights suggesting that this was the best in predicting events. Adding 2h-PG to models including FPG or APG with other co-variates yielded continuous net reclassification improvement (NRI) of 0.22 (p = 0.026) and 0.27 (p = 0.005) and categorical NRI of 0.09 (p = 0.032) and 0.12 (p = 0.014), respectively. Adding 2 h-PG to models including only FPG, only APG and both yielded integrated discrimination improvement of 0.012 (p = 0.015), 0.022 (p = 0.001) and 0.013 (p = 0.014), respectively.
Conclusions
AGT is under-diagnosed on current guidelines. 2h-PG is a better predictor of prognosis compared to APG and FPG.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Current guidelines [1, 2] do not recommend routine use of oral glucose tolerance test (OGTT) to identify abnormal glucose tolerance (AGT) in patients without known diabetes mellitus (DM) admitted with acute coronary syndromes (ACS). These guidelines are not based on prognostic studies [3,4,5,6,7]. It is reasonable to suggest that the most important measure of the glucometabolic state would be the one that determines long-term prognosis after ACS.
Elevated admission plasma glucose (APG), fasting plasma glucose (FPG), admission glycosylated haemoglobin (HbA1c) [8,9,10,11,12] and newly diagnosed AGT [13,14,15,16,17] after myocardial infarction (MI) and ACS in patients without known DM adversely affect prognosis. However, the ability of APG, FPG and 2-h post-load plasma glucose (2h-PG) to predict post-ACS prognosis in same group of patients without known DM has not been evaluated. Studies exploring relationship between abnormal APG, FPG or 2h-PG and prognosis, have done so using dichotomous groupings, e.g. those above and below a cutoff point [13] or conventional classifications of normal (NGT) or impaired glucose tolerance (IGT) and new DM (NDM) [14,15,16,17,18,19,20,21] rather than a study of the predictability of these measurements as continuous variables. Furthermore, information on the independent effect of 2h-PG on prognosis is limited [14, 15, 19].
In the present study, we evaluate the effect of the current guidelines on the prevalence of new AGT in patients with ACS and compare the predictive value of APG, FPG and 2h-PG on prognosis after MI in patients without known DM.
Materials and methods
As reported [17], we retrospectively analysed standard dataset collected locally for the Myocardial Infarction National Audit Project (MINAP) on 768 consecutive post-MI [22] survivors admitted between November 2005 and October 2008 without known DM who underwent pre-discharge OGTT. This study includes patients for whom APG, FPG and 2h-PG were available.
“Known DM” was diagnosed from history, i.e. the patient had been informed of the diagnosis by a physician before the admission or was on anti-diabetic treatment. HbA1c was not used in diagnosing pre-hospital diabetes as it was not recommended in contemporary guidance [23,24,25]. FPG and OGTT were done on/after the third day of admission. We defined admission hyperglycaemia (AH) as APG ≥ 7.8 mmol/l [5] and DM as APG > 11.1 mmol/l [26]. The patients were classified as normal glucose tolerance (NGT), impaired fasting glucose (IFG), IGT and NDM as follows: normal glucose tolerance (NGT): FPG < 6.1 mmol/l and a 2-h PG < 7.8 mmol/l; impaired fasting glucose (IFG): FPG 6.1–6.9 mmol/l and 2-h PG < 7.8 mmol/l; IGT: FPG < 7 mmol/l and 2-h PG 7.8–11 mmol/l. NDM: FPG ≥ 7.0 and/or 2-h PG ≥ 11.1 mmol/l. The patients were divided into quartiles of 2h-PG. The patients with IGT and NDM were advised lifestyle modification including diet, physical activity and referred to the diabetologists for appropriate out-patients management.
All participants were followed up for a median of 48 months for outcomes. Completeness of follow-up was ensured by manual review of hospital and general practice records. The first occurrence of an adverse event was obtained from hospital and general practice records and confirmed by the office of public health intelligence. The major adverse cardiovascular event (MACE) was defined as cardiovascular death, non-fatal re-infarction or non-haemorrhagic stroke. Cardiovascular death was defined as death from MI, heart failure or sudden death. A non-fatal re-infarction was a non-fatal MI occurring later than 72 h after the index infarction. Stroke was defined as a neurological deficit persisting > 24 h as observed by a physician with radiological confirmation. As this study retrospectively analysed routinely collected anonymised data on standard clinical practice for MINAP, the East Yorkshire and North Lincolnshire Research Ethics Committee confirmed that formal patient consent and ethical approval was not required [17].
Continuous variables are presented as mean ± SD and median (interquartile range, IR) and categorical variables as counts and proportions (%). The baseline characteristics of quartiles were compared using one-way analysis of variance and Kruskal–Wallis test for parametric and nonparametric data, respectively, for continuous variables and Chi-squared test for categorical variables. Correlations were assessed with Spearman’s rank correlation coefficient (ρ). Event-free survival curves were estimated by the Kaplan–Meier method compared using the log-rank test. Cox proportional-hazards regression was used to analyse the effect of several variables on event-free survival. Age, gender, smoking status, hypercholesterolaemia, hypertension, history of previous MI, diagnosis at discharge, discharge prescription of aspirin, clopidogrel, beta-blockers, angiotensin-converting enzyme inhibitors and statins, revascularisation status, and glucometabolic status were “entered” into the model. Hazard ratios (HRs) and 95% confidence intervals (CIs) are reported. Multicollinearity was examined using variance inflation factor (VIF) (MedCalc Statistical Software version 17.0.4, Ostend, Belgium) and variables with VIF < 4 were included in the same model.
Nested models were compared using χ2 likelihood ratio tests to determine whether the logistic regression models including APG, FPG and 2 h PG provided a significantly better fit than those with variables individually, in pairs and vice versa. Nested and non-nested models containing one of either APG, FPG, 2h-PG or a combination were compared using the corrected Akaike’s information criterion (AICc), δAICc, Akaike weights (wi) and evidence ratios to estimate the probability that a given model is the best fitting model of those studied [27, 28].
Logistic regression analysis of models including APG, FPG and 2h-PG, individually and in combination, along with the other above covariates was used to compute the predicted probabilities of MACE. The incremental predictive value of adding 2h-PG to models with APG and FPG was analysed from these predicted probabilities using several measures: categorical (cNRI) and category-free continuous net reclassification improvement (NRI>0) and integrated discrimination improvement (IDI). In the absence of clearly pre-defined clinical risk thresholds for the models, we opted not to use arbitrary cutoffs for risk. Instead, the predicted probabilities for the most restricted model were divided into quartiles to define the risk categories for calculating categorical NRI. The event (NRIe) and non-event NRI (NRIne) were defined as net percentage of persons with and without the event of interest correctly assigned a higher and lower predicted risk, respectively. The overall NRI is the sum of NRIe and NRIne reported as a number. The IDI was defined as the mean difference in predicted risks between those with and without events.
Results
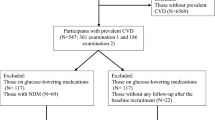
Of the 674 patients included, 70.3% had normal APG (Fig. 1). Of those without AH, 35.0% had IGT and 15.2% had NDM. Of those with AH, 79.0% had normal FPG, of which 47.5 and 18.4% had IGT and NDM, respectively. AGT would be missed in 52.0% patients with AH without OGTT. If AH was not considered, 89.3% had normal FPG. Of these, 38.6 and 14.3% had IGT and NDM, respectively. Thus, IGT and NDM would be diagnosed only in 1.3 and 4.9% patients on following CG130 [1] and 2.5 and 7.1% patients on following ESC Guidance [2], respectively.
The baseline characteristics of patients in each 2h-PG quartile were similar (Table 1). About 76% of patients presenting with STEMI were thrombolysed of which about 23% needed rescue PCI. Another 2% had PPCI. The rest were ineligible for thrombolysis and were not reperfused. Of the STEMI patients that did not undergo revascularisation acutely, 29% had ischaemia-driven revascularisation (PCI in 72%). All patients with NSTEMI had coronary angiogram and 41% were revascularised (92% had PCI). Overall 42% of patients were revascularised (PCI 37%, CABG 5%). About 36 and 7% patients with STEMI and 38 and 3% with NSTEMI underwent PCI and CABG, respectively. All the patients in the 1st quartile and 78.7% in the 2nd quartile had NGT; 21.3% in the 2nd quartile, all patients in the 3rd quartile and 22.7% in the 4th quartile had IGT and the rest in the 4th quartile had NDM. FPG was < 6.1 mmol/l in 83.3% of patients with 2h-PG ≥ 7.8 mmol/l.
MACE and non-fatal MIs were higher in the upper glucose quartiles (Table 2). Event-free survival significantly reduced with increasing quartiles of 2h-PG even below the conventional threshold for DM (Fig. 2). As the multicollinearity between FPG, APG and 2h-PG was low (VIF: FPG 1.56, 2h-PG 1.50 and APG 1.32), they were included into Cox proportional hazard regression models individually and in combinations. When APG, FPG or 2h-PG was included individually with other covariates (Table 3), 2h-PG independently predicted all, FPG predicted some, but APG did not predict any outcomes. The risk of adverse events increased by 9–19% for each mmol/l rise in 2h-PG and by 18–44% for each increasing quartile of 2h-PG. In a model including FPG, 2h-PG and APG, 2h-PG consistently remained an independent predictor of survival (Table 4) free of MACE (HR 1.12, CI 1.04–1.20, p = 0.0012), all cause mortality (HR 1.17, CI 1.05–1.30, p = 0.0039), cardiovascular mortality (HR 1.17, CI 1.02–1.33, p = 0.0205) and non-fatal MI (HR 1.10, CI 1.01–1.20, p = 0.0291) but neither FPG nor APG predicted events. In addition, age, previous history of MI, beta-blocker and revascularisation status independently predicted MACE-free survival.
Nested models were compared using likelihood ratio tests to determine whether logistic regression models that included 2h-PG provided a significantly better fit than those limited to the APG, FPG or its combination (Online Resource Table 1). Addition of 2h-PG improved ability of a model including FPG to predict MACE (χ2 = 16.01, df = 1, p = 0.0001), all deaths (χ2 = 7.75, df = 1, p = 0.005), cardiovascular deaths (χ2 = 4.90, df = 1, p = 0.027) and myocardial infarction (χ2 = 8.64, df = 1, p = 0.003). Addition of 2h-PG to models including FPG and APG improved the ability of the later to predict MACE (χ2 = 17.36, df = 1, p = 0.000), all deaths (χ2 = 7.85, df = 1, p = 0.005), cardiovascular death (χ2 = 6.04, df = 1, p = 0.014) and MI (χ2 = 8.57, df = 1, p = 0.003). However, addition of FPG or APG to a model including 2h-PG did not improve its predictability.
The model including 2h-PG as the only measure of the glucometabolic state with other covariates had the lowest AICc and the highest wi suggesting that these models were the best in predicting all events (Online Resource Table 2). The δAICc suggests that addition of FPG or APG to these models worsen the AICc. Models with FPG or APG alone or in combination are inadequate. On comparing non-nested models (Online Resource Table 3) containing FPG, APG and 2h-PG, the later consistently had the lowest AICc. It also has a 98, 71, 66 and 82% chance of being the “best” model among these for predicting MACE, all deaths, cardiovascular deaths and MI, respectively.
The models including APG, FPG or 2h-PG as the only glycaemic measure correctly predicted 54 (40.3%), 65 (48.5%) and 68 (50.7%) events and 330 (61.1%) 356 (65.9%) and 343 (76.2%) of non-events. The addition of 2h-PG to models including FPG or APG with other co-variates to calculate risk of MACE at the end of follow-up led to a NRI>0 (Online Resource Table 4) of 0.22 (p = 0.026) and 0.27 (p = 0.005), respectively. Adding 2h-PG to a model including FPG and APG led to a NRI>0 of 0.19 (p = 0.046). Addition of either FPG or APG to a model including 2h-PG did not significantly improve net reclassification. Similarly addition of 2h-PG to models including FPG or APG led to a categorical NRI (Online Resource Table 5) of 0.09 (p = 0.032) and 0.12 (p = 0.014), respectively. Addition of either FPG or APG to a model including 2h-PG did not significantly improve net reclassification. Adding 2h-PG to models including only FPG, only APG and both yielded IDI (Online Resource Table 6) of 0.012 (p = 0.015), 0.022 (p = 0.001) and 0.013 (p = 0.014), respectively.
Discussion
Our study suggests that (1) AGT after an MI is under-diagnosed if current guidelines are followed, (2) FPG, but not APG, when considered alone independently predicts post-MI prognosis, (3) FPG ceases to be an independent predictor when included with 2h-PG in the same model and (4) 2h-PG may be a better independent predictor of prognosis compared to APG and FPG.
The prevalence of AGT resembles Euro Heart Survey [29] suggesting a true estimate. AGT is underestimated without OGTT [5, 14, 17, 30, 31]. If CG130 [1] is followed, 70% of our patients would not have further tests. This proportion would increase if higher threshold of APG was used for AH. As plasma glucose is overestimated early after MI [16, 32], it is likely that number of patients with abnormal FPG would decrease if more patients were tested later thus reducing the number of patients undergoing OGTT even further. If ESC Guidance [2] is followed, 89% of our patients would not have OGTT. HbA1c is unlikely to be raised in all of these patients with normal FPG. Thus, a large proportion of these patients with normal HbA1c and FPG would not be offered further testing. Thus, AGT after an MI would be substantially under-diagnosed if current guidelines are followed.
Current Guidelines are not based on prognostic studies [3,4,5,6,7]. This is the first study to assess the relative importance of APG, FPG and 2h-PG in determining post-MI prognosis in the same patients. Studies suggesting adverse post-MI prognosis in newly diagnosed AGT [13,14,15,16,17, 19, 20] have not shown 2h-PG to be independent predictor of event-free survival. Moreover, the cutoffs defining glucometabolic categories suggested by WHO and ADA for epidemiological purposes may be somewhat arbitrary soon after an MI. As increasing plasma glucose is likely to affect post-MI prognosis as a continuum, it was important to test the relative ability of these measurements as continuous variables in predicting outcomes. Increasing tertiles of FPG even below conventional levels of abnormality independently affects prognosis [33]. The risk of events increases with each increasing quartile of 2h-PG, in our study. The 2h-PG independently affected outcomes even when included in the same model as the FPG and APG.
The MACE rates in our study are similar to those previously reported [13,14,15,16]. The differing definitions of MI [13,14,15,16] and MACE [14, 15] used in studies render comparisons of MACE rate between studies less meaningful. Revascularisation status predicted MACE-free survival. The revascularisation rate was similar to the GRACE registry [34]. Compared to the EHS-ACS II survey [35], PCI rates were lower in STEMI, as most patients were thrombolysed, but similar in NSTEMI patients.
Epidemiological studies suggest that 2h-PG is better than FPG alone at identifying increased prognostic risk [31, 36, 37]. The relative value of FPG, APG and 2h-PG in predicting post-MI prognosis in the same population of patients had not been tested. Tamita et al. [14, 19] showed that neither APG nor FPG independently predicted MACE; the effect of 2h-PG was not reported. FPG may be a better predictor of prognosis than APG [33, 38]. Ravid et al. [39] suggested FPG was more important in predicting the course of the MI, than the results of OGTT. In our study, adding 2h-PG to models including APG and/or FPG significantly improved their ability to predict prognosis. The models containing 2h-PG yielded best AIC and demonstrated a very high probability of representing the best model. Adding 2h-PG to logistic regression models containing FPG significantly improved the net reclassification and the integrated discrimination of these models. Thus, 2h-PG may be a more powerful predictor of event-free survival than FPG or APG. The increased macrovascular morbidity associated with higher 2h-PG rather than FPG seen here may be related to progression of atherosclerosis demonstrated with post-challenge rather than fasting hyperglycaemia [40,41,42,43,44].
Whether OGTT after MI reflects “true” glucometabolic state is debated. The pre-discharge glucometabolic category may [16, 20, 32] or may not [45, 46] change with time. The infarct size and timing of OGTT may influence its ability to predict long-term glucometabolic status [16, 32, 45,46,47]. Glucometabolic abnormality is overestimated within the first 24 h after STEMI [16, 32]. However, OGTT done at or after 5 days reliably predicts long-term glucometabolic state [45, 46]. This may be related to the subsidence of the acute responses between 2 and 5 days with no further decrease thereafter [46]. OGTT was done at least 3 days after the index event in our patients, and 60% patients had NSTEMI. These two opposing influences may have limited the effect of stress dysglycaemia on our results. However, the influence of the timing of the OGTT on the measured glucose levels cannot completely discounted. The accuracy of pre-discharge OGTT in diagnosing NDM or IGT is pertinent for studies using OGTT to categorise patients into these groups [13,14,15,16,17, 19, 20]. As pre-discharge 2h-PG much below the conventional abnormal thresholds predicted risk of MACE irrespective of the categorisation of patients, the long-term reproducibility of these categorisations may be less relevant when assessing prognostic risk.
HbA1c was not measured as per guidance [23,24,25]. Prevalence of HbA1c ≥ 6.5% is 5–7% in similar populations [6, 7, 9, 10]. Thus, most of our patients with normal FPG and HbA1c would not qualify for OGTT [1, 2]. Consequently, a large proportion of AGT would be missed. HbA1c has predicted post-MI prognosis in some [9, 48,49,50] but not all studies [10, 51,52,53,54]. The 2h-PG, but not HbA1c, predicted prognosis in studies comparing the two [10, 53]. Kowalczyk et al. suggest that the HbA1c may be useful in further risk stratifying patients diagnosed with new AGT but do not report the effect of HbA1c on prognosis of patients without AGT [55]. This suggests that usefulness of HbA1c in determining post-MI prognosis is seemingly unclear. HbA1c < 6.5% would leave many patients with undiagnosed AGT and unidentified risk of future adverse events according to current guideline. HbA1c ≥ 6.5% may not predict risk. Under both conditions, an OGTT may be useful to determine prognosis.
This study has important clinical implications. Firstly, as 2h-PG is the best glucometabolic measure that independently predicts post-MI prognosis in patients without pre-existing diabetes, it would be reasonable to do a pre-discharge OGTT for these patients. An OGTT 4–5 days after an MI may be recommended to determine long-term post-MI prognosis. Secondly, measuring APG and FPG alone may not suffice. Thirdly, OGTT would prevent the underdiagnosis of AGT, an important adverse prognostic maker after MI, and thus may need to be included in the guidelines.
This study has the limitations of an observational study using retrospective analysis of data collected from a single centre. Although national death register was not consulted directly, a linked general practice database was used. Information recorded incompletely could not be used in statistical models. Exclusion of small number of patients, albeit for valid reasons, and mainly Caucasian study population could affect the generalizability of the results. The effect of random glycaemic fluctuations or stress hyperglycaemia on the results cannot be excluded. However, as pre-discharge 2h-PG predicted post-MI outcomes, the reproducibility of these measurements and its relation to long-term glucometabolic status may be less relevant when assessing prognostic risk. These results from a retrospective analysis of data may have to be confirmed in a prospective study. Addition of HbA1c as a glycaemic marker along with APG, FPG and 2h-PG may be helpful.
Change history
05 March 2018
Unfortunately, name of co-author “Thozhukat” was misspelled in the original publication and the same is corrected here. The original article has been corrected.
References
National Institute for Health and Care Excellence. Clinical guideline [CG130]: Hyperglycaemia in acute coronary syndromes: management. https://www.niceorguk/guidance/cg130
Ryden L, Grant PJ, Anker SD et al (2013) ESC Guidelines on diabetes, pre-diabetes, and cardiovascular diseases developed in collaboration with the EASD: the Task Force on diabetes, pre-diabetes, and cardiovascular diseases of the European Society of Cardiology (ESC) and developed in collaboration with the European Association for the Study of Diabetes (EASD). Eur Heart J 34:3035–3087
Senthinathan A, Kelly V, Dzingina M et al (2011) Hyperglycaemia in acute coronary syndromes: summary of NICE guidance. BMJ 343:d6646
Bartnik M, Ryden L, Malmberg K et al (2007) Oral glucose tolerance test is needed for appropriate classification of glucose regulation in patients with coronary artery disease: a report from the Euro Heart Survey on Diabetes and the Heart. Heart 93:72–77
de Mulder M, Oemrawsingh RM, Stam F, Boersma E, Umans VA (2012) Comparison of diagnostic criteria to detect undiagnosed diabetes in hyperglycaemic patients with acute coronary syndrome. Heart 98:37–41
Hage C, Lundman P, Ryden L, Mellbin L (2013) Fasting glucose, HbA1c, or oral glucose tolerance testing for the detection of glucose abnormalities in patients with acute coronary syndromes. Eur J Prev Cardiol 20:549–554
Doerr R, Hoffmann U, Otter W et al (2011) Oral glucose tolerance test and HbA(1)c for diagnosis of diabetes in patients undergoing coronary angiography: [corrected] the Silent Diabetes Study. Diabetologia 54:2923–2930
Tenerz A, Nilsson G, Forberg R et al (2003) Basal glucometabolic status has an impact on long-term prognosis following an acute myocardial infarction in non-diabetic patients. J Intern Med 254:494–503
Timmer JR, Hoekstra M, Nijsten MW et al (2011) Prognostic value of admission glycosylated hemoglobin and glucose in nondiabetic patients with ST-segment-elevation myocardial infarction treated with percutaneous coronary intervention. Circulation 124:704–711
Pararajasingam G, Hofsten DE, Logstrup BB et al (2016) Newly detected abnormal glucose regulation and long-term prognosis after acute myocardial infarction: Comparison of an oral glucose tolerance test and glycosylated haemoglobin A1c. Int J Cardiol 214:310–315
Aggarwal B, Shah GK, Randhawa M et al (2016) Utility of glycated hemoglobin for assessment of glucose metabolism in patients with ST-segment elevation myocardial infarction. Am J Cardiol 117:749–753
Gustafsson I, Kistorp CN, James MK et al (2007) Unrecognized glycometabolic disturbance as measured by hemoglobin A1c is associated with a poor outcome after acute myocardial infarction. Am Heart J 154:470–476
Kitada S, Otsuka Y, Kokubu N et al (2010) Post-load hyperglycemia as an important predictor of long-term adverse cardiac events after acute myocardial infarction: a scientific study. Cardiovasc Diabetol 9:75
Tamita K, Katayama M, Takagi T et al (2007) Impact of newly diagnosed abnormal glucose tolerance on long-term prognosis in patients with acute myocardial infarction. Circ J 71:834–841
Bartnik M, Malmberg K, Norhammar A et al (2004) Newly detected abnormal glucose tolerance: an important predictor of long-term outcome after myocardial infarction. Eur Heart J 25:1990–1997
Knudsen EC, Seljeflot I, Abdelnoor M et al (2011) Impact of newly diagnosed abnormal glucose regulation on long-term prognosis in low risk patients with ST-elevation myocardial infarction: a follow-up study. BMC Endocr Disord 11:14
George A, Bhatia RT, Buchanan GL et al (2015) Impaired glucose tolerance or newly diagnosed diabetes mellitus diagnosed during admission adversely affects prognosis after myocardial infarction: an observational study. PLoS ONE 10:e0142045
Lenzen M, Ryden L, Ohrvik J et al (2006) Diabetes known or newly detected, but not impaired glucose regulation, has a negative influence on 1-year outcome in patients with coronary artery disease: a report from the Euro Heart Survey on diabetes and the heart. Eur Heart J 27:2969–2974
Tamita K, Katayama M, Takagi T et al (2012) Newly diagnosed glucose intolerance and prognosis after acute myocardial infarction: comparison of post-challenge versus fasting glucose concentrations. Heart 98:848–854
Mazurek M, Kowalczyk J, Lenarczyk R et al (2012) The prognostic value of different glucose abnormalities in patients with acute myocardial infarction treated invasively. Cardiovasc Diabetol 11:78
Ritsinger V, Tanoglidi E, Malmberg K et al (2014) Sustained prognostic implications of newly detected glucose abnormalities in patients with acute myocardial infarction: long-term follow-up of the Glucose Tolerance in Patients with Acute Myocardial Infarction cohort. Diabetes Vasc Dis Res 12:23
Thygesen K, Alpert JS, White HD (2007) Universal definition of myocardial infarction. Eur Heart J 28:2525–2538
Ryden L, Standl E, Bartnik M et al (2007) Guidelines on diabetes, pre-diabetes, and cardiovascular diseases: executive summary. The Task Force on Diabetes and Cardiovascular Diseases of the European Society of Cardiology (ESC) and of the European Association for the Study of Diabetes (EASD). Eur Heart J 28:88–136
American Diabetes Association (1997) Report of the expert committee on the diagnosis and classification of diabetes mellitus. Diabetes Care 20:1183–1197
Genuth S, Alberti KG, Bennett P et al (2003) Follow-up report on the diagnosis of diabetes mellitus. Diabetes Care 26:3160–3167
American Diabetes Association (2012) Standards of medical care in diabetes–2012. Diabetes Care 35(Suppl 1):S11–S63
Y Sakamoto, M Ishiguro, G Kitagawa (1986) Akaike information criterion statistics. Springer, Netherlands
Wagenmakers EJ, Farrell S (2004) AIC model selection using Akaike weights. Psychon Bull Rev 11:192–196
Bartnik M, Ryden L, Ferrari R et al (2004) The prevalence of abnormal glucose regulation in patients with coronary artery disease across Europe. The Euro Heart Survey on diabetes and the heart. Eur Heart J 25:1880–1890
Norhammar A, Tenerz A, Nilsson G et al (2002) Glucose metabolism in patients with acute myocardial infarction and no previous diagnosis of diabetes mellitus: a prospective study. Lancet 359:2140–2144
The DECODE Study Group (2001) Glucose tolerance and cardiovascular mortality: comparison of fasting and 2-hour diagnostic criteria. Arch Intern Med 161:397–405
Knudsen EC, Seljeflot I, Abdelnoor M et al (2009) Abnormal glucose regulation in patients with acute ST-elevation myocardial infarction-a cohort study on 224 patients. Cardiovasc Diabetol 8:6
Suleiman M, Hammerman H, Boulos M et al (2005) Fasting glucose is an important independent risk factor for 30-day mortality in patients with acute myocardial infarction: a prospective study. Circulation 111:754–760
Avezum A, Makdisse M, Spencer F et al (2005) Impact of age on management and outcome of acute coronary syndrome: observations from the Global Registry of Acute Coronary Events (GRACE). Am Heart J 149:67–73
Mandelzweig L, Battler A, Boyko V et al (2006) The second Euro Heart Survey on acute coronary syndromes: characteristics, treatment, and outcome of patients with ACS in Europe and the Mediterranean Basin in 2004. Eur Heart J 27:2285–2293
Smith NL, Barzilay JI, Shaffer D et al (2002) Fasting and 2-hour postchallenge serum glucose measures and risk of incident cardiovascular events in the elderly: the Cardiovascular Health Study. Arch Intern Med 162:209–216
Qiao Q, Pyorala K, Pyorala M et al (2002) Two-hour glucose is a better risk predictor for incident coronary heart disease and cardiovascular mortality than fasting glucose. Eur Heart J 23:1267–1275
Sinnaeve PR, Steg PG, Fox KA et al (2009) Association of elevated fasting glucose with increased short-term and 6-month mortality in ST-segment elevation and non-ST-segment elevation acute coronary syndromes: the Global Registry of Acute Coronary Events. Arch Intern Med 169:402–409
Ravid M, Berkowicz M, Sohar E (1975) Hyperglycemia during acute myocardial infarction. A six-year follow-up study. JAMA 233:807–809
Ando T, Okada S, Niijima Y et al (2010) Impaired glucose tolerance, but not impaired fasting glucose, is a risk factor for early-stage atherosclerosis. Diabet Med 27:1430–1435
Choi ES, Rhee EJ, Choi JH et al (2010) The association of brachial-ankle pulse wave velocity with 30-minute post-challenge plasma glucose levels in korean adults with no history of type 2 diabetes. Korean Diabetes J 34:287–293
Mellen PB, Bittner V, Herrington DM (2007) Post-challenge glucose predicts coronary atherosclerotic progression in non-diabetic, post-menopausal women. Diabet Med 24:1156–1159
Hanefeld M, Koehler C, Henkel E et al (2000) Post-challenge hyperglycaemia relates more strongly than fasting hyperglycaemia with carotid intima-media thickness: the RIAD Study. Risk factors in impaired glucose tolerance for atherosclerosis and diabetes. Diabet Med 17:835–840
Temelkova-Kurktschiev TS, Koehler C, Henkel E et al (2000) Postchallenge plasma glucose and glycemic spikes are more strongly associated with atherosclerosis than fasting glucose or HbA1c level. Diabetes Care 23:1830–1834
Wallander M, Malmberg K, Norhammar A, Ryden L, Tenerz A (2008) Oral glucose tolerance test: a reliable tool for early detection of glucose abnormalities in patients with acute myocardial infarction in clinical practice: a report on repeated oral glucose tolerance tests from the GAMI study. Diabetes Care 31:36–38
Tenerz A, Norhammar A, Silveira A et al (2003) Diabetes, insulin resistance, and the metabolic syndrome in patients with acute myocardial infarction without previously known diabetes. Diabetes Care 26:2770–2776
Hage C, Malmberg K, Ryden L, Wallander M (2010) The impact of infarct type on the reliability of early oral glucose tolerance testing in patients with myocardial infarction. Int J Cardiol 145:259–260
Moura FA, Figueiredo VN, Teles BS et al (2015) Glycosylated hemoglobin is associated with decreased endothelial function, high inflammatory response, and adverse clinical outcome in non-diabetic STEMI patients. Atherosclerosis 243:124–130
Liu XJ, Wan ZF, Zhao N et al (2015) Adjustment of the GRACE score by Hemoglobin A1c enables a more accurate prediction of long-term major adverse cardiac events in acute coronary syndrome without diabetes undergoing percutaneous coronary intervention. Cardiovasc Diabetol 19:110
Geng J, Zhang Y, Wang B et al (2017) Glycosylated hemoglobin levels and clinical outcomes in nondiabetic patients with coronary artery disease: a meta-analysis. Medicine (Baltimore) 96:e6784
Shin D, Ahn J, Cha KS et al (2016) Impact of initial glycosylated hemoglobin level on cardiovascular outcomes in prediabetic patients with ST-segment elevation myocardial infarction undergoing primary percutaneous coronary intervention. Coron Artery Dis 27:40–46
Lazzeri C, Valente S, Chiostri M et al (2015) Glycated haemoglobin and long-term mortality in patients with ST Elevation Myocardial Infarction. J Cardiovasc Med (Hagerstown) 16:404–408
Shahim B, De Bacquer D, De Backer G et al (2017) The prognostic value of fasting plasma glucose, two-hour postload glucose, and HbA1c in patients with coronary artery disease: a report from EUROASPIRE IV—a survey from the European Society of Cardiology. Diabetes Care 40:1233–1240
Tailakh MA, Friger M, Zahger D et al (2017) Prospective study of the impact of diabetes mellitus newly diagnosed by glycated hemoglobin on outcomes in patients undergoing percutaneous coronary intervention. Eur J Intern Med 37:69–74
Kowalczyk J, Mazurek M, Zielinska T et al (2015) Prognostic significance of HbA1c in patients with AMI treated invasively and newly detected glucose abnormalities. Eur J Prev Cardiol 22:798–806
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare they have no conflict of interest.
Ethical approval
All procedures performed in study involving human participants were in accordance with the ethical standards of the institutional research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed consent
For this type of study formal consent is not required.
Additional information
Managed by Massimo Federici.
The original version of this article was revised: The co-author name “Thozhukat” was misspelled in the original publication and the same is corrected here.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Chattopadhyay, S., George, A., John, J. et al. Two-hour post-challenge glucose is a better predictor of adverse outcome after myocardial infarction than fasting or admission glucose in patients without diabetes. Acta Diabetol 55, 449–458 (2018). https://doi.org/10.1007/s00592-018-1114-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00592-018-1114-2